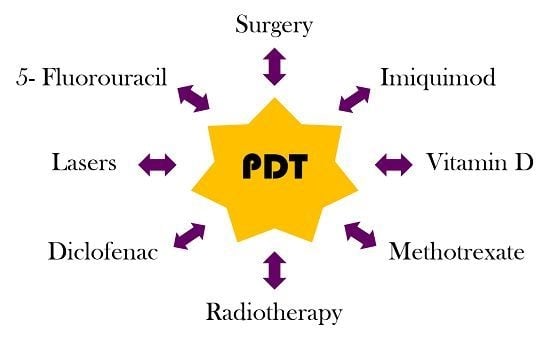
Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer
Abstract
:1. Skin Cancer
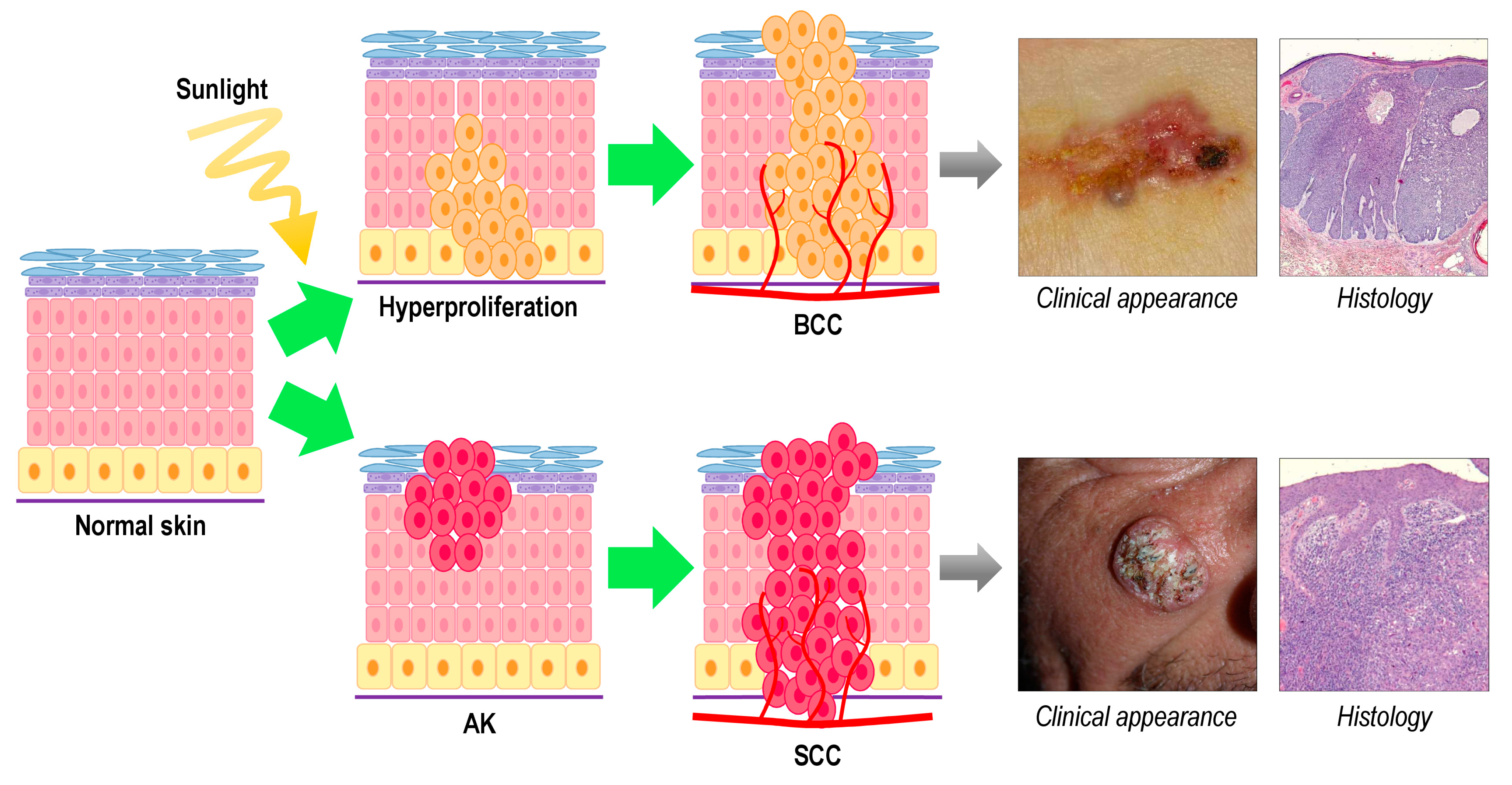
2. Photodynamic Therapy for Non-Melanoma Skin Cancer
2.1. Limitations of Photodynamic Therapy (PDT)
2.2. Resistance to PDT
3. Combined Treatments with PDT in NMSC

3.1. Actinic Keratosis
| PS (PDT) | Coadyuvant | n (Patients) | Results | Adverse Effects | Reference |
|---|---|---|---|---|---|
| ALA | 5-fluorouracil | 15 | At 1 month and at 1 year post-PDT + 5-FU treatment, 90% of treated AKs had resolved in all but one patient. | Not reported | [76] |
| ALA | 5-fluorouracil | 3 | PDT + 5-FU treatment is more effective, minimizes the recurrence of areas of field cancerization and improves the appearance of the skin, in comparison with PDT alone | Not reported | [77] |
| ALA | Imiquimod | 3 | At 7 and 11 months after PDT + imiquimod treatment, 2 patients showed complete clearance of AKs | Skin reactions such as severe erythema, scaling, and crusting | [80] |
| ALA | Imiquimod | 25 | At month 12, median lesion reduction was 89.9% after PDT + imiquimod and 74.5% after PDT | Severe local skin reactions in most participants: erythema, flaking, scaling and dryness | [78] |
| MAL | Imiquimod | 105 | Complete clinical response: 37.5% after PDT + imiquimod, 10% after PDT and 27% after imiquimod.
Histological response (absence of AK): 84% after PDT + imiquimod, 55% after PDT and 79% after imiquimod. Complete clinicopathologic response: 34% after PDT + imiquimod, 10% after PDT and 27% after imiquimod | 90% of patients who received PDT were very satisfied with treatment versus 61% of patients who received imiquimod | [79] |
| ALA | Diclofenac | 10 | At 12 months, significantly fewer AK were seen in the PDT + diclofenac group compared with PDT alone | Pain during PDT was greater in the PDT + Diclofenac group | [83] |
| ALA | Ingenol mebutate | 24 | Mean reduction of the number of Aks:
| Not reported | [84] |
| MAL-PDT: conventional (cPDT) and daylight (dPDT) | Ablative Fractional Laser (AFL) | 16 | Complete response rates:
| Erythema and crusting were more intense following AFL-dPDT than dPDT or cPDT | [85] |
3.2. Squamous Cell Carcinoma
3.3. Basal Cell Carcinoma
| MODEL | Photosensitizer (PDT) | Co-Adjuvant | Results | Side Effects | Reference |
|---|---|---|---|---|---|
| Patients: 13 BD | ALA | Surgery | 100% complete response. No recurrence in 1 year | Moderate pain, mild local swelling, hyperpigmentation. | [86] |
| Patient: 1 BD | ALA | Imiquimod | No recurrence in 1 year | Pain, burning sensation, erythema, intermittent episodes of scaling and dryness. | [87] |
| Patients: 4 BD | ALA | Radiotherapy | 100% complete response. no recurrence in 1 year | Improve radiotherapy side effects. | [89] |
| Patients: 22 BD | ALA | CO2 Laser | Combined therapy: 72.73% (8/11) complete remission, 1 recurrence (9%) after 1 month. CO2 Laser: 63.63% (7/11) complete remission, 5 recurrence (45.45%) after 6 months | Local side effects included mild to moderate edema, erosion, ulceration, delayed healing, prolonged pain, and scarring. | [90] |
| Patients: 5 SCC | ALA | Surgery | No recurrence in 6 months. 2 cases experienced recurrence in 1 year | Moderate pain, mild local swelling, hyperpigmentation. | [86] |
| Number of Tumors | Photosensitizer (PDT) | Co-Adjuvant | Results | Reference |
|---|---|---|---|---|
| 10 BCCs and 8 SCCs | ALA | Surgery | 4 BCC complete response. 14 lesions reduction in lesion area. 2 lesions increased. No recurrence after TFD + Surgery. | [103] |
| 6 Morpheaform BCCs | MAL | Surgery | 30%–50% reduction in volume after PDT. No recurrence in two years and a half. | [104] |
| 32 BCCs | ALA | Surgery | No recurrence in one year. | [86] |
| 1 nodular BCC | MAL | Imiquimod | 50% reduction in volume after PDT. No recurrence 15 months. | [105] |
| 3 giant BCCs | MAL | Imiquimod | 20%–40% reduction in volume after the combined treatment. All needed surgery. | [43] |
| 34 BCCs | ALA | Imiquimod | 60% healing after PDT, and 75% after the combined one. | [106] |
| 1 BCC | MAL | Imiquimod | No recurrence in two years | [107] |
| 3 recurrent nodular BCCs on each patient (194 patients) | ALA | Er:YAG laser | Effectivity: 94.85% PDT, 91.75% laser, 98.9% after combined treatment. | [108] |
| 75 BCCs | MAL | Er:YAG laser or diode laser | Effectivity: 81.2% PDT, 94.7% Er:YAG laser and 100% PDT diode laser. | [109] |
| 13 nodular BCCs | MAL | CO2 laser | No recurrence. Mild hypopigmentation in 2 cases and mild discomfort with PDT. | [110] |
| 56 nodular BCCs | MAL | Diode laser | Efficiency: 80.4% control group, 92.9% after combined treatment. | [111] |
| 177 BCCs | MAL | CO2 laser | Efficiency: 97.1% with combined treatment. Mild hypopigmentation was occasionally seen and some discomfort with PDT. | [112] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, B.; He, Y.Y. Recent advances in the prevention and treatment of skin cancer using photodynamic therapy. Expert Rev. Anticancer Ther. 2010, 10, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, A.; Kunze, U.; Schafer, T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: Incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer). Br. J. Dermatol. 2003, 149, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, N.; Waldmann, A.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J. Investig. Dermatol. 2014, 134, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sidoroff, M.D.; Thaler, P. Taking treatment decisions in non-melanoma skin cancer—The place for topical photodynamic therapy (PDT). Photodiagn. Photodyn. Ther. 2010, 7, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Ericson, M.B.; Wennberg, A.M.; Larkö, O. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Ther. Clin. Risk Manag. 2008, 4, 1–9. [Google Scholar] [PubMed]
- Koyuncuer, A. Histopathological evaluation of non-melanoma skin cancer. World J. Surg. Oncol. 2014, 12, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Berking, C.; Hauschild, A.; Kölbl, O.; Mast, G.; Gutzmer, R. Basal cell carcinoma-treatments for the commonest skin cancer. Dtsch. Arztebl. Int. 2014, 111, 389–395. [Google Scholar] [PubMed]
- Blanplain, C.; Fuchs, E. Stem cell plasticity. Plasticity of epithelial stem cell in tissue regeneration. Science 2014, 344, 1243–1253. [Google Scholar]
- Dlugosz, A.; Merlino, G.; Yuspa, S.H. Progress in cutaneous cancer research. J. Investig. Dermatol. Symp. Proc. 2002, 7, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.D. Squamous cell cancer: A practical approach. Semin. Cutan. Med. Surg. 1998, 17, 80–95. [Google Scholar] [CrossRef]
- Kolk, A.; Wolff, K.D.; Smeets, R.; Kesting, M.; Hein, R.; Eckert, A.W. Melanotic and non-melanotic malignancies of the face and external ear—A review of current treatment concepts and future options. Cancer Treat. Rev. 2014, 40, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Martorell-Calatayud, A.; Sanmartín, O.; Cruz, J.; Guillén, C. Cutaneous squamous cell carcinoma: Defining the high-risk variant. Actas Dermosifiliogr. 2013, 104, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, G.F.; Bouwes, J.N.; Euvrard, S. Organ transplantation and skin cancer: Basic problems and new perspectives. Exp. Dermatol. 2010, 19, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Cockerell, C.J. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J. Am. Acad. Dermatol. 2000, 42, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Figueras, M.T.; Carrato, C.; Sáenz, X.; Puig, L.; Musulen, E.; Ferrándiz, C.; Ariza, A. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Frankel, A.; Goldenberg, G. An update on nonmelanoma skin cancer. J. Clin. Aesthet. Dermatol. 2011, 4, 20–27. [Google Scholar] [PubMed]
- Kauvar, A.N.; Cronin, T., Jr.; Roenigk, R.; Hruza, G.; Bennett, R. Consensus for nonmelanoma skin cancer treatment: Basal cell carcinoma, including a cost analysis of treatment methods. Dermatol. Surg. 2015, 41, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, C.; Fonseca-Capdevila, E.; García-Diez, A.; Guillén-Barona, C.; Belinchón-Romero, I.; Redondo-Bellón, P.; Moreno-Giménez, J.C.; Senán, R. Spanish adaptation of the European guidelines for the evaluation and treatment of actinic keratosis. Actas Dermosifiliogr. 2014, 105, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A. Photodynamic therapy for nonmelanoma skin cancer—And more? Arch. Dermatol. 2004, 140, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Marmur, E.S.; Schmults, C.D.; Goldberg, D.J. A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatol. Surg. 2004, 30, 264–271. [Google Scholar] [PubMed]
- Kormeili, T.; Yamauchi, P.S.; Lowe, N.J. Topical photodynamic therapy in clinical dermatology. Br. J. Dermatol. 2004, 150, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P. Methyl aminolaevulinate-photodynamic therapy: A review of clinical trials in the treatment of actinic keratoses and nonmelanoma skin cancer. Br. J. Dermatol. 2007, 156, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Braathen, L.R.; Szeimies, R.M.; Basset-Seguin, N.; Bissonnette, R.; Foley, P.; Pariser, D.; Roelandts, R.; Wennberg, A.M.; Morton, C.A. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. J. Am. Acad. Dermatol. 2007, 56, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, J.M.; Schreml, S.; Kohl, E.A.; Karrer, S.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology. J. Dtsch. Dermatol. Ges. 2010, 8, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.; Szeimies, R.M.; Sidoroff, A.; Wennberg, A.M.; Basset-Seguin, N.; Calzavara-Pinton, P.; Gilaberte, Y.; Hofbauer, G.; Hunger, R.; Karrer, S.; et al. European Dermatology Forum Guidelines on topical photodynamic therapy. Eur. J. Dermatol. 2015, 25, 296–311. [Google Scholar]
- Choudhary, S.; Nouri, K.; Elsaie, M.L. Photodynamic therapy in dermatology: A review. Lasers Med. Sci. 2009, 24, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Warloe, T.; Kroon, S.; Funk, J.; Helsing, P.; Soler, A.M.; Stang, H.J.; Vatne, O.; Mørk, C. Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.; Abrahamse, H. Cell death pathways and phthalocyanine as an efficient agent for photodynamic cancer therapy. Int. J. Mol. Sci. 2015, 16, 10228–10241. [Google Scholar] [CrossRef]
- Blume, J.E.; Oseroff, A.R. Aminolevulinic acid photodynamic therapy for skin cancers. Dermatol. Clin. 2007, 25, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, G.; Farrar, M.D.; Cotterell, L.; Andrew, S.; Tosca, A.D.; Watson, R.E.; Rhodes, L.E. Topical photodynamic therapy significantly reduces epidermal Langerhans cells during clinical treatment of basal cell carcinoma. Br. J. Dermatol. 2012, 166, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Szeimies, R.M.; Sidoroff, A; Braathen, L.R. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications-actinic keratoses, Bowen’s disease, basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Kasche, A.; Luderschmidt, S.; Ring, J.; Hein, R. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J. Drugs Dermatol. 2006, 5, 353–356. [Google Scholar] [PubMed]
- Halldin, C.B.; Gillstedt, M.; Paoli, J.; Wennberg, A.M.; Gonzalez, H. Predictors of pain associated with photodynamic therapy: A retrospective study of 658 treatments. Acta Derm. Venereol. 2011, 91, 545–551. [Google Scholar] [PubMed]
- Attili, S.K.; Dawe, R.; Ibbotson, S. A review of pain experienced during topical photodynamic therapy-our experience in Dundee. Photodiagn. Photodyn. Ther. 2011, 8, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Gholam, P.; Denk, K.; Sehr, T.; Enk, A.; Hartmann, M. Factors influencing pain intensity during topical photodynamic therapy of complete cosmetic units for actinic keratoses. J. Am. Acad. Dermatol. 2010, 63, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Borroni, R.G.; Carugno, A.; Rivetti, N.; Arbustini, E.; Brazzelli, V. Risk of acute postoperative hypertension after topical photodynamic therapy for non-melanoma skin cancer. Photodermatol. Photoimmunol. Photomed. 2013, 29, 73–77. [Google Scholar] [CrossRef] [PubMed]
- McKay, K.M.; Sambrano, B.L.; Fox, P.S.; Bassett, R.L.; Chon, S.; Prieto, V.G. Thickness of superficial basal cell carcinoma (sBCC) predicts imiquimod efficacy: A proposal for a thickness-based definition of sBCC. Br. J. Dermatol. 2013, 169, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Roozeboom, M.H.; van Kleef, L.; Arits, A.H.; Mosterd, K.; Winnepenninckx, V.J.; van Marion, A.M.; Nelemans, P.J.; Kelleners-Smeets, N.W. Tumor thickness and adnexal extension of superficial basal cell carcinoma (sBCC) as determinants of treatment failure for methyl aminolevulinate (MAL)-photodynamic therapy (PDT), imiquimod, and 5-fluorouracil (FU). J. Am. Acad. Dermatol. 2015, 73, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Mørk, C.; Foss, O.A. Pre-treatment deep curettage can significantly reduce tumour thickness in thick Basal cell carcinoma while maintaining a favourable cosmetic outcome when used in combination with topical photodynamic therapy. J. Skin Cancer 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.P.; Kurachi, C.; Inada, N.M.; Moriyama, L.T.; Salvio, A.G.; Vollet Filho, J.D.; Pires, L.; Buzzá, H.H.; de Andrade, C.T.; Greco, C.; et al. Experience and BCC subtypes as determinants of MAL-PDT response: Preliminary results of a national Brazilian project. Photodiagn. Photodyn. Ther. 2014, 11, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Madan, V.; West, C.A.; Murphy, J.V.; Lear, J.T. Sequential treatment of giant basal cell carcinomas. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, C.; Goerz, G.; Ruzicka, T. Photodynamic therapy in dermatology. Arch. Dermatol. 1998, 134, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Wolf, P.; Wulf, H.C.; Warloe, T.; Fritsch, C.; Rhodes, L.E.; Kaufmann, R.; de Rie, M.; Legat, F.J.; Stender, I.M.; et al. Topical methyl aminolaevulinate photodynamic therapy in patients with basal cell carcinoma prone to complications and poor cosmetic out-come with conventional treatment. Br. J. Dermatol. 2003, 149, 1242–1249. [Google Scholar] [CrossRef]
- Rhodes, L.E.; de Rie, M.A.; Leifsdottir, R.; Yu, R.C.; Bachmann, I.; Goulden, V.; Wong, G.A.; Richard, M.A.; Anstey, A.; Wolf, P. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch. Dermatol. 2007, 143, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.M.; Ibbotson, S.; Murrell, D.F.; Rubel, D.; Frambach, Y.; de Berker, D.; Dummer, R.; Kerrouche, N.; Villemagne, H. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8–20 mm), with a 12-month follow-up. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Vinciullo, C.; Elliott, T.; Francis, D.; Gebauer, K.; Spelman, L.; Nguyen, R.; Weightman, W.; Sheridan, A.; Reid, C.; Czarnecki, D.; et al. Photodynamic therapy with topical methyl aminolaevulinate for “difficult-to-treat” basal cell carcinoma. Br. J. Dermatol. 2005, 152, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Whitehurst, C.; McColl, J.H.; Moore, J.V.; MacKie, R.M. Photodynamic therapy for large or multiple patches of bowen disease and basal cell carcinoma. Arch. Dermatol. 2001, 137, 319–324. [Google Scholar] [PubMed]
- Fantini, F.; Greco, A.; del Giovane, C.; Cesinaro, A.M.; Venturini, M.; Zane, C.; Surrenti, T.; Peris, K.; Calzavara-Pinton, P.G. Photodynamic therapy for basal cell carcinoma: Clinical and pathological determinants of response. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.; Meyer-Gonzalez, T.; Herrera-Acosta, E.; Bosch, R.; Castillo, R.; Herrera, E. Photodynamic therapy in the treatment of extensive Bowen’s disease. J. Dermatol. Treat. 2012, 23, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Stables, G.I.; Stringer, M.R.; Robinson, D.J.; Ash, D.V. Large patches of Bowen’s disease treated by topical aminolaevulinic acid photodynamic therapy. Br. J. Dermatol. 1997, 136, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R.; Capezzera, R.; Parrinello, G.; Specchia, C.; Zane, C. Methyl aminolevulinate-based photodynamic therapy of Bowen’s disease and squamous cell carcinoma. Br. J. Dermatol. 2008, 159, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, S.K.; Cho, K.H.; Kim, Y.C. Huge Bowen’s disease: A pitfall of topical photodynamic therapy. Photodiagn. Photodyn. Ther. 2013, 10, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Campbell, S.; Gupta, G.; Keohane, S.; Lear, J.; Zaki, I.; Walton, S.; Kerrouche, N.; Thomas, G.; Soto, P. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: A multicentre, randomized controlled study. Br. J. Dermatol. 2006, 155, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, R.; Spelman, L.; Weightman, W.; Reifenberger, J.; Szeimies, R.M.; Verhaeghe, E.; Kerrouche, N.; Sorba, V.; Villemagne, H.; Rhodes, L.E. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br. J. Dermatol. 2008, 158, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Birnie, A.J.; Eedy, D.J. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen’s disease) 2014. Br. J. Dermatol. 2014, 170, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Nissen, C.V.; Philipsen, P.A.; Wulf, H.C. PpIX formation after topical application of methyl aminolevulinate and BF-200 ALA declines with age. Br. J. Dermatol. 2015, 173, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Tehranchinia, Z.; Rahimi, H.; Ahadi, M.S.; Ahadi, M.S. Aminolevulinic Acid-photodynamic therapy of Basal cell carcinoma and factors affecting the response to treatment: A clinical trial. Indian J. Dermatol. 2013, 58. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.P.; Gottesman, M. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 47–76. [Google Scholar] [PubMed]
- Hombach-Klonisch, S.; Natarajan, S.; Thanasupawat, T.; Medapati, M.; Pathak, A.; Ghavami, S.; Klonisch, T. Mechanisms of therapeutic resistance in cancer (stem) cells with emphasis on thyroid cancer cells. Front. Endocrinol. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Dimassi, S.; Abou-Antoun, T.; El-Sibai, M. Cancer cell resistance mechanisms: A mini review. Clin. Transl. Oncol. 2014, 16, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Maydan, E.; Nooothet, P.K.; Goldman, M.P. Case reports: Development of a keratoacanthoma after topical photodynamic therapy with 5-aminolevolunic acid. J. Drugs Dermatol. 2006, 5, 804–806. [Google Scholar] [PubMed]
- Fiechter, S.; Skaria, A.; Nievergelt, H.; Anex, R.; Borradori, L.; Parmentier, L. Facial basal cell carcinomas recurring after photodynamic therapy: A retrospective analysis of histological subtypes. Dermatology 2012, 224, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Bardazzi, F.; Loi, C.; Magnano, M.; Burtica, E.C.; Giordano, F.; Patrizi, A. Methyl-aminolevulinic acid photodynamic therapy for actinic keratoses: An useful treatment or a risk factor? A retrospective study. J. Dermatol. Treat. 2015, 26, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. More Adventures in Photodynamic Therapy. Int. J. Mol. Sci. 2015, 16, 15188–15193. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; di Venosa, G.; Hasan, T.; Batlle, A. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.; Piva, T.J. The UV response of the skin: A review of the MAPK, NF-κB and TNF-α signal transduction pathways. Arch. Dermatol. Res. 2010, 302, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Kuźbicki, Ł.; Lange, D.; Stanek-Widera, A.; Chwirot, B.W. Different expression of cyclooxygenase-2 (COX-2) in selected nonmelanocytic human cutaneous lesions. Folia Histochem. Cytobiol. 2011, 49, 381–388. [Google Scholar]
- Müller-Decker, K. Cyclooxygenase-dependent signaling is causally linked to non-melanoma skin carcinogenesis: Pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011, 30, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Walls, B.; Jordan, L.; Diaz, L.; Miller, R. Targeted therapy for cutaneous oncology: A review of novel treatment options for non-melanoma skin cancer: Part I. J. Drugs Dermatol. 2014, 13, 947–952. [Google Scholar] [PubMed]
- Bahner, J.D.; Bordeaux, J.S. Non-melanoma skin cancers: Photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin. Dermatol. 2013, 31, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.J. Treatment of actinic keratoses with sequential combination of 5-fluorouracil and photodynamic therapy. J. Drugs Dermatol. 2005, 4, 161–163. [Google Scholar] [PubMed]
- Martin, G. Prospective, case-based assessment of sequential therapy with topical Fluorouracil cream 0.5% and ALA-PDT for the treatment of actinic keratosis. J. Drugs Dermatol. 2011, 10, 372–378. [Google Scholar] [PubMed]
- Shaffelburg, M. Treatment of actinic keratoses with sequential use of photodynamic therapy; and imiquimod 5% cream. J. Drugs Dermatol. 2009, 8, 35–39. [Google Scholar] [PubMed]
- Serra-Guillén, C.; Nagore, E.; Hueso, L.; Traves, V.; Messeguer, F.; Sanmartín, O.; Llombart, B.; Requena, C.; Botella-Estrada, R.; Guillén, C. A randomized pilot comparative study of topical methyl aminolevulinate photodynamic therapy vs. imiquimod 5% vs. sequential application of both therapies in immunocompetent patients with actinic keratosis: Clinical and histologic outcomes. J. Am. Acad. Dermatol. 2012, 66, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Held, L.; Eigentler, T.K.; Leiter, U.; Garbe, C.; Berneburg, M.J. Effective combination of photodynamic therapy and imiquimod 5% cream in the treatment of actinic keratoses: Three cases. Biomed. Res. Int. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Akita, Y.; Kozaki, K.; Nakagawa, A.; Saito, T.; Ito, S.; Tamada, Y.; Fujiwara, S.; Nishikawa, N.; Uchida, K.; Yoshikawa, K.; et al. Cyclooxygenase-2 is a possible target of treatment approach in conjunction with photodynamic therapy for various disorders in skin and oral cavity. Br. J. Dermatol. 2004, 151, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Bagazgoitia, L.; Cuevas, J.; Juarranz, A.; Jaén, P. Photodynamic therapy reduces the histological features of actinic damage and the expression of early oncogenic markers. Br. J. Dermatol. 2011, 165, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Van der Geer, S.; Krekels, G.A. Treatment of actinic keratoses on the dorsum of the hands: ALA-PDT vs. diclofenac 3% gel followed by ALA-PDT. A placebo-controlled, double-blind, pilot study. J. Dermatol. Treat. 2009, 20, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Nestor, M.S.; Newburger, J.; Park, H.; Swenson, N. Treatment of facial actinic keratoses with aminolevulinic acid photodynamic therapy (ALA-PDT) or ingenol mebutate 0.015% gel with and without prior treatment with ALA-PDT. J. Drugs Dermatol. 2014, 13, 1353–1356. [Google Scholar] [PubMed]
- Togsverd-Bo, K.; Lei, U.; Erlendsson, A.M.; Taudorf, E.H.; Philipsen, P.A.; Wulf, H.C.; Skov, L.; Hædersdal, M. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—A randomized controlled trial. Br. J. Dermatol. 2015, 172, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.G.; Wang, Y.Y.; Yang, Y.D.; Zhang, X.C.; Gao, Y.; Yang, Y.; Zhang, J.B.; Li, G.L. Efficacy of topical ALA-PDT combined with excision in the treatment of skin malignant tumor. Photodiagn. Photodyn. Ther. 2014, 11, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, E.; Lallas, A.; Apalla, Z.; Ioannides, D. Treatment of giant Bowen’s disease with sequential use of photodynamic therapy and imiquimod cream. Photodermatol. Photoimmunol. Photomed. 2011, 27, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Zuo, L.; Shang, L.; Bazan, J.G. Radiotherapy treatment for nonmelanoma skin cancer. Expert Rev. Anticancer Ther. 2015, 15, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Watanabe, D.; Akita, Y.; Kawamura, T.; Tamada, Y.; Matsumoto, Y. Treatment efficiency of combining photodynamic therapy and ionizing radiation for Bowen’s disease. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, Y.X.; Zheng, J.C.; Sun, P.; Yang, Z.Y.; Li, Y.L.; Liu, X.Y.; Li, Q.; Liu, W. Photodynamic therapy in combination with CO2 laser for the treatment of Bowen’s disease. Lasers Med. Sci. 2015, 30, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Watanabe, D.; Akita, Y.; Nakano, A.; Yamashita, N.; Kuhara, T.; Yanagishita, T.; Takeo, T.; Tamada, Y.; Matsumoto, Y. Etretinate enhances the susceptibility of human skin squamous cell carcinoma cells to 5-aminolaevulic acid-based photodynamic therapy. Clin. Exp. Dermatol. 2009, 34, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Honari, G.; Hasan, T.; Elson, P.; Maytin, E.V. Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin. Cancer Res. 2009, 15, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Boniol, M.; Tosti, G.; Magi, S.; Medri, M.; Stanganelli, I.; Palli, D.; Assedi, M.; Marmol, V.D.; Gandini, S. Vitamin D and melanoma and non-melanoma skin cancer risk and prognosis: A comprehensive review and meta-analysis. Eur. J. Cancer 2014, 50, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Larriba, M.J.; Muñoz, A. Vitamin D and colon cancer. Endocr. Relat. Cancer 2012, 19, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Uhmann, A.; Niemann, H.; Lammering, B.; Henkel, C.; Hess, I.; Nitzki, F.; Fritsch, A.; Prüfer, N.; Rosenberger, A.; Dullin, C.; et al. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol. Cancer Ther. 2011, 10, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Cicarma, E.; Tuorkey, M.; Juzeniene, A.; Ma, L.W.; Moan, J. Calcitriol treatment improves methyl aminolaevulinate-based photodynamic therapy in human squamous cell carcinoma A431 cells. Br. J. Dermatol. 2009, 161, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Wilson, C.; Hasan, T.; Maytin, E.V. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011, 71, 6040–6050. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rollakanti, K.R.; Horst, R.L.; Hasan, T.; Maytin, E.V. Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma. Photochem. Photobiol. 2014, 90, 1126–1135. [Google Scholar] [PubMed]
- Rollakanti, K.; Anand, S.; Maytin, E.V. Topical calcitriol prior to photodynamic therapy enhances treatment efficacy in non-melanoma skin cancer mouse models. Proc. SPIE Int. Soc. Opt. Eng. 2015, 2, 1–13. [Google Scholar]
- Gilaberte, Y.; Milla, L.; Salazar, N.; Vera-Alvarez, J.; Kourani, O.; Damian, A.; Rivarola, V.; Roca, M.J.; Espada, J.; González, S.; et al. Cellular intrinsic factors involved in the resistance of squamous cell carcinoma to photodynamic therapy. J. Investig. Dermatol. 2014, 134, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carpio, P.A.; Trelles, M.A. The role of epidermal growth receptor in photodynamic therapy: A review of the literature and proposal for future investigations. Lasers Med. Sci. 2010, 25, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Weyergang, A.; Selbo, P.K.; Berg, K. Sustained ERK inhibition by EGFR targeting therapies is a predictive factor for synergistic cytotoxicity with PDT as neoadjuvant therapy. Biochim. Biophys. Acta 2013, 1830, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, G.; Brandt, M.G.; Jordan, K.; Doyle, P.C.; Yu, E.; Moore, C.C. Using photodynamic therapy as a neoadjuvant treatment in the surgical excision of nonmelanotic skin cancers: Prospective study. J. Otolaryngol. Head Neck Surg. 2011, 40, 82–89. [Google Scholar]
- Torres, T.; Fernandes, I.; Costa, V.; Selores, M. Photodynamic therapy as adjunctive therapy for morpheaform basal cell carcinoma. Acta Dermatovenerol. Alp. Pannonica Adriat. 2011, 20, 23–25. [Google Scholar] [PubMed]
- Devirgiliis, V.; Panasiti, V.; Curzio, M.; Gobbi, S.; Rossi, M.; Roberti, V.; Calvieri, S. Complete remission of nodular basal cell carcinoma after combined treatment with photodynamic therapy and imiquimod 5% cream. Dermatol. Online J. 2008, 14, 25. [Google Scholar] [PubMed]
- Osiecka, B.; Jurczyszyn, K.; Ziółkowski, P. The application of Levulan-based photodynamic therapy with imiquimod in the treatment of recurrent basal cell carcinoma. Med. Sci. Monit. 2012, 18, 5–9. [Google Scholar] [CrossRef]
- Requena, C.; Messeguer, F.; Llombart, B.; Serra-Guillén, C.; Guillén, C. Facial extensive recurrent basal cell carcinoma: Successful treatment with photodynamic therapy and imiquimod 5% cream. Int. J. Dermatol. 2012, 51, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Smucler, R.; Vlk, M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg. Med. 2008, 40, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Smucler, R.; Kriz, M.; Lippert, J.; Vlk, M. Ultrasound guided ablative-laser assisted photodynamic therapy of basal cell carcinoma (US-aL-PDT). Photomed. Laser Surg. 2012, 30, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, I.S.; Shokrollahi, K.; James, W.; Mishra, A.; Lohana, P.; Murison, M.C. Combined CO2 laser with photodynamic therapy for the treatment of nodular basal cell carcinomas. Ann. Plast. Surg. 2007, 59, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Lippert, J.; Smucler, R.; Vlk, M. Fractional carbon dioxide laser improves nodular basal cell carcinoma treatment with photodynamic therapy with methyl 5-aminolevulinate. Dermatol. Surg. 2013, 39, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, K.; Javed, M.; Aeuyung, K.; Ghattaura, A.; Whitaker, I.S.; O’Leary, B.; James, W.; Murison, M. Combined carbon dioxide laser with photodynamic therapy for nodular and superficial basal cell carcinoma. Ann. Plast. Surg. 2014, 73, 552–558. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucena, S.R.; Salazar, N.; Gracia-Cazaña, T.; Zamarrón, A.; González, S.; Juarranz, Á.; Gilaberte, Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int. J. Mol. Sci. 2015, 16, 25912-25933. https://doi.org/10.3390/ijms161025912
Lucena SR, Salazar N, Gracia-Cazaña T, Zamarrón A, González S, Juarranz Á, Gilaberte Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. International Journal of Molecular Sciences. 2015; 16(10):25912-25933. https://doi.org/10.3390/ijms161025912
Chicago/Turabian StyleLucena, Silvia Rocío, Nerea Salazar, Tamara Gracia-Cazaña, Alicia Zamarrón, Salvador González, Ángeles Juarranz, and Yolanda Gilaberte. 2015. "Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer" International Journal of Molecular Sciences 16, no. 10: 25912-25933. https://doi.org/10.3390/ijms161025912
APA StyleLucena, S. R., Salazar, N., Gracia-Cazaña, T., Zamarrón, A., González, S., Juarranz, Á., & Gilaberte, Y. (2015). Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. International Journal of Molecular Sciences, 16(10), 25912-25933. https://doi.org/10.3390/ijms161025912