Gentamicin Arrests Cancer Cell Growth: The Intriguing Involvement of Nuclear Sphingomyelin Metabolism
Abstract
:1. Introduction
2. Results
2.1. Lymphocyte and SUP-T1 Cell Composition
2.2. What Gentamicin Does in Non-Hodgkin’s T-Cell Human Lymphoblastic Lymphoma Cells
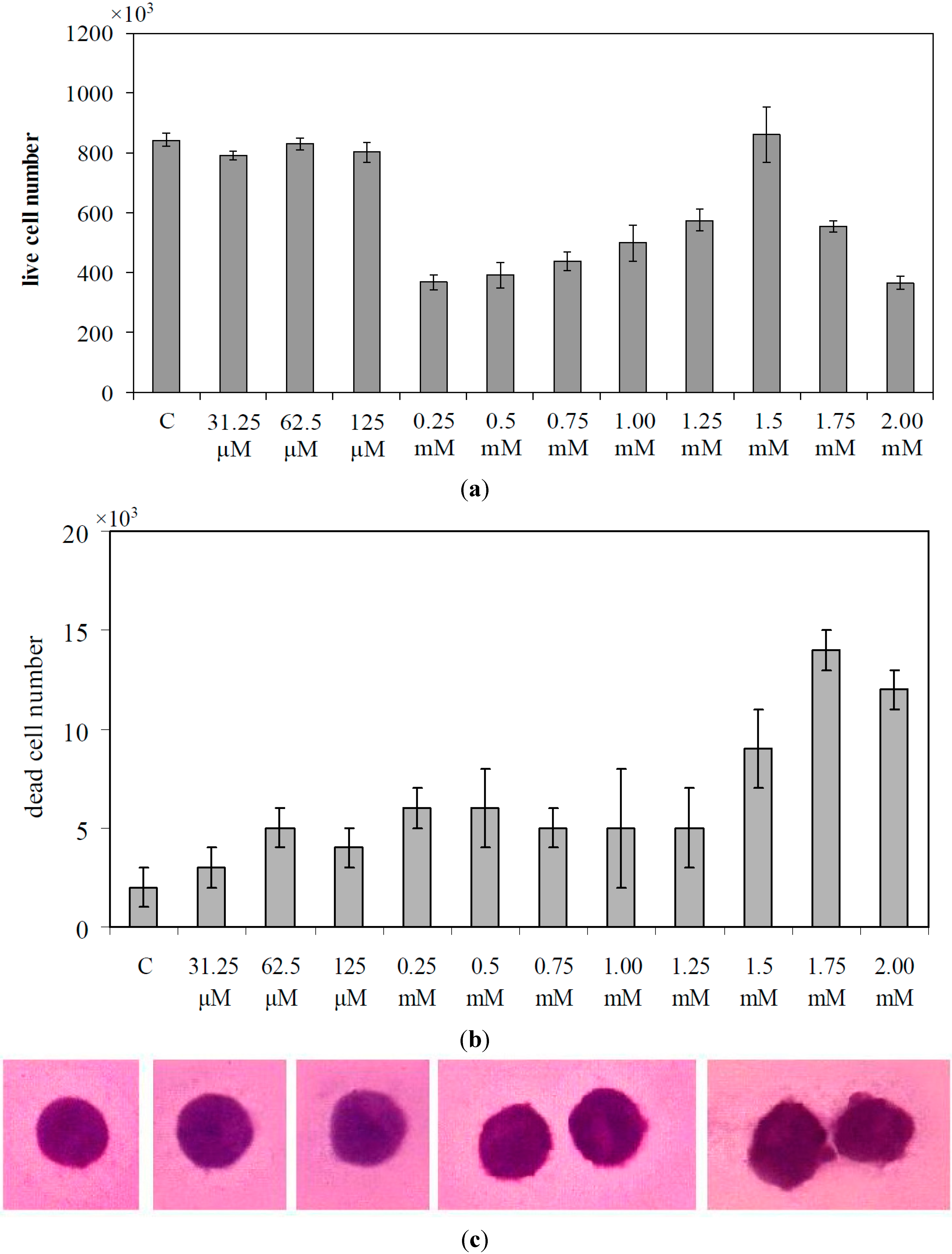


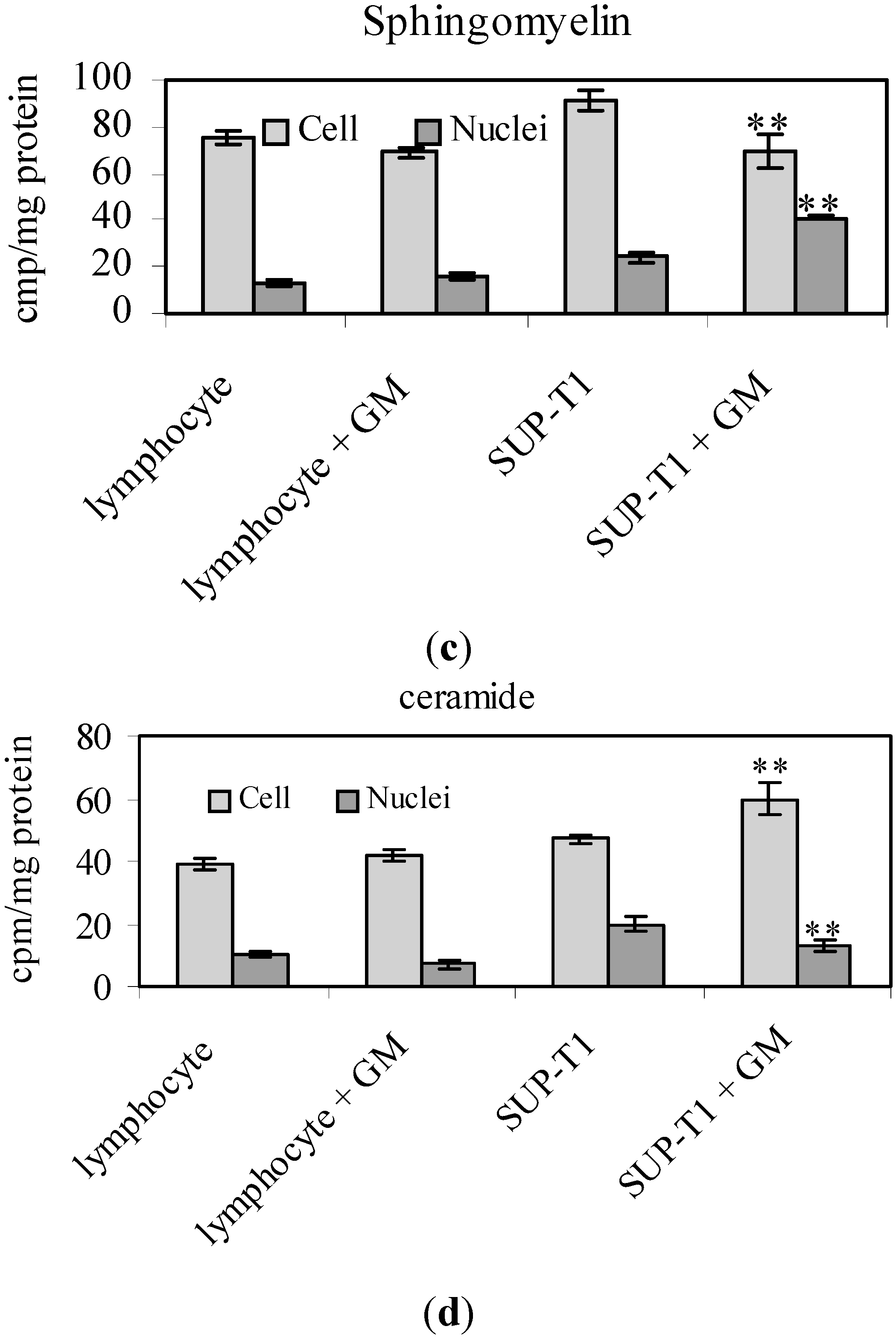
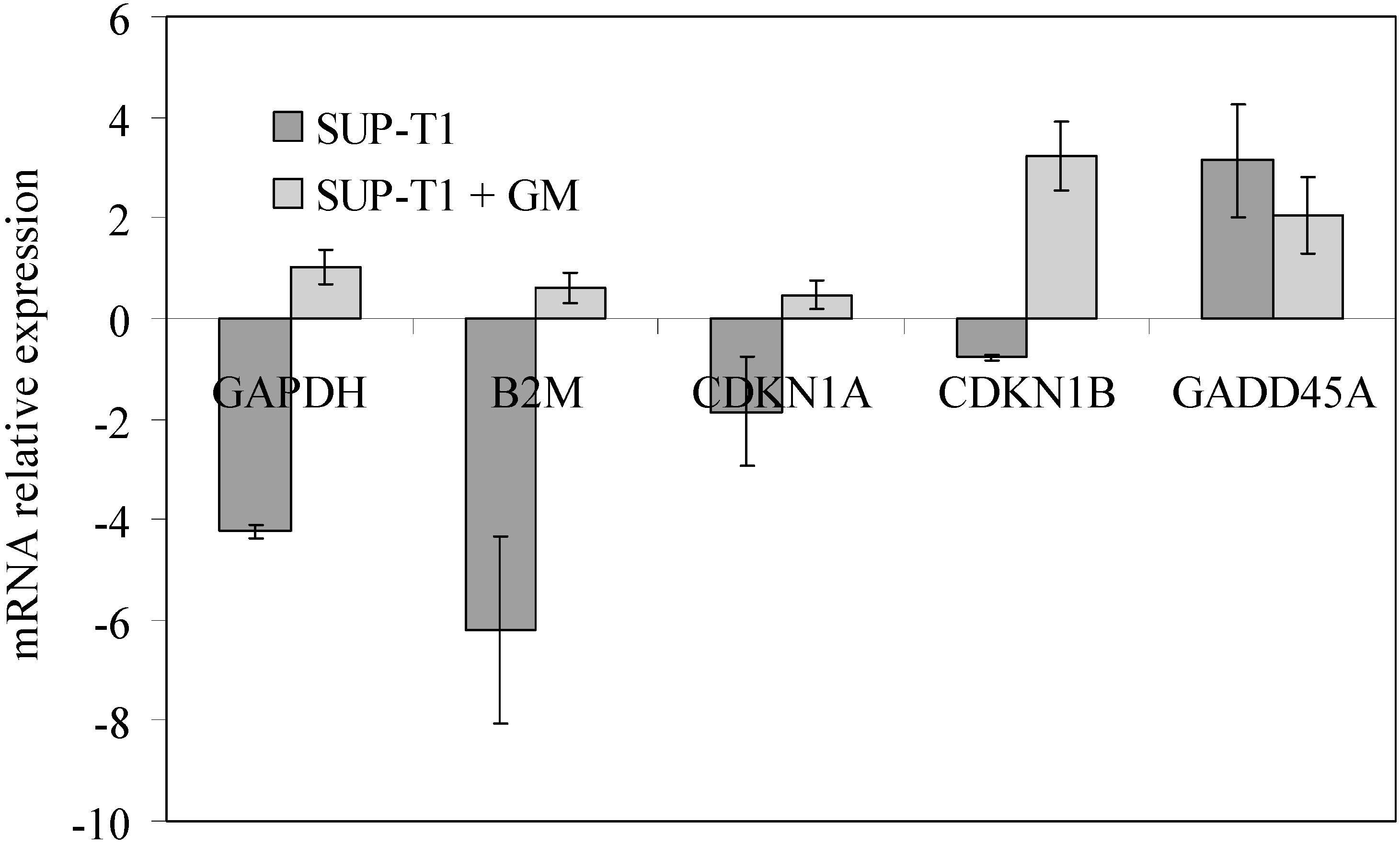
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Lymphocyte and SUP-T1 Culture
4.3. Cell Treatments
4.4. Nuclei Purification
4.5. Biochemical Determinations
4.6. Cell Morphology
4.7. Phosphatidylcholine-Specific Phospholipase C Assay
4.8. Sphingomyelinase Assay
4.9. Sphingomyelin-Synthase Assay
4.10. [3H]-Sphingomyelin and Ceramide Level
4.11. Reverse Transcription Quantitative PCR
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cuccarese, M.F.; Singh, A.; Amiji, M.; O’Doherty, G.A. A novel use of gentamicin in the ROS-mediated sensitization of NCI-H460 lung cancer cells to various anticancer agents. ACS Chem. Biol. 2013, 8, 2771–2777. [Google Scholar] [PubMed]
- Denamur, S.; Tyteca, D.; Marchand-Brynaert, J.; van Bambeke, F.; Tulkens, P.M.; Courtoy, P.J.; Mingeot-Leclercq, M.P. Role of oxidative stress in lysosomal membrane permeabilization and apoptosis induced by gentamicin, an aminoglycoside antibiotic. Free Radic. Biol. Med. 2011, 51, 1656–1665. [Google Scholar] [PubMed]
- Zhipeng, W.; Li, L.; Qibing, M.; Linna, L.; Yuhua, R.; Rong, Z. Increased expression of heat shock protein (HSP)72 in a human proximal tubular cell line (HK-2) with gentamicin-induced injury. J. Toxicol. Sci. 2006, 31, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Fathiazad, F.; Khaki, A.; Ahmadnejad, B. Protective effect of ginger on gentamicin-induced apoptosis in testis of rats. Adv. Pharm. Bull. 2012, 2, 197–200. [Google Scholar] [PubMed]
- Coffin, A.B.; Rubel, E.W.; Raible, D.W. Bax, Bcl2, and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line. J. Assoc. Res. Otolaryngol. 2013, 14, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.N.; Tabuchi, K.; Nakamagoe, M.; Nakayama, M.; Nishimura, B.; Hara, A. Ceramide/sphingomyelin cycle involvement in gentamicin-induced cochlear hair cell death. Arch. Toxicol. 2014, in press. [Google Scholar]
- Van der Luit, A.H.; Vink, S.R.; Klarenbeek, J.B.; Perrissoud, D.; Solary, E.; Verheij, M.; van Blitterswijk, W.J. A new class of anticancer alkylphospholipids uses lipid rafts as membrane gateways to induce apoptosis in lymphoma cells. Mol. Cancer Ther. 2007, 6, 2337–2345. [Google Scholar]
- Bezombes, C.; Grazide, S.; Garret, C.; Fabre, C.; Quillet-Mary, A.; Müller, S.; Jaffrézou, J.P.; Laurent, G. Rituximab antiproliferative effect in B-lymphoma cells is associated with acid-sphingomyelinase activation in raft microdomains. Blood 2004, 104, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.C.; Zheng, H.; Zhong, J. Tumor cell resistance to apoptosis due to a defect in the activation of sphingomyelinase and the 24 kDa apoptotic protease (AP24). FASEB J. 1996, 10, 325–332. [Google Scholar] [PubMed]
- Claus, R.A.; Dorer, M.J.; Bunck, A.C.; Deigner, H.P. Inhibition of sphingomyelin hydrolysis: Targeting the lipid mediator ceramide as a key regulator of cellular fate. Curr. Med. Chem. 2009, 16, 1978–2000. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Viola Magni, M.P. The role of intranuclear lipids. Biol. Cell. 2004, 96, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; La Porta, C.A.; Cataldi, S.; Magni, M.V. Nuclear sphingomyelin-synthase and protein kinase C δ in melanoma cells. Arch. Biochem. Biophys. 2005, 438, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Garcia Rodriguez, L.A.; Gonzalez-Perez, A. Use of antibiotics and risk of breast cancer. Am. J. Epidemiol. 2005, 161, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Rossini, A.; Rumio, C.; Sfondrini, L.; Tagliabue, E.; Morelli, D.; Miceli, R.; Mariani, L.; Palazzo, M.; Ménard, S.; Balsari, A. Influence of antibiotic treatment on breast carcinoma development in proto-neu transgenic mice. Cancer Res. 2006, 66, 6219–6224. [Google Scholar]
- Cummings, M.; Sarveswaran, J.; Homer-Vanniasinkam, S.; Burke, D.; Orsi, N.M. Glyceraldehyde-3-phosphate dehydrogenase is an inappropriate housekeeping gene for normalising gene expression in sepsis. Inflammation 2014, 37, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Leisner, T.M.; Moran, C.; Holly, S.P.; Parise, L.V. CIB1 prevents nuclear GAPDH accumulation and non-apoptotic tumor cell death via AKT and ERK signaling. Oncogene 2013, 32, 4017–4027. [Google Scholar] [CrossRef] [PubMed]
- Palomero, T.; Couronné, L.; Khiabanian, H.; Kim, M.Y.; Ambesi-Impiombato, A.; Perez-Garcia, A.; Carpenter, Z.; Abate, F.; Allegretta, M.; Haydu, J.E.; et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat. Genet. 2014, 46, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Newbold, A.; Salmon, J.M.; Martin, B.P.; Stanley, K.; Johnstone, R.W. The role of p21waf1/cip1 and p27Kip1 in HDACi-mediated tumor cell death and cell cycle arrest in the Eμ-myc model of B-cell lymphoma. Oncogene 2013. [Google Scholar] [CrossRef]
- Bustany, S.; Tchakarska, G.; Sola, B. Cyclin D1 regulates p27Kip1 stability in B cells. Cell Signal. 2011, 23, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Plyusnina, E.; Shaposhnikov, M.; Shilova, L.; Kazachenok, A.; Zhavoronkov, A. The role of D-GADD45 in oxidative, thermal and genotoxic stress resistance. Cell Cycle 2012, 11, 4222–4241. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Codini, M.; Cascianelli, G.; Tringali, S.; Tringali, A.R.; Lazzarini, A.; Floridi, A.; Bartoccini, E.; Garcia-Gil, M.; Lazzarini, R.; et al. Nuclear lipid microdomain as resting place of dexamethasone to impair cell proliferation. Int. J. Mol. Sci. 2014, 15, 19832–19846. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Lazzarini, A.; Lazzarini, R.; Floridi, A.; Damaskopoulou, E.; Curcio, F.; Cataldi, S. Nuclear lipid microdomain as place of interaction between sphingomyelin and DNA during liver regeneration. Int. J. Mol. Sci. 2013, 14, 6529–6541. [Google Scholar] [CrossRef] [PubMed]
- Albi, E. The role of intranuclear lipids in health and disease. Clin. Lipidol. 2011, 6, 59–69. [Google Scholar] [CrossRef]
- Pugliese, L.; Bernardini, I.; Pacifico, N.; Peverini, M.; Damaskopoulou, E.; Cataldi, S.; Albi, E. Severe hypocholesterolaemia is often neglected in haematological malignancies. Eur. J. Cancer 2010, 46, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Bartoccini, F.; Cascianelli, G.; Voccoli, V.; Caviglia, M.G.; Magni, M.P.; Garcia-Gil, M.; Albi, E. Effect of 1α,25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus 2010, 20, 696–705. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Cascianelli, G.; Villani, M.; Tosti, M.; Marini, F.; Bartoccini, E.; Magni, M.V.; Albi, E. Lipid microdomains in cell nucleus. Mol. Biol. Cell 2008, 19, 5289–5295. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Rossi, G.; Viola Magni, M.; Toller, M.; Casani, S.; Perrella, G. The nuclear ceramide/diacylglycerol balance depends on the physiological state of thyroid cells and changes during UV-C radiation-induced apoptosis. Arch. Biochem. Biophys. 2008, 478, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Bartoccini, E.; Magni, M.V.; Marini, F.; Mazzoni, F.; Rainaldi, G.; Evangelista, M.; Garcia-Gil, M. Nuclear sphingomyelin pathway in serum deprivation-induced apoptosis of embryonic hippocampal cells. J. Cell Physiol. 2006, 206, 189–195. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codini, M.; Cataldi, S.; Ambesi-Impiombato, F.S.; Lazzarini, A.; Floridi, A.; Lazzarini, R.; Curcio, F.; Beccari, T.; Albi, E. Gentamicin Arrests Cancer Cell Growth: The Intriguing Involvement of Nuclear Sphingomyelin Metabolism. Int. J. Mol. Sci. 2015, 16, 2307-2319. https://doi.org/10.3390/ijms16022307
Codini M, Cataldi S, Ambesi-Impiombato FS, Lazzarini A, Floridi A, Lazzarini R, Curcio F, Beccari T, Albi E. Gentamicin Arrests Cancer Cell Growth: The Intriguing Involvement of Nuclear Sphingomyelin Metabolism. International Journal of Molecular Sciences. 2015; 16(2):2307-2319. https://doi.org/10.3390/ijms16022307
Chicago/Turabian StyleCodini, Michela, Samuela Cataldi, Francesco Saverio Ambesi-Impiombato, Andrea Lazzarini, Alessandro Floridi, Remo Lazzarini, Francesco Curcio, Tommaso Beccari, and Elisabetta Albi. 2015. "Gentamicin Arrests Cancer Cell Growth: The Intriguing Involvement of Nuclear Sphingomyelin Metabolism" International Journal of Molecular Sciences 16, no. 2: 2307-2319. https://doi.org/10.3390/ijms16022307
APA StyleCodini, M., Cataldi, S., Ambesi-Impiombato, F. S., Lazzarini, A., Floridi, A., Lazzarini, R., Curcio, F., Beccari, T., & Albi, E. (2015). Gentamicin Arrests Cancer Cell Growth: The Intriguing Involvement of Nuclear Sphingomyelin Metabolism. International Journal of Molecular Sciences, 16(2), 2307-2319. https://doi.org/10.3390/ijms16022307





