Gold-Coated Superparamagnetic Nanoparticles for Single Methyl Discrimination in DNA Aptamers
Abstract
:1. Introduction
| Name | Sequence |
|---|---|
| TBA1 | HS-5’-T15GGTTGGTGTGGTTGG-3’ |
| TBA2 | HS-5’-T5AGTCCGTGGTAGGGCAGGTTGGGGTGACT-3’ |
| O6-MeG-TBA1 | HS-5’-T15GGTTGMeGTGTGGTTGG-3’ |
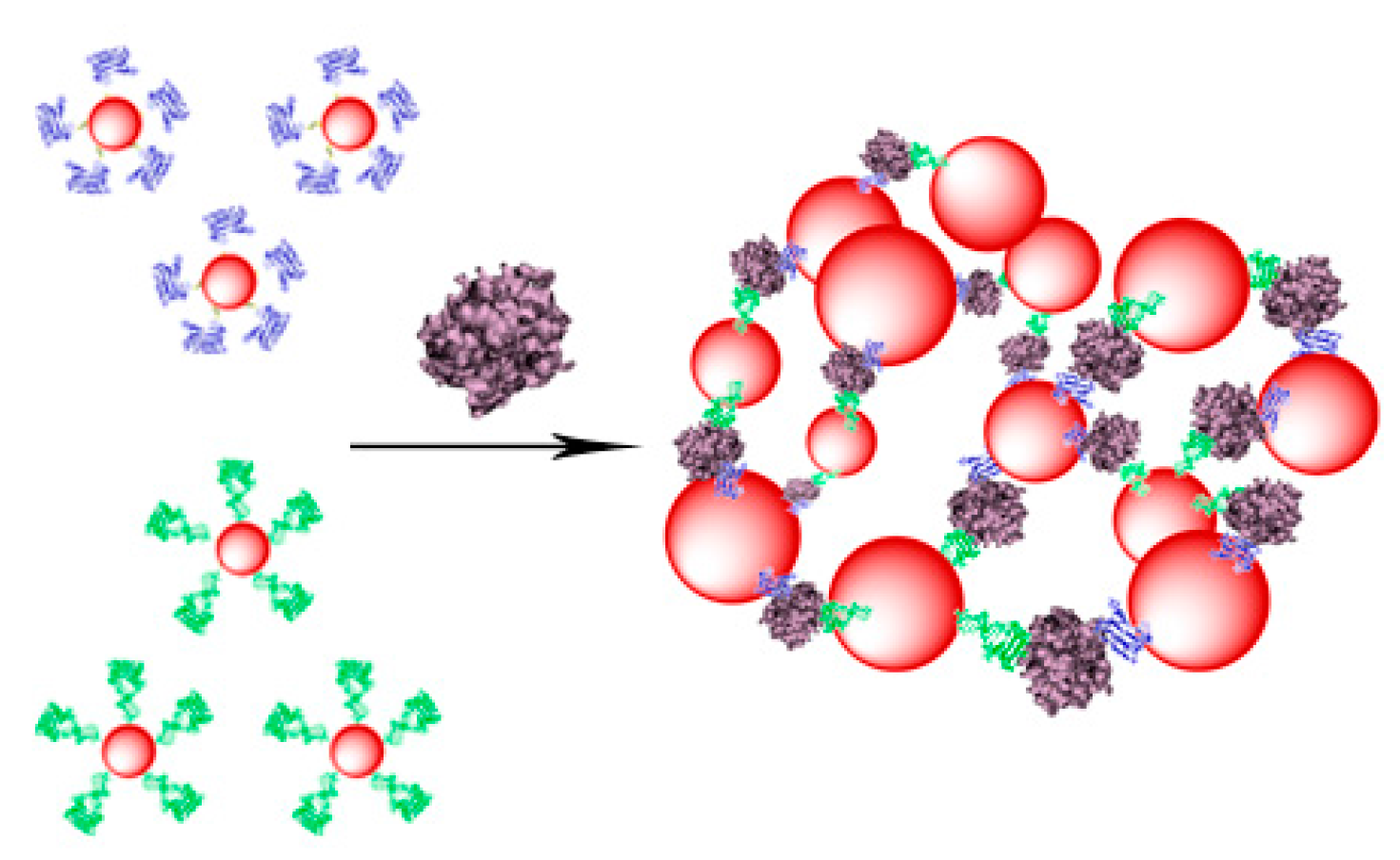
2. Results and Discussion
2.1. Preparation of Gold Superparamagnetic Iron Oxide Nanoparticles (AuSPION)
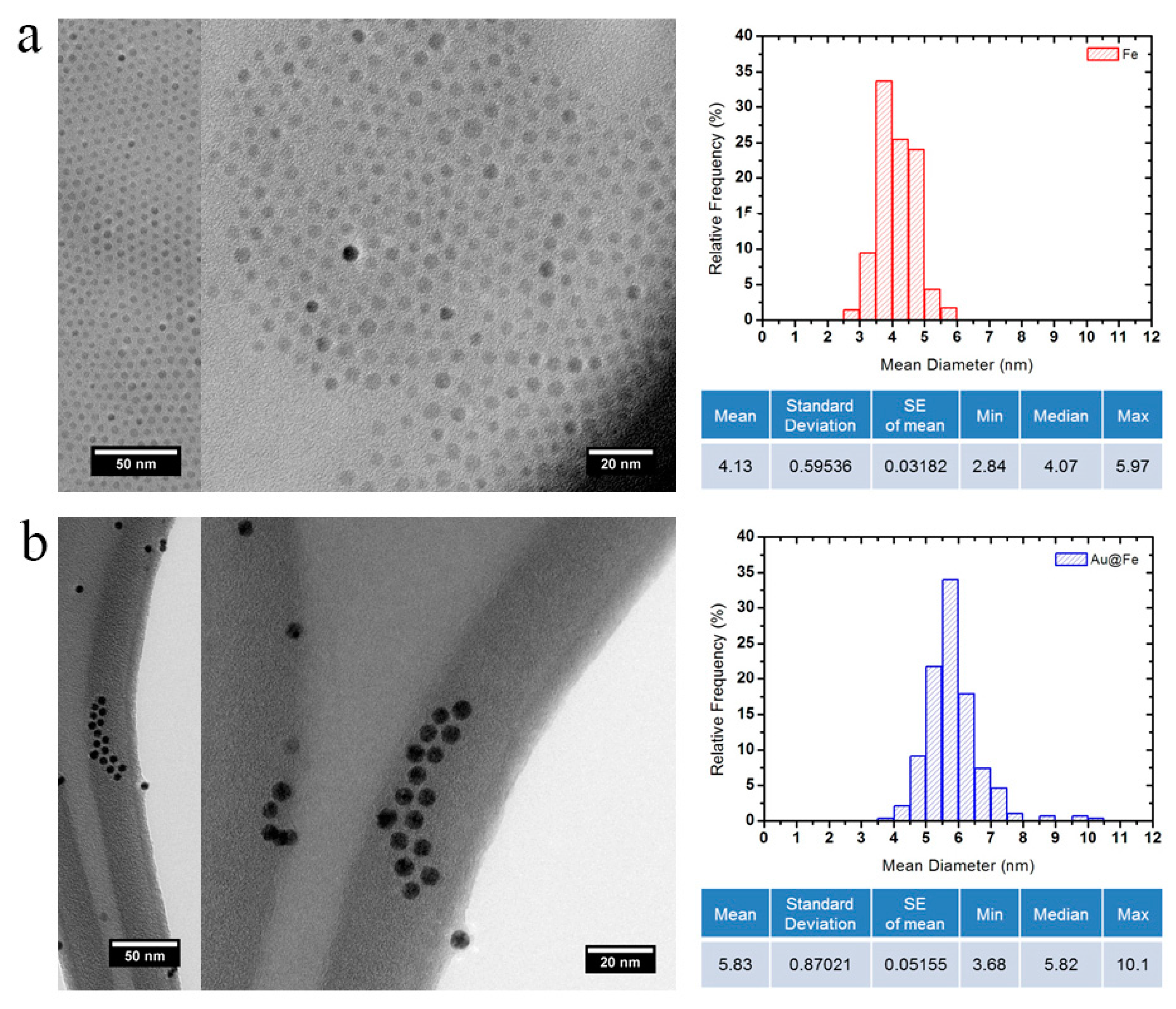
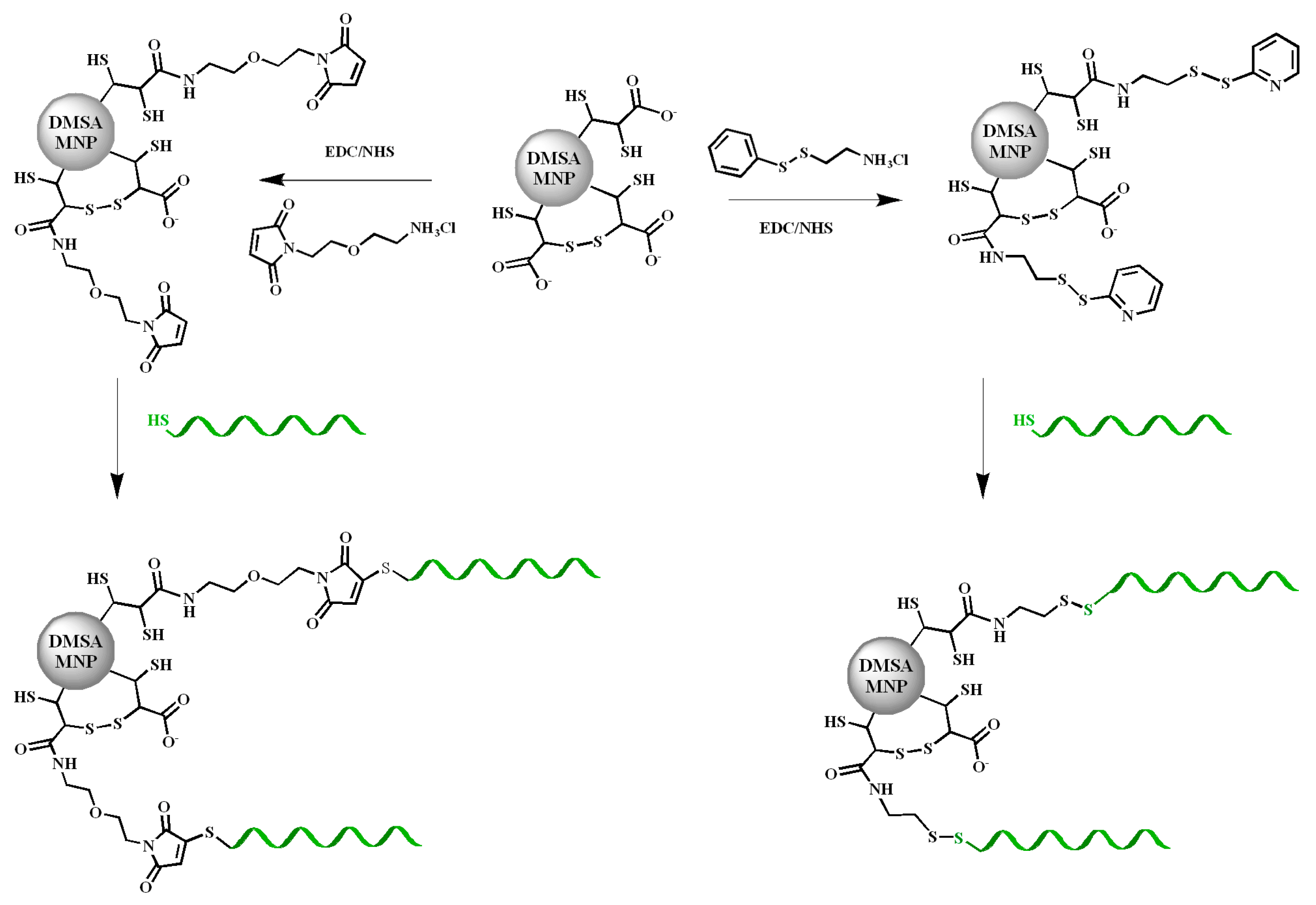
2.2. Conjugation of the AuNPs, AuSPIONs and SPIONs with TBA1, TBA2 and O6-MeG-TBA1
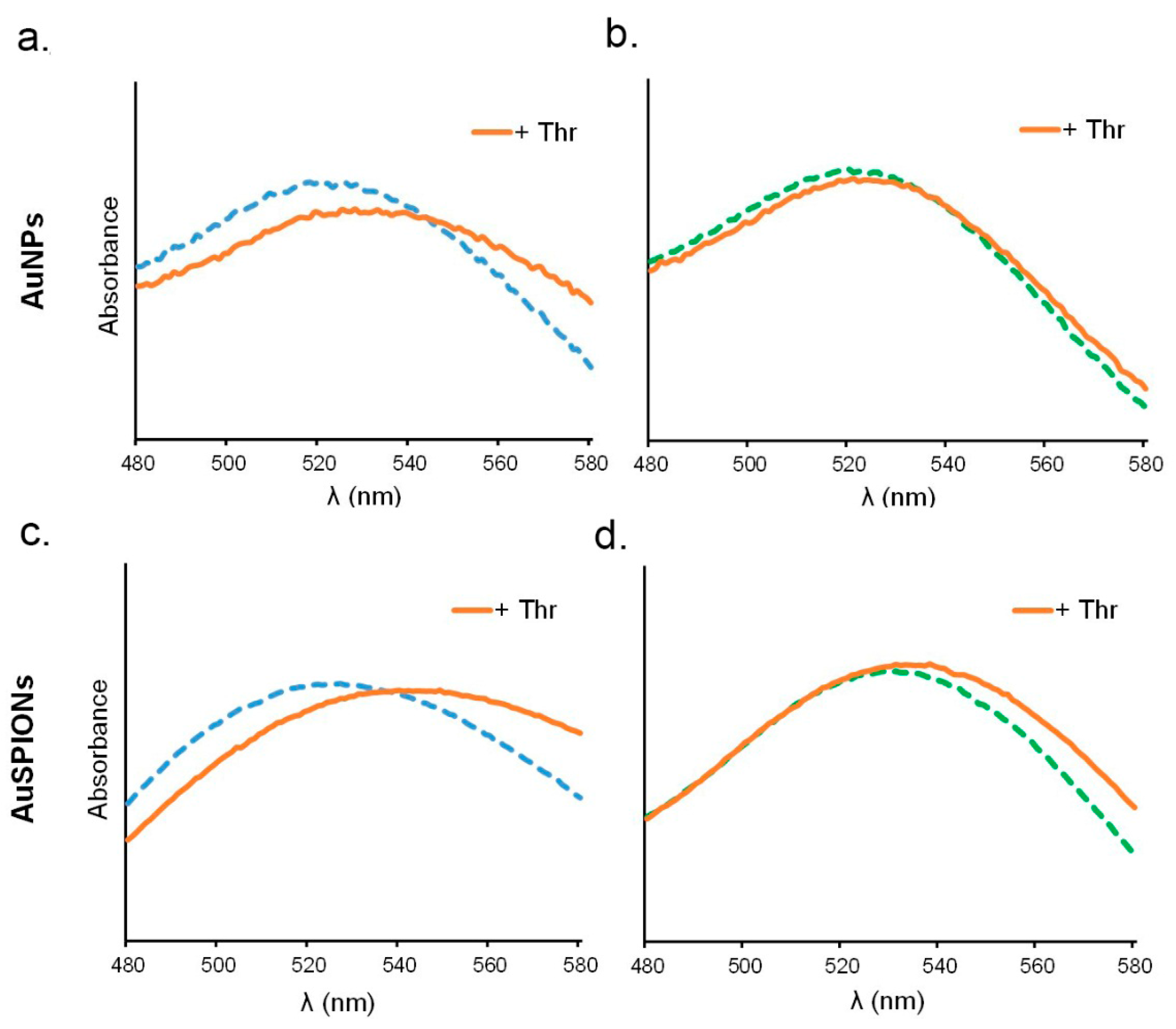
2.3. UV Study of the Complex Formation between α-Thrombin and AuNPs or AuSPIONs Functionalized with TBAs
2.4. DLS Study of the Complex Formation between α-Thrombin and NPs-TBAs
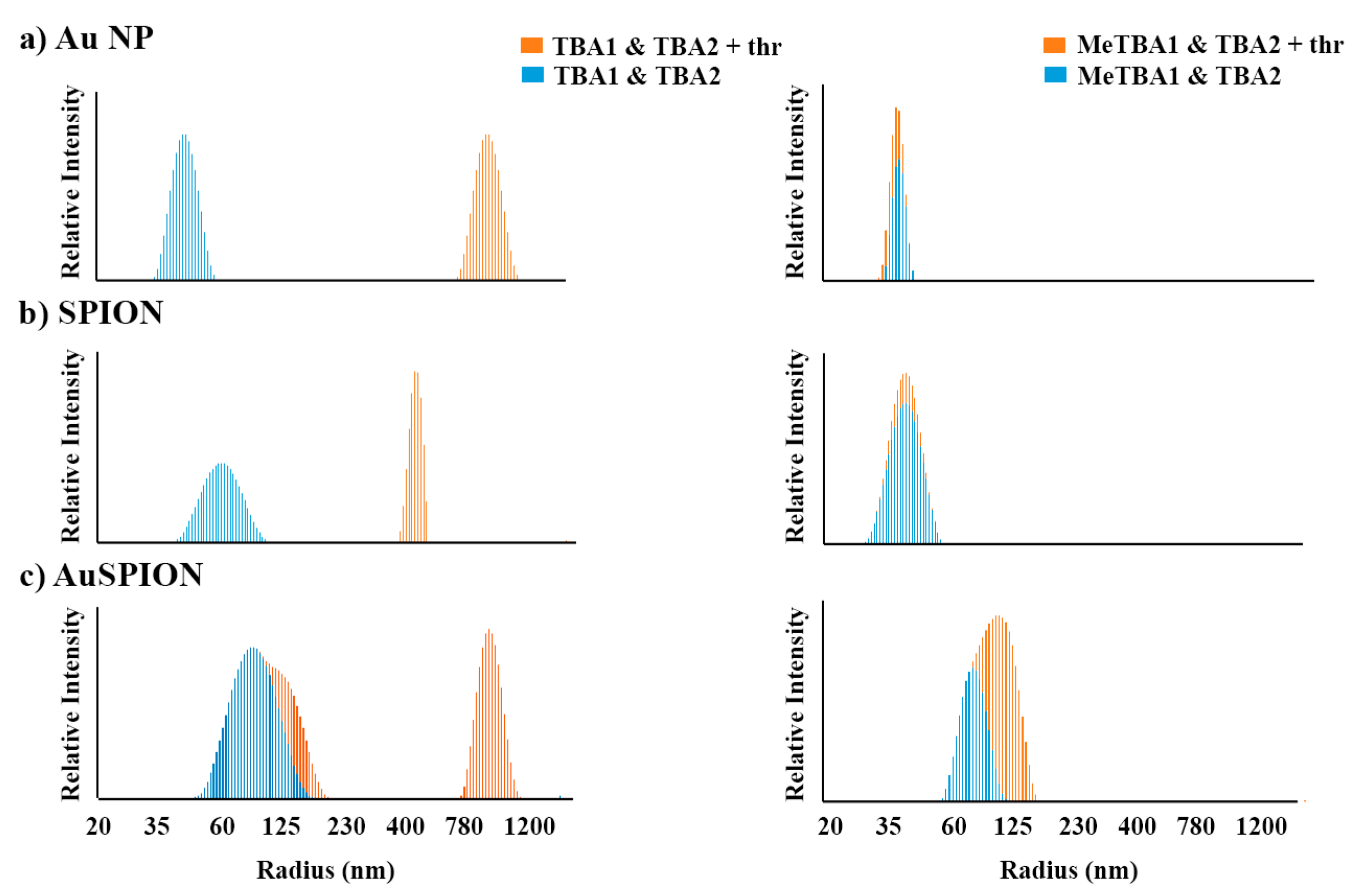
| Nanoparticles Type | Without α-Thrombin HD (nm) | With α-Thrombin HD (nm) | |
|---|---|---|---|
| AuNPs | TBA1 & TBA2 | 38 ± 5 | 947 ± 283 |
| O6-MeG-TBA1 & TBA2 | 38 ± 5 | 56 ± 7 | |
| SPIONs | TBA1 & TBA2 | 36 ± 5 | 781 ± 185 |
| O6-MeG-TBA1 & TBA2 | 39.5 ± 11 | 39.5 ± 11 | |
| AuSPIONs | TBA1 & TBA2 | 91 ± 25 | 633 ± 225 |
| O6-MeG-TBA1 & TBA2 | 91 ± 25 | 109 ± 34 | |
2.5. Magnetic Resonance Imaging
| Nanoparticles Type | Concentration of α-Thrombin | ||
|---|---|---|---|
| 0 | 5 nM | ||
| SPIONs | TBA1 & TBA2 | 50 ± 3 ms | 40 ± 3 ms |
| O6-MeG-TBA1 & TBA2 | 51 ± 4 ms | 51 ± 5 ms | |
| AuSPIONs. | TBA1 & TBA2 | 70 ± 1 ms | 62 ± 1 ms |
| O6-MeG-TBA1 & TBA2 | 63 ± 2 ms | 62 ± 1 ms | |
3. Experimental Section
3.1. Chemicals
3.2. Instrumentation
3.3. Oligonucleotides Synthesis
3.4. Synthesis of SPIONs
3.5. Synthesis of Maleimide Linker
3.6. Synthesis of Thiopyridinyl Linker (PDA*HCl)
3.7. Functionalization of DMSA Coated FexOy Nanoparticles with Maleimide or PDA*HCl Linker
3.8. Synthesis of AuSPIONs
3.8.1. Synthesis of FexOy MNP as Seeds
3.8.2. Reduction of Au–Acetate (Coating)
3.9. Functionalization of the Different Type of Nanoparticles
3.9.1. Gold Nanoparticles (AuNPs)
3.9.2. Superparamagnetic Iron Oxide Nanoparticles (SPION)
3.9.3. Gold Superparamagnetic Iron Oxide Nanoparticles (AuSPION)
3.10. Studies of α-Thrombin Interactions with TBA-Functionalized AuNPs and AuSPIONs by UV
3.11. Studies of α-Thrombin Interaction with TBAs Nanoparticles (AuNPS, SPIONs and AuSPIONs) by DLS
3.12. Studies of α-Thrombin Interaction with TBAs Nanoparticles (SPIONs and AuSPIONs) by MRI
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Subbiah, R.; Veerapandian, M.; Yun, K.S. Nanoparticles: Functionalization and multifunctional applications in biomedical sciences. Curr. Med. Chem. 2010, 17, 4559–4577. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Singh, A.V. Biophysicochemical perspective of nanoparticle compatibility: A critically ignored parameter in nanomedicine. J. Nanosci. Nanotechnol. 2014, 14, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Willner, B.; Willner, I. Biomolecule-nanoparticle hybrids as functional units for nanobiotechnology. Chem. Commun. 2007, 4, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Storhoff, J.J.; Mirkin, C.A. Programmed materials synthesis with DNA. Chem. Rev. 1999, 99, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Brook, M.A.; Li, Y. Design of gold nanoparticle-based colorimetric biosensing assays. ChemBioChem 2008, 9, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. In. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366. [Google Scholar] [CrossRef] [PubMed]
- Teichroeb, J.H.; Forrest, J.A.; Ngai, V.; Jones, L.W. Anomalous thermal denaturing of proteins adsorbed to nanoparticles. Eur. Phys. J. E Soft Matter 2006, 21, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M. Novel plasmonic field-enhanced nanoassay for trace detection of proteins. Biosens. Bioelectron. 2014, 55, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Yu, L.; Fang, Z.; Zeng, L. An enhanced strip biosensor for rapid and sensitive detection of histone methylation. Anal. Chem. 2013, 85, 9343–9349. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, Z.; Ju, H.; Yan, F. Triplex signal amplification for electrochemical DNA biosensing by coupling probe-gold nanoparticles-graphene modified electrode with enzyme functionalized carbon sphere as tracer. Biosens. Bioelectron. 2012, 33, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Dheen, S.T.; Yip, W.C.; Ong, C.N.; Bay, B.H.; Lanry Yung, L.Y. The induction of epigenetic regulation of pros1 gene in lung fibroblasts by gold nanoparticles and implications for potential lung injury. Biomaterials 2011, 32, 7609–7615. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.Y.; Xu, H.; Penn, S.G.; Cromer, R. Sers nanoparticles: A new optical detection modality for cancer diagnosis. Nanomedicine 2007, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H.; Kneipp, J. Surface-enhanced raman scattering in local optical fields of silver and gold nanoaggregates-from single-molecule raman spectroscopy to ultrasensitive probing in live cells. Acc. Chem. Res. 2006, 39, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chiuman, W.; Brook, M.A.; Li, Y. Simple and rapid colorimetric biosensors based on DNA aptamer and noncrosslinking gold nanoparticle aggregation. Chembiochem 2007, 8, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Truong, P.L.; Anh, N.H.; Sim, S.J. Single gold nanoplasmonic sensor for clinical cancer diagnosis based on specific interaction between nucleic acids and protein. Biosens. Bioelectron. 2015, 67, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Jin, H.Y.; He, X.P.; James, T.D.; Chen, G.R.; Long, Y.T. Colorimetric and plasmonic detection of lectins using core-shell gold glyconanoparticles prepared by copper-free click chemistry. ACS Appl. Mater. Interfaces 2015, 7, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Unnikrishnan, B.; Li, Y.J.; Huang, C.C. Functional gold nanoparticles coupled with microporous membranes: A flow controlled assay for colorimetric visualization of proteins. Analyst 2014, 139, 5977–5982. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Sandhyarani, N. Detection of glucose using immobilized bienzyme on cyclic bisureas-gold nanoparticle conjugate. Anal. Biochem. 2014, 459, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhu, C.; Liu, J.; Yan, M.; Yang, S.; Chen, A. Aunp-based colorimetric aptasensor for rapid detection of six organophosphorus pesticides. Environ. Toxicol. Chem. 2015, 34, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Satpati, A.K.; Reddy, A.V. Electrochemically deposited gold nanoparticles on a carbon paste electrode surface for the determination of mercury. J. AOAC Int. 2015, 98, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.A.; Yoon, J.H.; Gurudatt, N.G.; Won, M.S.; Shim, Y.B. Ultrasensitive cytosensing based on an aptamer modified nanobiosensor with a bioconjugate: Detection of human non-small-cell lung cancer cells. Biosens. Bioelectron. 2015, 74, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.H.; Pi, J.; Lin, X.; Li, B.; Li, A.; Yang, P.H.; Cai, J. Gold nanoprobes-based resonance rayleigh scattering assay platform: Sensitive cytosensing of breast cancer cells and facile monitoring of folate receptor expression. Biosens. Bioelectron. 2015, 74, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Formisano, N.; Miodek, A.; Jain, A.; di Lorenzo, M.; Pula, G.; Estrela, P. Plasmonic ruler on field-effect devices for kinase drug discovery applications. Biosens. Bioelectron. 2015, 71, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. Molecular imaging in cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite nanoparticles for medical mr imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in mr imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Elizondo, G.; Wittenberg, J.; Rabito, C.A.; Bengele, H.H.; Josephson, L. Ultrasmall superparamagnetic iron oxide: Characterization of a new class of contrast agents for mr imaging. Radiology 1990, 175, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Bagalkot, V.; Vasilliou, C.C.; Gu, F.; Alexis, F.; Zhang, L.; Shaikh, M.; Yuet, K.; Cima, M.J.; Langer, R.; et al. Superparamagnetic iron oxide nanoparticle-aptamer bioconjugates for combined prostate cancer imaging and therapy. ChemMedChem 2008, 3, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, G.K.; Irudayaraj, J. A nanoparticle-based immobilization assay for prion-kinetics study. J. Nanobiotechnol. 2006, 4, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kouassi, G.K.; Irudayaraj, J. Magnetic and gold-coated magnetic nanoparticles as a DNA sensor. Anal. Chem. 2006, 78, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.V.; Mazumdar, D.; Lu, Y. Mri detection of thrombin with aptamer functionalized superparamagnetic iron oxide nanoparticles. Bioconjug. Chem. 2008, 19, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics 2013, 3, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Sabale, S.; Jadhav, V.; Khot, V.; Zhu, X.; Xin, M.; Chen, H. Superparamagnetic MFe2O4 (m = Ni, Co, Zn, Mn) nanoparticles: Synthesis, characterization, induction heating and cell viability studies for cancer hyperthermia applications. J. Mater. Sci. Mater. Med. 2015, 26, 5466. [Google Scholar] [CrossRef] [PubMed]
- Hervault, A.; Thanh, N.T. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Tung le, D.; Maenosono, S.; Walti, C.; Thanh, N.T. Synthesis of core-shell gold coated magnetic nanoparticles and their interaction with thiolated DNA. Nanoscale 2010, 2, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed core-shell Fe3O4@Au nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, L.; Manzoni, L.; Paduano, L.; Flori, A.; Kusmic, C.; de Marchi, D.; Casciaro, S.; Conversano, F.; Lombardi, M.; Positano, V.; et al. Iron oxide-gold core-shell nanoparticles as multimodal imaging contrast agent. IEEE Sens. J. 2013, 13, 7. [Google Scholar] [CrossRef]
- Pal, S.; Morales, M.; Mukherjee, P.; Srikantha, H. Synthesis and magnetic properties of gold coated iron oxide nanoparticles. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- León-Félix, L.; Chaker, J.; de Souza Parise, M.; Coaquira, J.A.H.; de Los Santos Valladares, L.; Bustamante, A.; Garg, V.; Oliveira, A.; Morais, P. Synthesis and characterization of uncoated and gold-coated magnetite nanoparticles. Hyperfine Interact. 2014, 224, 12. [Google Scholar] [CrossRef]
- Tasset, D.M.; Kubik, M.F.; Steiner, W. Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. J. Mol. Biol. 1997, 272, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Gallego, I.; Manning, B.; Eritja, R.; Fabrega, C. DNA origami as a DNA repair nanosensor at the single-molecule level. Angew. Chem. Int. Ed. 2013, 52, 7747–7750. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Aviño, A.; Ruiz, F.M.; Eritja, R.; Fabrega, C. Development of a novel fluorescence assay based on the use of the thrombin-binding aptamer for the detection of o-alkylguanine-DNA alkyltransferase activity. J. Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Salas, G.; Casado, C.; Teran, F.J.; Miranda, R.; Serna, C.J.; Puerto Morales, M. Controlled synthesis of uniform magnetite nanocrystals with high-quality properties for biomedical applications. J. Mater. Chem. 2012, 22, 21065. [Google Scholar] [CrossRef]
- Marciello, M.; Connord, V.; Veintemillas-Verdaguer, S.; Andrés Vergés, M.; Carrey, J.; Respaud, M.; Serna, C.J.; Puerto Morales, M. Large scale production of biocompatible magnetite nancrystals with high saturation magnetization values through green aqueous synthesis. J. Mater. Chem. B 2013, 1, 5995. [Google Scholar] [CrossRef]
- Latorre, A.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Somoza, A. Multifunctionalization of magnetic nanoparticles for controlled drug release: A general approach. Eur. J. Med. Chem. 2014, 82, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Feau, C.; Klein, E.; Dosche, C.; Kerth, P.; Lebeau, L. Synthesis and characterization of coumarin-based europium complexes and luminescence measurements in aqueous media. Org. Biomol. Chem. 2009, 7, 5259–5270. [Google Scholar] [CrossRef] [PubMed]
- Posch, C.; Latorre, A.; Crosby, M.B.; Celli, A.; Latorre, A.; Vujic, I.; Sanlorenzo, M.; Green, G.A.; Weier, J.; Zekhtser, M.; et al. Detection of gnaq mutations and reduction of cell viability in uveal melanoma cells with functionalized gold nanoparticles. Biomed. Microdevices 2015, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.W.; Boutin, R.H.; Nedelman, M.A.; Lister-James, J.; Dean, R.T. Enhanced kidney clearance with an ester-linked 99m Tc-radiolabeled antibody Fab'-chelator conjugate. Bioconjug. Chem. 1990, 1, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools. Chem. Res. Toxicol. 2011, 24, 618–639. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tintoré, M.; Mazzini, S.; Polito, L.; Marelli, M.; Latorre, A.; Somoza, Á.; Aviñó, A.; Fàbrega, C.; Eritja, R. Gold-Coated Superparamagnetic Nanoparticles for Single Methyl Discrimination in DNA Aptamers. Int. J. Mol. Sci. 2015, 16, 27625-27639. https://doi.org/10.3390/ijms161126046
Tintoré M, Mazzini S, Polito L, Marelli M, Latorre A, Somoza Á, Aviñó A, Fàbrega C, Eritja R. Gold-Coated Superparamagnetic Nanoparticles for Single Methyl Discrimination in DNA Aptamers. International Journal of Molecular Sciences. 2015; 16(11):27625-27639. https://doi.org/10.3390/ijms161126046
Chicago/Turabian StyleTintoré, Maria, Stefania Mazzini, Laura Polito, Marcello Marelli, Alfonso Latorre, Álvaro Somoza, Anna Aviñó, Carme Fàbrega, and Ramon Eritja. 2015. "Gold-Coated Superparamagnetic Nanoparticles for Single Methyl Discrimination in DNA Aptamers" International Journal of Molecular Sciences 16, no. 11: 27625-27639. https://doi.org/10.3390/ijms161126046
APA StyleTintoré, M., Mazzini, S., Polito, L., Marelli, M., Latorre, A., Somoza, Á., Aviñó, A., Fàbrega, C., & Eritja, R. (2015). Gold-Coated Superparamagnetic Nanoparticles for Single Methyl Discrimination in DNA Aptamers. International Journal of Molecular Sciences, 16(11), 27625-27639. https://doi.org/10.3390/ijms161126046