Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Biochemical and Histopathological Pulmonary Changes in Mice
Abstract
:1. Introduction
2. Results
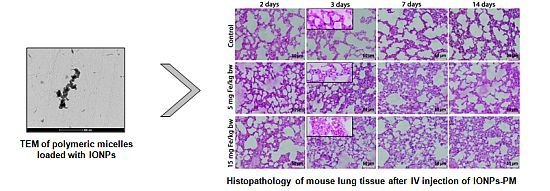
2.1. Characterization of Polymeric Micelles Loaded with Magnetic NPs
| Sample | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| Pristine IONPs | 12.5 | – |
| DSPE-PEG micelles | 14.9 | −30.1 |
| DSPE-PEG micelles loaded with IONPs | 21.8 | −28.7 |
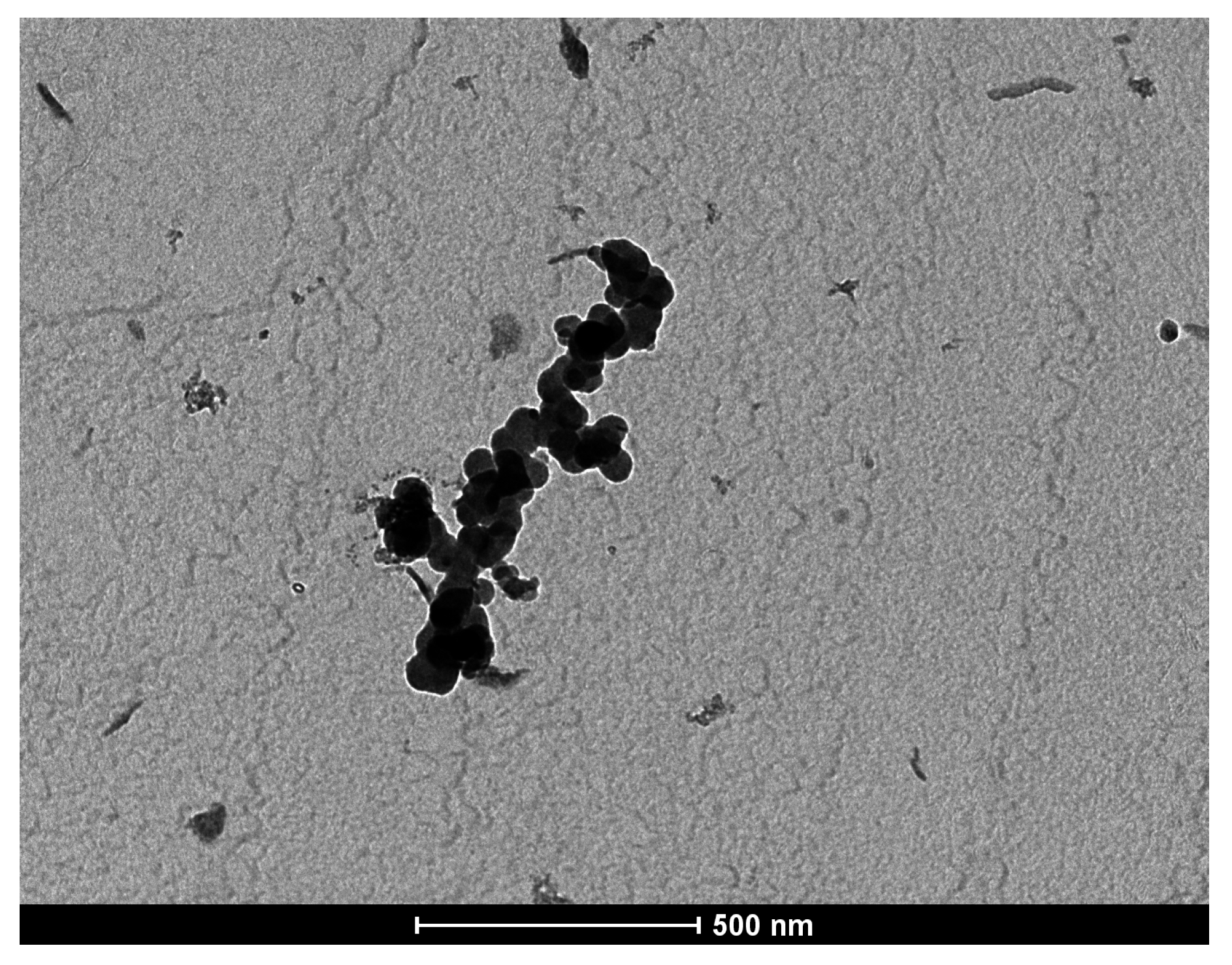
2.2. Morphopathological Changes in Mice Lung Tissue
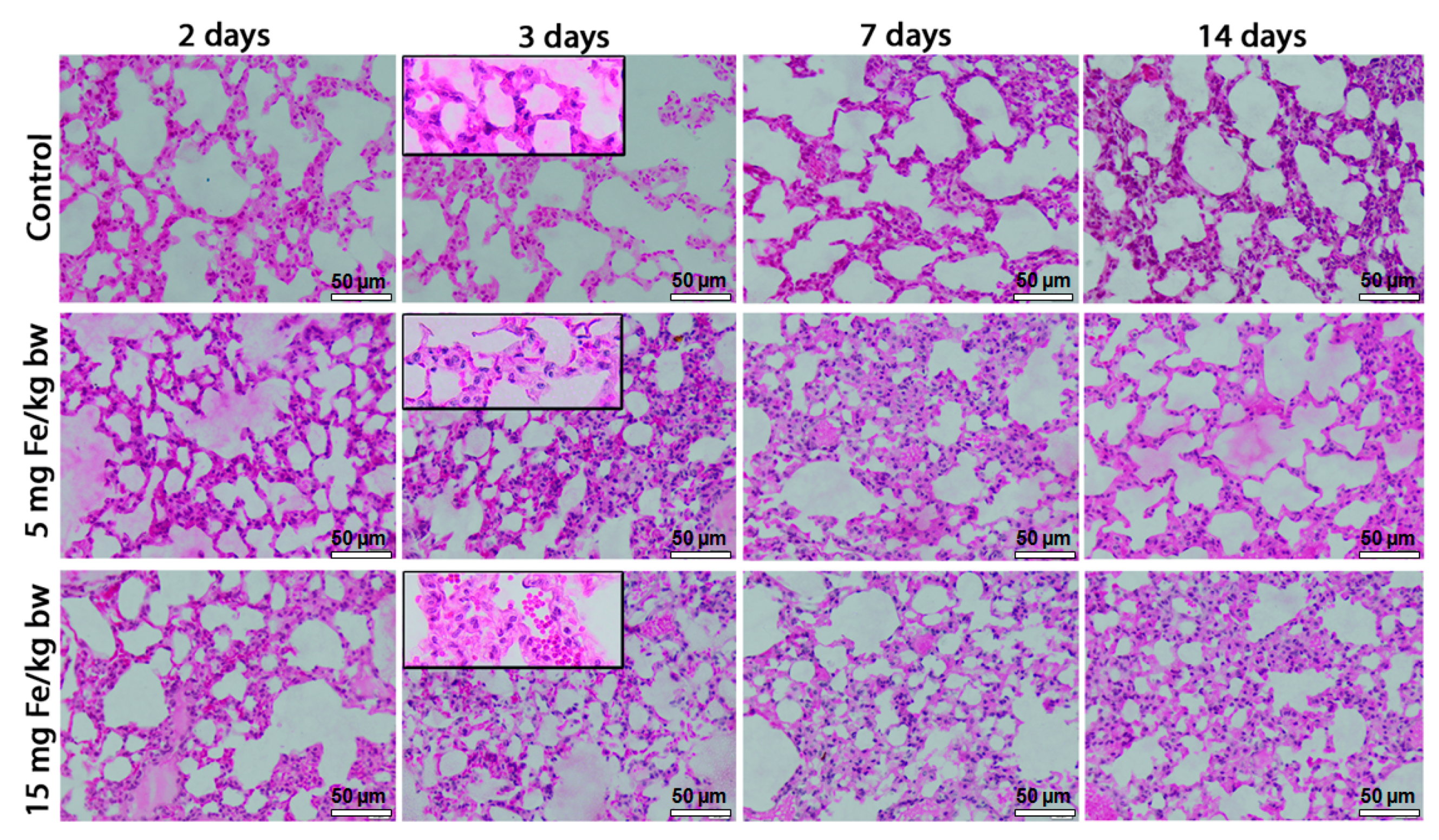
2.3. Total LDH Activity
2.4. Activity of Oxidative Stress Related Enzymes
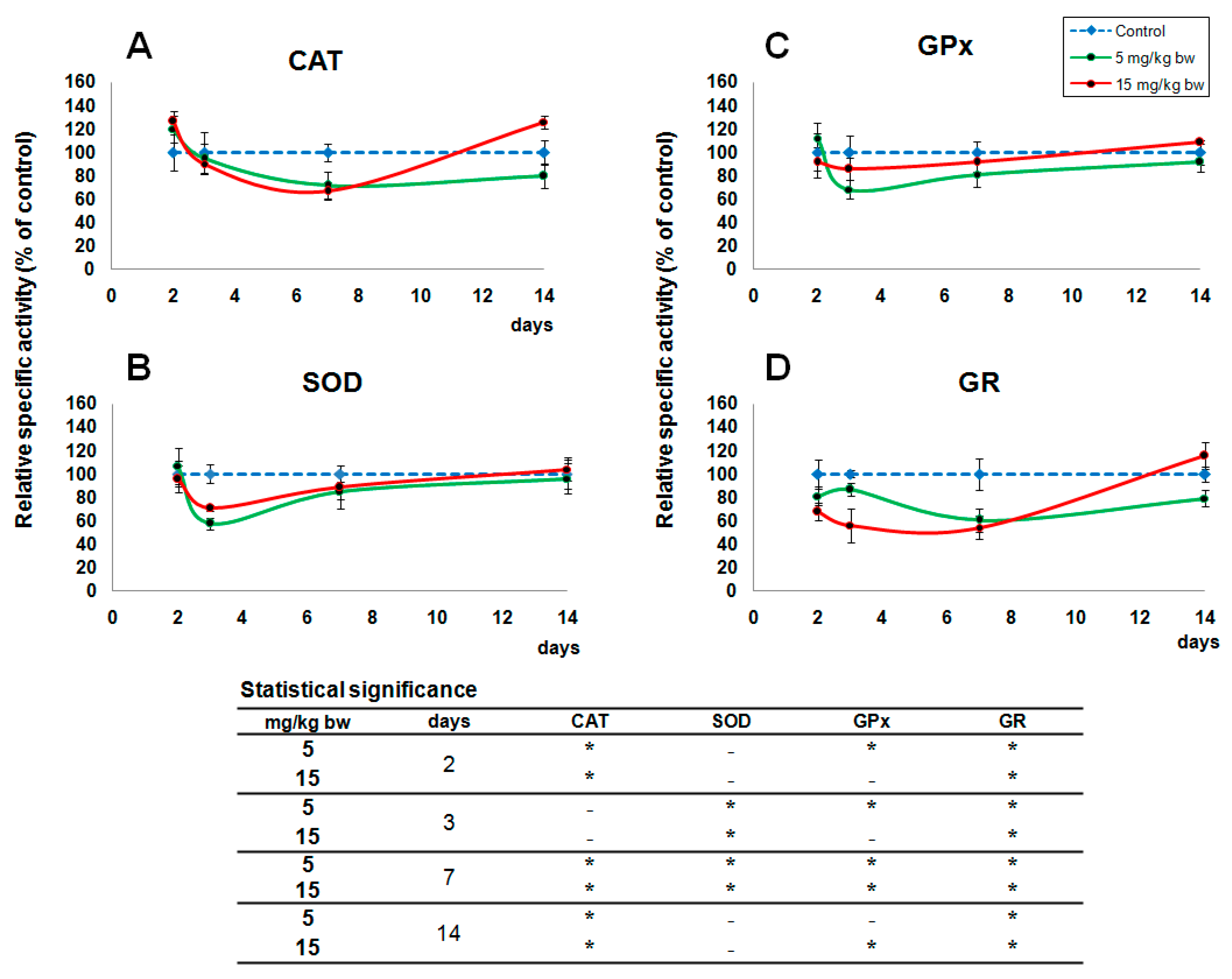
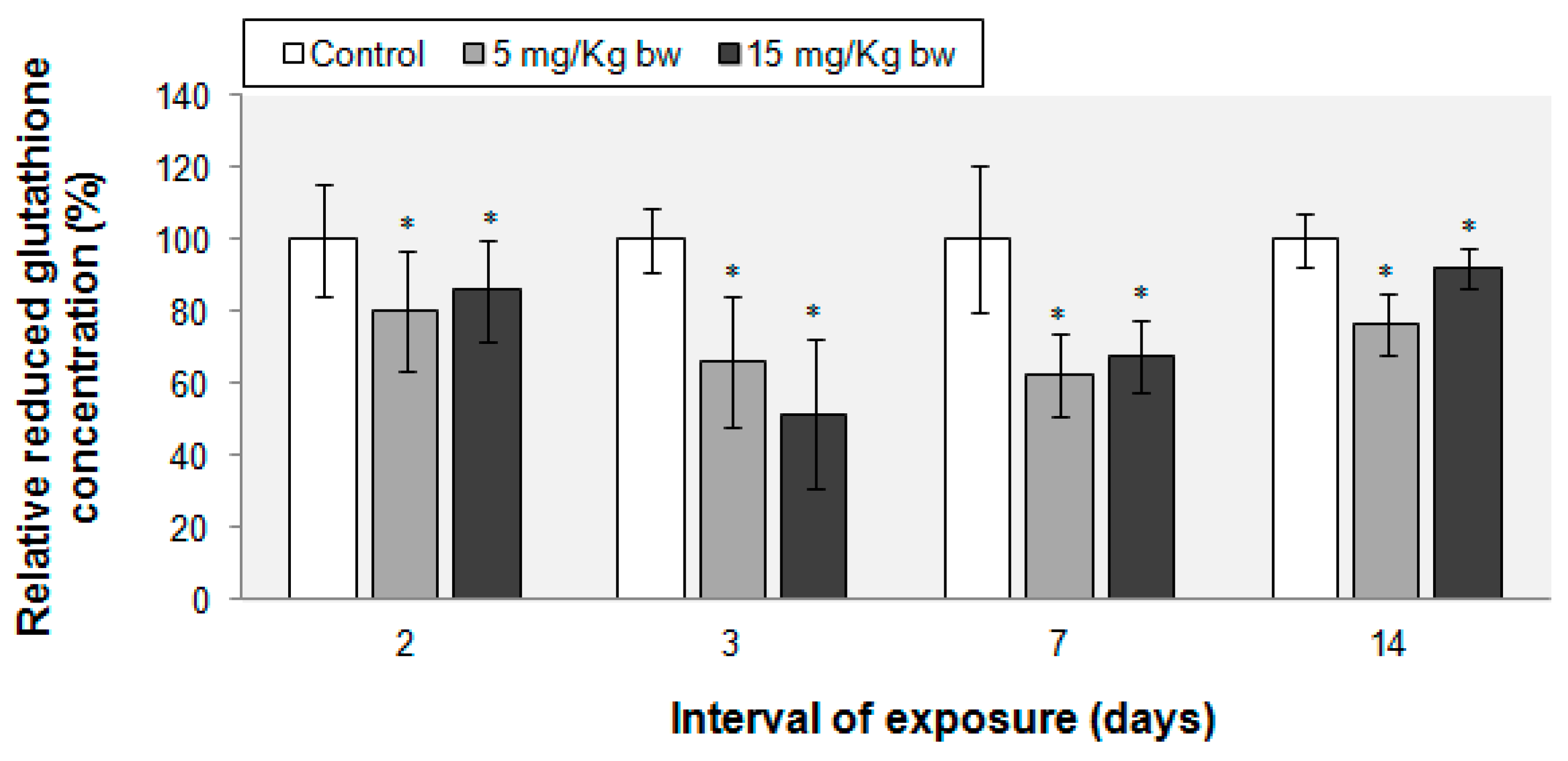
2.5. Variations in Reduced Glutathione (GSH) Content
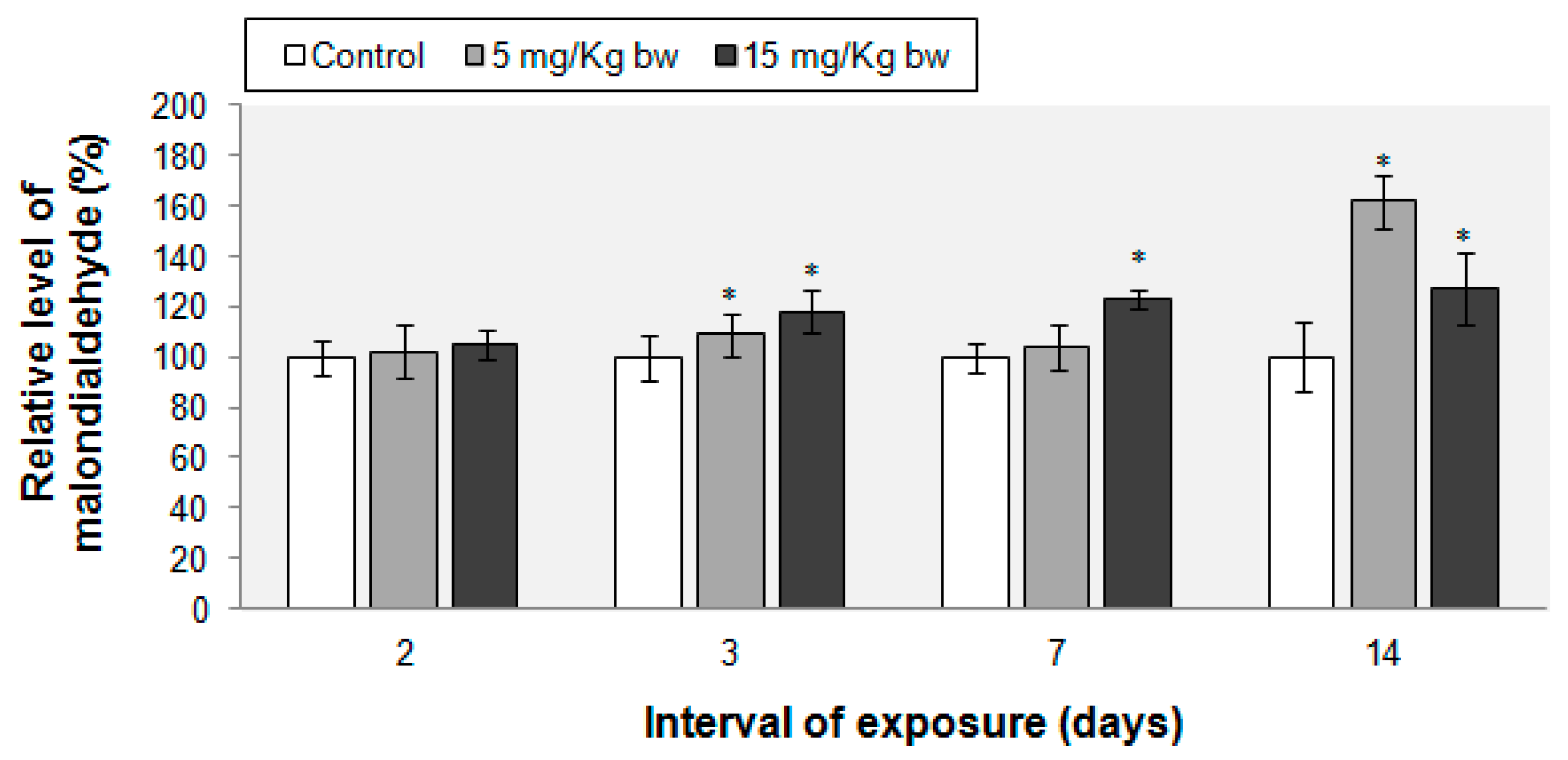
2.6. Lipid Peroxidation Evaluation
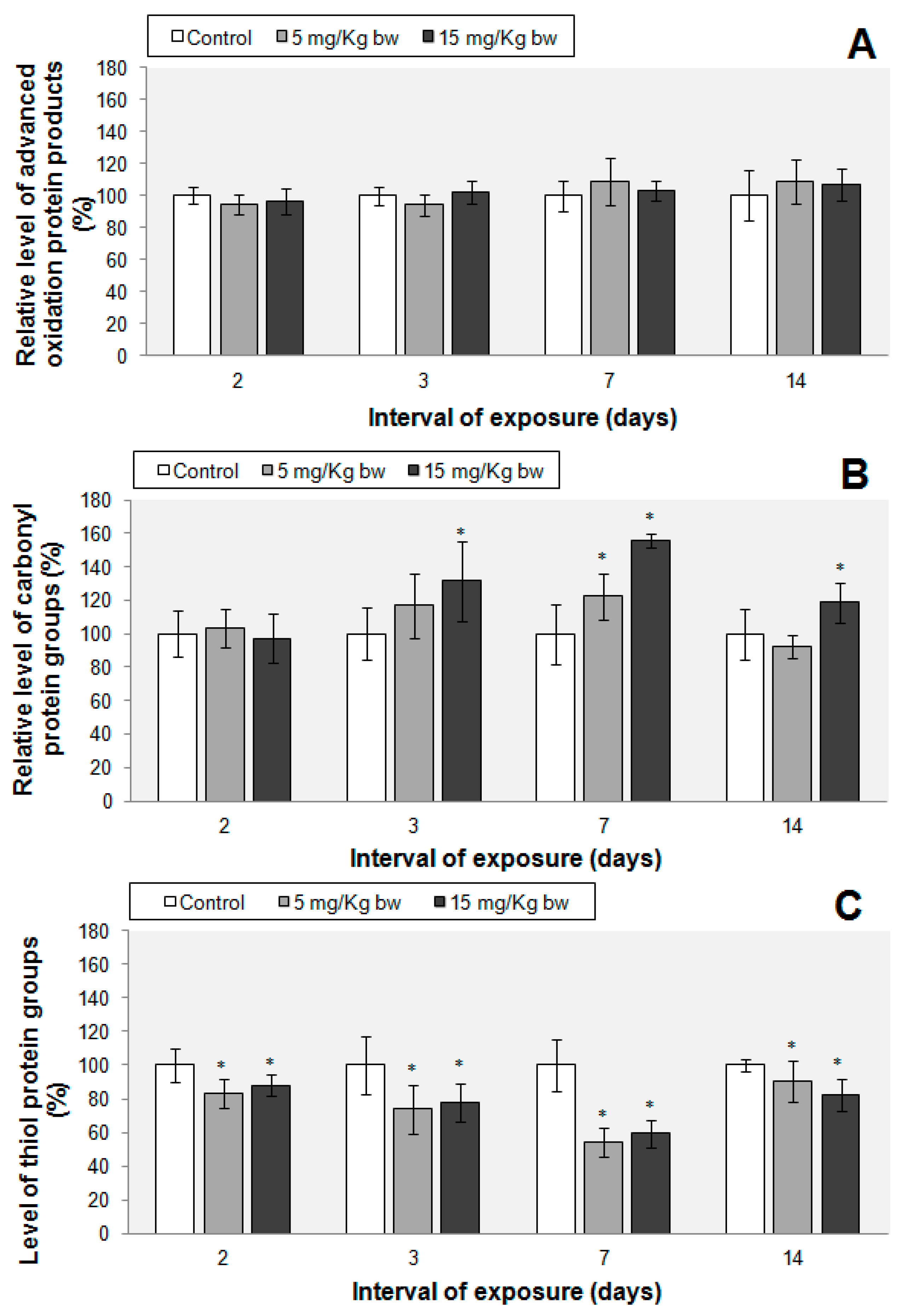
2.7. Protein Oxidations Markers
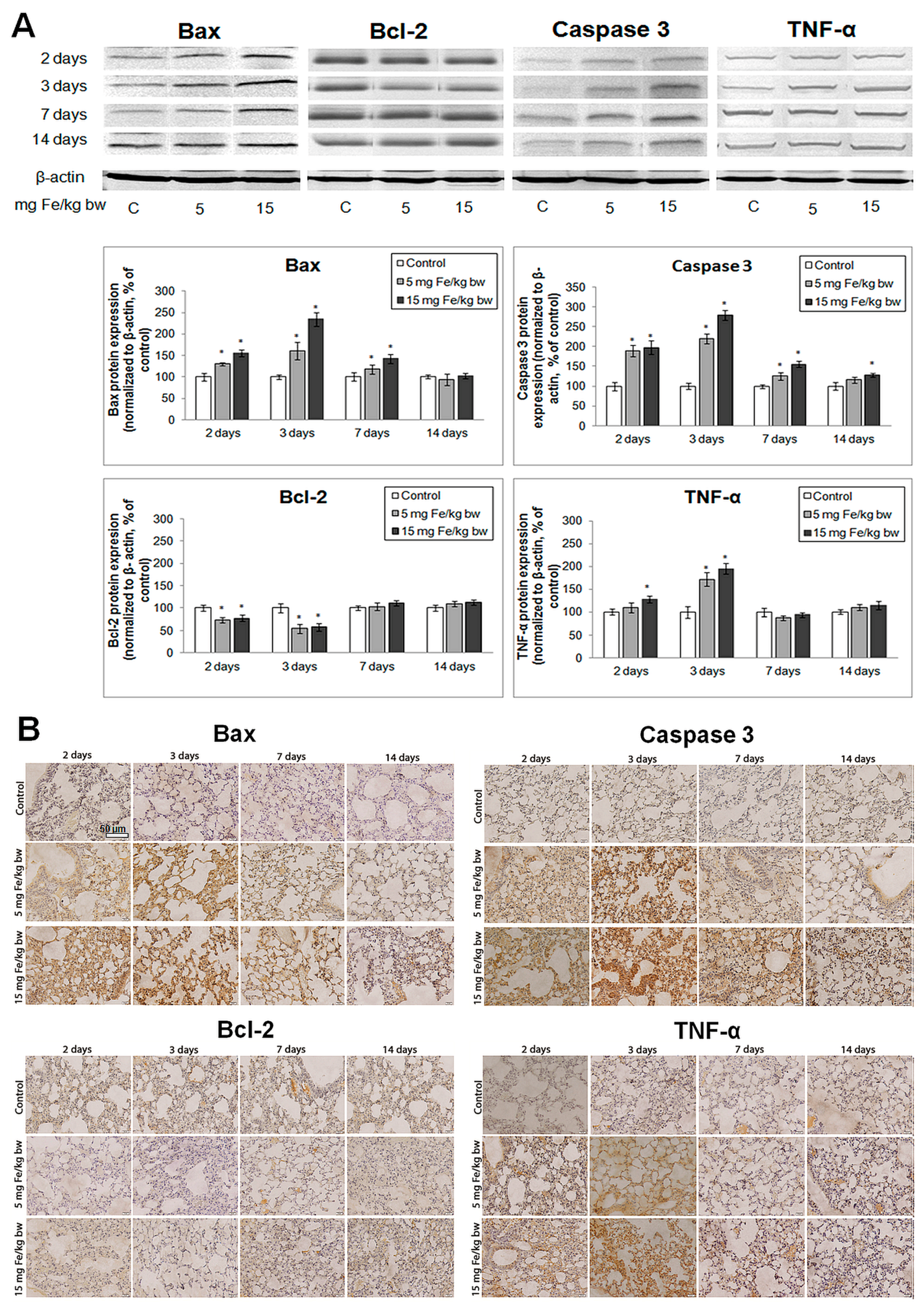
2.8. Evaluation of Apoptotic Markers
3. Discussion
4. Experimental Section
4.1. Reagents
4.2. Synthesis of Magnetic IONPs
4.3. Preparation of Polymeric Micelles Loaded with IONPs
4.4. Characterization of Polymeric Micelles Loaded with IONPs
4.5. Experimental Animals and Treatment
4.6. Histopathology
4.7. Tissue Extract Preparation and Protein Determination
4.8. Measurement of Total LDH Activity
4.9. Enzymatic Activities
4.10. Reduced Glutathione Quantification
4.11. Lipid Peroxidation Measurement
4.12. Proteins Oxidation Assays
- Advanced oxidation protein products (AOPP) levels in the mouse lung tissue were evaluated according to the method of Witko et al. (1992) [73]. Briefly, a volume of 200 µL of protein extract was mixed with 10 µL of 1.16 M KI in a 96-well plate and allowed to stand for 5 min at room temperature. Subsequently, 20 µL of glacial acetic acid were added and, after 10 min, the optical density was read at 340 nm. Chloramine T was used as a standard. The results were calculated as µmoles of AOPP/mg of protein and expressed as % of controls.
- Protein carbonyl groups (CO) levels were measured according to the Fields and Dixon’s method (1971) [74], which is based on the reaction of 2,4-dinitrophenylhydrazine (DNPH) with protein carbonyls resulting in hydrazones. A volume of 500 µL protein extract was incubated with 500 µL of 10 mM DNPH (in 2 M HCl), for 1 h, at room temperature. The proteins were then precipitated with 500 µL ice-cold 20% TCA and centrifuged at 13,000 rpm for 3 min. The pellets were washed three times with 500 µL ethanol:ethyl acetate mixture and dissolved in 600 µL 1 M NaOH. The samples absorbance was read at 370 nm and the concentration expressed in nmoles CO/mg protein was calculated using a molar extinction coefficient of 22.000 M−1·cm−1. Finally the concentrations were expressed as % of controls.
- Protein sulfhydryl groups (-SH) were determined according to the method of Riener et al. (2002) [75]. Briefly, the protein extract (100 µL) was deproteinized with an equal volume of 20% TCA and centrifuged for 10 min at 10,000 rpm, at 4 °C. The pellet was dissolved in 20 µL of 1 M NaOH. Before reading the optical density at 324 nm, the soluble pellets were incubated for 5 min with 730 µL of 0.4 M Tris-HCl buffer, pH 9 and 30 µL of 4 mM 4,4′-dithiodipyridine (DTDP). The concentration of protein sulfhydryl groups (nmoles/mg of protein) was quantified using a N-acetyl-cysteine standard curve and expressed as % of controls.
4.13. Immunological Techniques
4.13.1. Immunohistochemistry
4.13.2. Immunoblot Analysis
4.14. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.M.; Kim, D.K.; El Haj, A.J.; Dobson, J. Development of superparamagnetic iron oxide nanoparticles (SPIONS) for translation to clinical applications. IEEE Trans. Nanobiosci. 2008, 7, 298–305. [Google Scholar]
- Soenen, S.J.; Himmerlreich, U.; Nuytten, N.; Cuyper, M.D. Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials 2011, 32, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Juang, R.S. Biochemical and biomedical applications of multifunctional magnetic nanoparticles: A review. J. Nanopart. Res. 2011, 13, 4411–4430. [Google Scholar] [CrossRef]
- Bulte, J.W.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Wu, C.H.; Aronstam, R.S. Toxicity of transition metal oxide nanoparticles: Recent insights from in vitro studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Shokrgozar, M.A.; Milani, A.S.; Häfeli, U.O.; Stroeve, P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2010, 75, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Radu, M.; Dinu, D.; Sima, C.; Burlacu, R.; Hermenean, A.; Ardelean, A.; Dinischiotu, A. Magnetite nanoparticles induced adaptative mechanisms counteract cell death in human pulmonary fibroblasts. Toxicol. In Vitro 2015, 29, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Samim, M.; Abdin, M.Z.; Ahmed, F.J.; Maitra, A.N.; Prashant, C.K.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Holgersson, A.; Moller, L. Mechanisms related to the genotoxicity of particles in the subway and from other sources. Chem. Res. Toxicol. 2008, 21, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Suzuki, K.I.; Ishihara, A.; Kubo-Irie, M.; Fujimoto, R.; Tabata, M.; Oshio, S.; Nihei, Y.; Ihara, T.; Sugamata, M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J. Health Sci. 2009, 55, 95–102. [Google Scholar] [CrossRef]
- Gu, L.; Fang, R.H.; Sailor, M.J.; Park, J.H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano 2012, 6, 4947–4954. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Reed, K.L.; Warheit, D.B. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Szalay, B. Iron Oxide Nanoparticles and Their Toxicological Effects: In vitro and in vivo Studies. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2012. [Google Scholar]
- Zhuang, J.; Fan, K.; Gao, L.; Lu, D.; Feng, J.; Yang, D.; Gu, N.; Zhang, Y.; Liang, M.; Yan, X. Ex vivo detection of iron oxide magnetic nanoparticles in mice using their intrinsic peroxidase-mimicking activity. Mol. Pharm. 2012, 9, 1983–1939. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Hernández, Y.; Cabal, C.; González, E.; Veintemillas-Verdaguer, S.; Martínez, E.; Morales, M.P. Biodistribution and pharmacokinetics of uniformmagnetite nanoparticles chemically modified with polyethylene glycol. Nanoscale 2013, 5, 11400–11408. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.L.; Xu, Y.F.; Qi, X.R.; Maitani, Y.; Nagai, T. Superparamagnetic iron oxide nanoparticles stabilized by alginate: Pharmacokinetics, tissue distribution, and applications in detecting liver cancers. Int. J. Pharm. 2008, 354, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787–794. [Google Scholar]
- Raynal, I.; Prigent, P.; Peyramaure, S.; Najid, A.; Rebuzzi, C.; Corot, C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: Mechanisms and comparison of ferumoxides and ferumoxtran-10. Investig. Radiol. 2004, 39, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.A.; Petryk, A.A.; Giustini, A.J.; Hoopes, P.J. In vivobiodistribution of iron oxide nanoparticles: An overview. Proc. SPIE 2011. [Google Scholar] [CrossRef]
- Gao, Z.; Lukyanov, A.N.; Torchilin, V.P. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002, 2, 979–982. [Google Scholar] [CrossRef]
- Cinteza, L.O.; Ohulchanskyy, T.Y.; Sahoo, Y.; Bergey, E.J.; Pandey, R.K.; Prasad, P.N. Diacyllipid micelle-based nanocarrier for magnetically guided delivery of drugs in photodynamic therapy. Mol. Pharm. 2006, 3, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Marecos, E.; Bogdanov, A.; Weissleder, R. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology 2000, 214, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, L.-L.; Zeng, Y.; Liu, G. Toxicity of superparamagnetic iron oxide nanoparticles: Research strategies and implications for nanomedicine. Chin. Phys. B 2013, 22, 127503. [Google Scholar] [CrossRef]
- Patil, U.S.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.R.; Chrisey, D.B. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. Int. J. Mol. Sci. 2015, 6, 24417–24450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yu, M.K.; Jeong, Y.Y.; Kim, J.W.; Lee, K.; Phan, V.N.; Jon, S. Antibiofouling amphiphilic polymer-coated superparamagnetic iron oxide nanoparticles: Synthesis, characterization, and use in cancer imaging in vivo. J. Mater. Chem. 2009, 19, 6412–6417. [Google Scholar] [CrossRef]
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Turi, J.L.; Yang, F.; Garrick, L.M.; Garrick, M.D. Iron homeostasis in the lung. Biol. Res. 2006, 39, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Dunford, H.B. Oxidations of iron(II)/(III) by hydrogen peroxide total plasma malondialdehyde with mild derivatization conditions. Clin. Chem. 2002, 49, 690–692. [Google Scholar]
- Briley-Saebo, K.; Bjornerud, A.; Grant, D.; Ahlstrom, H.; Berg, T.; Kindberg, G.M. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: Implications for magnetic resonance imaging. Cell Tissue Res. 2004, 316, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.L. Human copper-containing superoxide dismutase of high molecular weight. Proc. Natl. Acad. Sci. USA 1982, 79, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, J.; Vízek, M. Oxidative stress in the lung tissue—Sources of reactive oxygen species and antioxidant defence. Prague Med. Rep. 2007, 108, 105–114. [Google Scholar] [PubMed]
- Bargagli, E.; Olivieri, C.; Bennett, D.; Prasse, A.; Muller-Quernheim, J.; Rottoli, P. Oxidative stress in the pathogenesis of diffuse lung diseases: A review. Respir. Med. 2009, 103, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharides coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef]
- Düssman, H.; Kögel, D.; Rehm, M.; Prehn, H.M. Mitochondrial membrane permeabilization and superoxide production during apoptosis. J. Biol. Chem. 2003, 278, 12645–12649. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moeller, L. Copper oxide nanoparticles are highly toxic: Acomparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.A.; Ali, D.; Alarafi, S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and Polymer Polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Lin, X.; Wang, S.; Li, L.; Liu, Y.; Ye, L.; Wang, G. Biological evaluation of Fe3O4-poly(l-lactide)-poly(ethylene glycol)-poly(l-lactide) magnetic microspheres prepared in supercritical CO2. Toxicol. Lett. 2012, 212, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kareyeva, A.V.; Grivennikova, V.G.; Vinogradov, A.D. Mitochondrial hydrogen peroxide production as determined by the pyridine nucleotide pool and its redox state. Biochem. Biophys. Acta 2012, 1817, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Kareyeva, A.V.; Grivennikova, V.G.; Cecchini, G.; Vinogradov, A.D. Molecular identification of the enzyme responsible for the mitochondrial NADH-supported ammonium-dependent hydrogen peroxide production. FEBS Lett. 2011, 585, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.M.; Cinteza, L.O.; Hermenean, A.; Dinischiotu, A. In vivo exposure of mice spleen to magnetic nanoparticles encapsulated in phospholipid polymeric micelles; an oxidative stress and structural approach. Dig. J. Nanomater. Biostruct. 2015, 10, 871–881. [Google Scholar]
- Zhu, M.T.; Feng, W.Y.; Wang, B.; Wang, T.C.; Gu, Y.Q.; Wang, M.; Wang, Y.; Ouyang, H.; Zhao, Y.L.; Chai, Z.F. Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology 2008, 247, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. Oxidative stress and lung inflammation in airway disease. Eur. J. Pharmacol. 2001, 429, 195–207. [Google Scholar] [CrossRef]
- Repine, J.E.; Bast, A.; Lankhorst, I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am. J. Respir. Crit. Care Med. 1997, 156, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, U.S.; Paulraj, R. Iron oxide nanoparticles induced oxidative damage in peripheral blood cells of rat. J. Biomed. Sci. Eng. 2015, 8, 274–286. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation and aging. Free Radic. Res. 2006, 40, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Drake, J.; Pocernich, C.; Butterfield, D.A. Protein Carbonyl Levels—An Assessment of Protein Oxidation. In Methods in Pharmacology and Toxicology: Methods in Biological Oxidative Stress; Hensley, K., Floyd, R.A., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2003; pp. 161–168. [Google Scholar]
- Kowaltowski, A.J.; Vercesi, A.E. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. 1999, 26, 463–471. [Google Scholar] [CrossRef]
- Forkert, P.G.; Custer, E.M.; Alpert, A.J.; Ansari, G.A.; Reynolds, E.S. Lactate dehydrogenase activity in mouse lung following 1,1-dichloroethylene: Index of airway injury. Exp. Lung Res. 1982, 4, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Siddiqui, M.A.; Farshori, N.N.; Ahamed, M.; Musarrat, J.; Al-Khedhairy, A.A. Synthesis, characterization and toxicological evaluation of iron oxide nanoparticles in human lung alveolar epithelial cells. Colloids Surf. B Biointerfaces 2014, 122, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Fattman, C.L. Apoptosis in pulmonary fibrosis: Too much or not enough? Antioxid. Redox Signal. 2008, 10, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, K. Involvement of epithelial cell apoptosis in interstitial lung diseases. Intern. Med. 2008, 47, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Hsin, Y.; Chen, C.; Huang, S.; Shih, T.; Lai, P.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.M.; Manjula, C.; GnanaKumar, G.; Kanjwal, M.A.; Sekar, T.V.; Paulmurugan, R.; Rajaguru, P. Oxidative stress-mediated cytotoxicity and apoptosis induction by TiO2 nanofibers in HeLa cells. Eur. J. Pharm. Biopharm. 2012, 81, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Ravichandran, P.; Copeland, C.L.; Gopikrishnan, R.; Biradar, S.; Goornavar, V.; Ramesh, G.T.; Hall, J.C. Magnetite induces oxidative stress and apoptosis in lung epithelial cells. Mol. Cell. Biochem. 2011, 363, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.C.; Aggarwal, B.B. TNF-induced signaling in apoptosis. J. Clin. Immunol. 1999, 19, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G. The mitochondrial production of reactive oxygen species: Mechanisms and implications in human pathology. IUBMB Life 2001, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. Br. Med. J. 2007, 334, 197. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Maina, T. Of mice and humans: Are they the same?—Implications in cancer translational research. J. Nucl. Med. 2010, 51, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.J. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin-Phenol reagents. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Borgmann, V.; Moon, T.; Laidler, K. Molecular kinetics of beef heart lactate dehydrogenase. Biochemistry 1974, 13, 5152–5158. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods of Enzymatic Analysis; Bergmayer, H.U., Ed.; FRG: Weinheim, Germany, 1984; pp. 673–684. [Google Scholar]
- Paoletti, F.; Aldinucci, D.; Mocali, A.; Caparrini, A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 1986, 154, 538–541. [Google Scholar] [CrossRef]
- Beutler, E. Glutathione peroxidase. In Red Cell Metabolism. A Manual of Biochemical Methods; Grune and Stratlon: Orlando, FL, USA, 1984; pp. 74–76. [Google Scholar]
- Goldberg, D.M.; Spooner, R.J. Glutathione reductase. In Methods of Enzymatic Analysis; Bergmayer, H.U., Ed.; Verlag Chemie: Dearfield Beach, FL, USA, 1983; pp. 258–265. [Google Scholar]
- Dinischiotu, A.; Stanca, L.; Gradinaru, D.; Petrache, S.N.; Radu, M.; Serban, A. Chapter 10. Lipid Peroxidation due toin vitro and in vivo exposure of biological samples to nanoparticles. In Oxidative Stress and Nanotechnology: Methods and Protocols, Methods in Molecular Biology; Armstrong, D., Bharali, D.J., Eds.; Springer Science+Business Media: New York, NY, USA, 2013; Volume 1028, pp. 155–164. [Google Scholar]
- Witko, V.; Nguyen, A.T.; Descamps-Latscha, B. Microtiter plate assay for phagocyte-derived taurine-chloramines. J. Clin. Lab. Anal. 1992, 6, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.; Dixon, H.B.F. Micro method for determination of reactive carbonyl groups in proteins and peptides, using 2,4-dinitrophenylhydrazine. Biochem. J. 1971, 121, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Riener, C.; Kada, G.; Gruber, H.J. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4′-dithiodipyridine. Anal. Bioanal. Chem. 2002, 373, 266–276. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, M., (Balas); Din, I.M., (Popescu); Hermenean, A.; Cinteză, O.L.; Burlacu, R.; Ardelean, A.; Dinischiotu, A. Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Biochemical and Histopathological Pulmonary Changes in Mice. Int. J. Mol. Sci. 2015, 16, 29417-29435. https://doi.org/10.3390/ijms161226173
Radu M (Balas), Din IM (Popescu), Hermenean A, Cinteză OL, Burlacu R, Ardelean A, Dinischiotu A. Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Biochemical and Histopathological Pulmonary Changes in Mice. International Journal of Molecular Sciences. 2015; 16(12):29417-29435. https://doi.org/10.3390/ijms161226173
Chicago/Turabian StyleRadu, Mihaela, (Balas), Ioana Mihaela Din, (Popescu), Anca Hermenean, Otilia Ludmila Cinteză, Radu Burlacu, Aurel Ardelean, and Anca Dinischiotu. 2015. "Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Biochemical and Histopathological Pulmonary Changes in Mice" International Journal of Molecular Sciences 16, no. 12: 29417-29435. https://doi.org/10.3390/ijms161226173
APA StyleRadu, M., (Balas), Din, I. M., (Popescu), Hermenean, A., Cinteză, O. L., Burlacu, R., Ardelean, A., & Dinischiotu, A. (2015). Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Biochemical and Histopathological Pulmonary Changes in Mice. International Journal of Molecular Sciences, 16(12), 29417-29435. https://doi.org/10.3390/ijms161226173