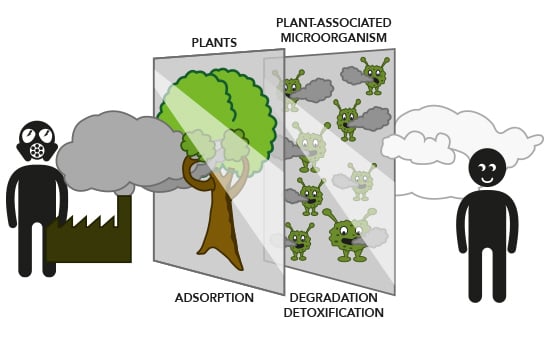
The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants
Abstract
:1. Introduction
| Pollutant | Averaging Period | Max Number of Exceedances | WHO Guideline |
|---|---|---|---|
| PM10 | 1 day | 3 | 50 μg/m3 |
| 1 year | NA | 20 μg/m3 | |
| PM2.5 | 1 day | 3 | 25 μg/m3 |
| 1 year | NA | 10 μg/m3 | |
| Ozone | Max daily 8 h | 0 | 100 μg/m3 |
| NOx | 1 h | 0 | 200 μg/m3 |
| 1 year | NA | 40 μg/m3 | |
| SOx | 10 min | NA | 500 μg/m3 |
| 1 day | 0 | 20 μg/m3 |
 ).
).
 ).
).
2. Particulate Matter
2.1. Definition and (Human) Toxicity
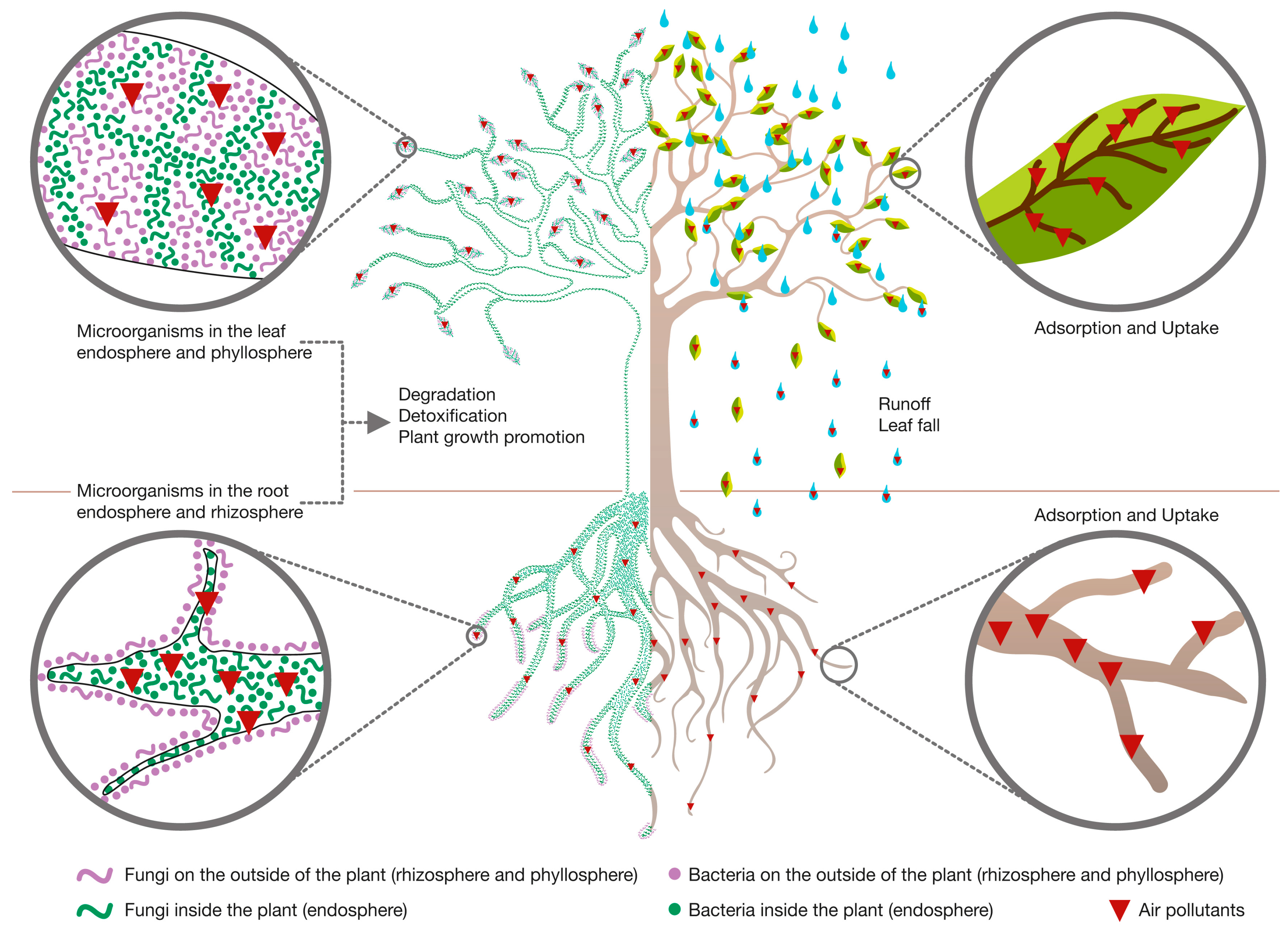
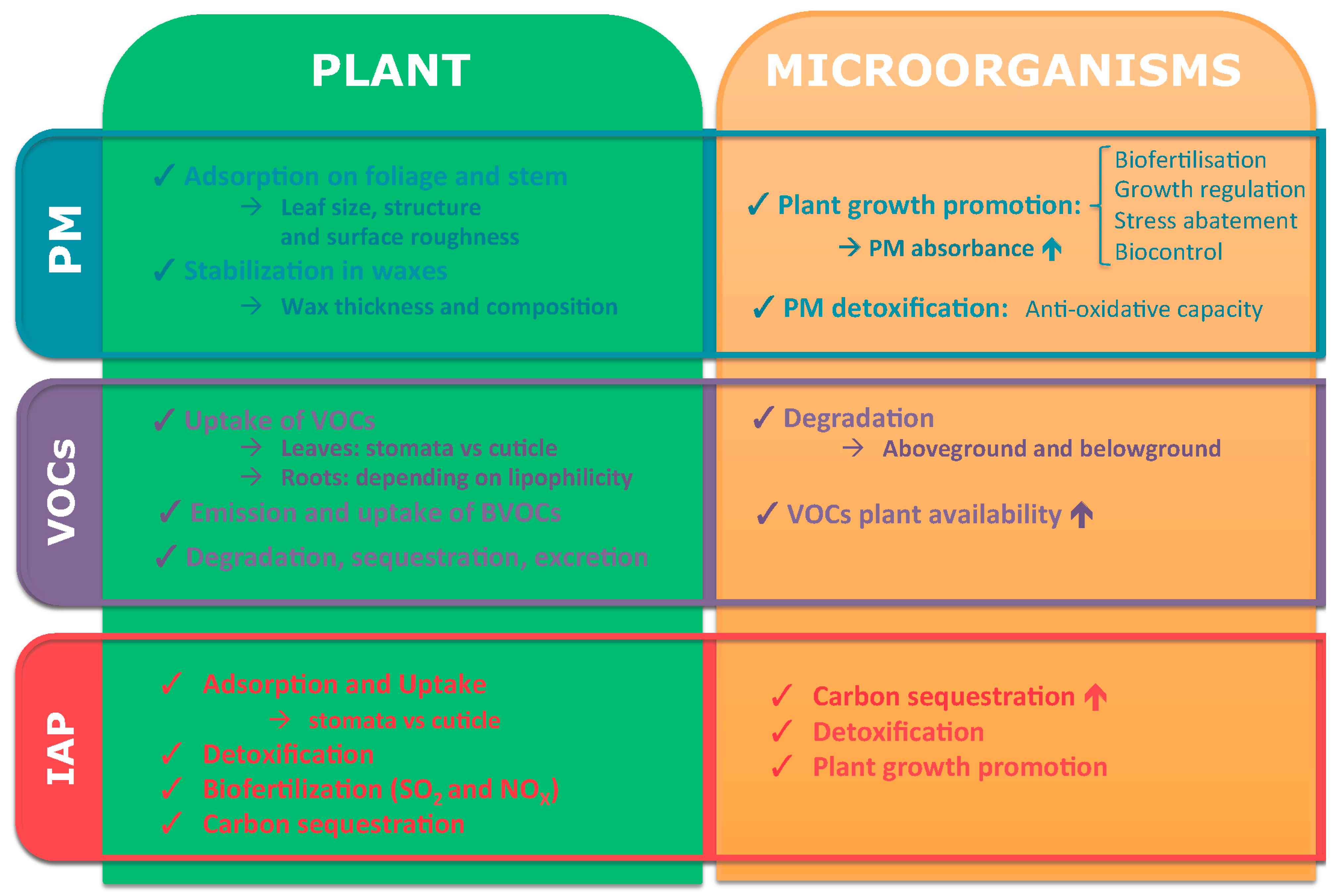
2.2. Role of Plants during PM Phytoremediation
2.3. Role of Plant-Associated Microorganisms during PM Phytoremediation
3. Volatile Organic Compounds (VOCs)
3.1. Definition and (Human) Toxicity
3.2. Role of Plants during VOCs’ Phytoremediation
3.3. Role of Plant-Associated Microorganisms during VOCs’ Phytoremediation
| Plants | Microbes | VOCs | References |
|---|---|---|---|
| Plant species used for phytoremediation | Bacterial groups with identified role in phytoremediation, predominantly Actinobacteria and Firmicutes | Aromatic and aliphatic hydrocarbons | Al-Awadhi et al. [136] |
| Peas, beans, tomatoes, and squash | Bacillus, Ochrobactrum, Enterobacter, Rhodococcus, Arthrobacter, Pontola, Nocardia, and Pseudoxanthomonas | n-Hexadecane, n-decosane, phenanthrene, and crude oil | Al-Awadhi et al. [137] |
| Halonemum strobilaceum | Ochrobactrum sp and Desulfovibrio sp. | Aliphatic and aromatic hydrocarbons | Al-Mailem et al. [138] |
| Bean and maize | Acinetobacter, Alcaligenes, and Rhodococcus. | Phenol | Sandhu et al. [139,140] |
| Ten evergreen ornamental plants | Acinetobacter, Pseudomonas, Pseudoxanthomonas, Mycobacterium | Acenaphthylene, acenaphthene, fluorine and phenanthrene | Yutthammo et al. [141] |
| Peas, beans, tomato and sunflower | Microbacterium spp., Rhodococcus spp., Citrobacter freundii | Crude oil, phenanthrene and n-octadecane | Ali et al. [142] |
| Sixteen cultivated and wild plant species from Kuwait | Flavobacterium, Halomonas, Arthrobacter, Marinobacter, Neisseria, Ralstonia, Ochrobactrumle, Exiguobacterium, Planomicrobium, Propionibacterium, Kocuria, Rhodococcus and Stenotrophomonas | Aromatic and aliphatic hydrocarbons | Ali et al. [143] |
| Anthocleista, Sarcophrynium, Canna, Colocassia, Musa, Cola, Citrus, Mangifera, Terminalia and Annona | Acinetobacter, Flavobacterium and Micrococcus | Diesel and kerosene | Ilori et al. [144] |
| American grass and broad beans | Rhodococcus and Pseudomonas | n-Alkanes, phenanthrene, naphthalene, and biphenyl | Sorkhoh et al. [145] |
| Six ornamental plants | Pseudomonas, Microbacterium, Rhizobium and Deinococcus | Phenanthrene | Waight et al. [146] |
| Azalea indica | Pseusomonas putida TVA8 | Toluene | De Kempeneer et al. [147] |
| Soybean, clover and Arabidopsis thaliana | Sphingomonas and Methylobacterium | Methanol (via proteomics) | Delmotte et al. [122] |
| Thirteen different plant species from Japan | Methylomonas, Methylosinus and Methylocystis | Methane | Iguchi et al. [148] |
| Four Prunus species | Sphingomonas and Methylobacterium | Methanol (via genomics) | Jo et al. [149] |
| Rice | Alpha, Beta and Gamma-proteobacteria, Actinobacteria, Bacteroidetes and Firmicutes | Methanol (via metaproteogenomics) | Knief et al. [121] |
| Arabidopsis thaliana | Hyphomicrobium | Chloromethane | Nadalig et al. [150] |
| Phaseolus vulgaris | Arthrobacter chlorophenolicus A6 | 4-chlorophenol | Scheublin et al. [151] |
| Foliage of an apple orchard | 3 Arthrobacter sp. | 4-chlorophenol | Scheublin and Leveau [152] |
4. Inorganic Air Pollutants (IAP)
4.1. Definition and (Human) Toxicity
4.2. Role of Plants during IAP Phytoremediation
4.3. Role of Plant-Associated Microorganisms during IAP Phytoremediation
5. State of the Art and Future Challenges
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.H.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93. [Google Scholar] [PubMed]
- Saravia, J.; You, D.; Thevenot, P.; Lee, G.I.; Shrestha, B.; Lomnicki, S.; Cormier, S.A. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014, 7, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Saravia, J.; Lee, G.I.; Lomnicki, S.; Dellinger, B.; Cormier, S.A. Particulate matter containing environmentally persistant free radicals and adverse infant respiratory health effects: A review. J. Biochem. Mol. Toxicol. 2013, 27, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Perez, L.; Künzli, N.; Munters, E.; Nemery, B. Public health importance of triggers of myocardial infarction: A comparative risk assessment. Lancet 2011, 377, 732–740. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Anderson, H.R.; Sunyer, J.; Ayres, J.; Baccini, M.; Vonk, J.M.; Boumghar, A.; Forastiere, F.; Forsberg, B.; Touloumi, G.; et al. Acute effects of particulate air pollution on respiratory admissions: Results from APHEA 2 project. Air pollution and health: A European Approach. Am. J. Respir. Crit. Care Med. 2001, 164, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Dominici, F.; Curriero, F.C.; Coursac, I.; Zeger, S.L. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. N. Eng. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Raz, R.; Roberts, A.L.; Lyall, K.; Hart, J.E.; Just, A.C.; Laden, F.; Weisskopf, M.G. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: A nested case-control analysis within the nurses’ health study II cohort. Environ. Health Perspect. 2015, 123, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Becerra, T.A.; Wilhelm, M.; Olsen, J.; Cockburn, M.; Ritz, B. Ambient air pollution and autism in Los Angeles county, California. Environ. Health Perspect. 2013, 121, 380–386. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency (EEA). Air Quality in Europe—2014. Available online: http://www.eea.europa.eu//publications/air-quality-in-europe-2014 (accessed on 22 October 2015).
- Myers, I.; Maynard, R.L. Polluted air—Outdoors and indoors. Occup. Med. 2005, 55, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Brack, C.L. Pollution mitigation and carbon sequestration by an urban forest. Environ. Pollut. 2002, 116, 195–200. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Saleem, M.; Hussain, S. Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotchnol. 2007, 25, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant–microbe partnerships for improving biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant-endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Mahmert, A.; Moissl-Eichinger, C. Beneficial effects of plant-associated microbes on indoor microbiomes and human health? Front. Microbiol. 2014, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wolverton, B.C. 2008 How to grow fresh air. In 50 Houseplants that Purify Your Home and Office; Weidenfeld & Nicolson: London, UK, 1997. [Google Scholar]
- Pastuszka, J.S. Effect of particulate aerosols on air quality—Methods of identification and assessment (Wplyw aerozoli ziarnistych na jakosc powietrza—Metody identyfikacji I oceny. Ekoprofit. Finanse Nauka Technol. Prawo). Ekoprofit. Financ. Sci. Technol. Law 2007, 2, 7–15. [Google Scholar]
- D’Amato, G.; Liccardi, G.; D’Amato, M.; Cazzola, M. Outdoor air pollution, climate changes and allergic bronchial asthma. Eur. Respir. J. 2002, 20, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Afshari, A.; Ekberg, L.E.; Matson, U. Characterization of indoor sources of fine and ultrafine particles a study conducted in a full-scale chamber. Indoor Air 2005, 15, 141–50. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Samet, J.M.; Romieu, I.; Bruce, N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax 2000, 55, 518–532. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Right to Healthy Indoor Air—Report on a WHO Meeting; World Health Organization (WHO): Bilthoven, The Netherlands, 2000. [Google Scholar]
- Gawronska, H.; Bakera, B. Phytoremediation of particulate matter from indoor air by Chlorophytum comosum L. plants. Air Qual. Atmos Health 2015, 8, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Masiol, M.; Hofer, A.; Squizzato, S.; Piazza, R.; Rampazzo, G.; Pavoni, B. Carcinogenic and mutagenic risk associated to airborne particle-phase polycyclic aromatic hydrocarbons: A source apportionment. Atmos. Environ. 2012, 60, 375–382. [Google Scholar] [CrossRef]
- Gehling, W.; Dellinger, B. Environmentally persistent free radicals and their lifetimes in PM2.5. Environ. Sci. Technol. 2013, 47, 8172–8178. [Google Scholar] [CrossRef] [PubMed]
- Louwies, T.; Nawrot, T.; Cox, B.; Dons, E.; Penders, J.; Provost, E.; Panis, L.I.; de Boever, P. Blood pressure changes in association with black carbon exposure in a panel of healthy adults are independent of retinal microcirculation. Environ. Int. 2015, 75, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Winckelmans, E.; Cox, B.; Martens, E.; Fierens, F.; Nemery, B.; Nawrot, T.S. Fetal growth and maternal exposure to particulate air pollution—More marked effects at lower exposure and modification by gestational duration. Environ. Res. 2015, 140, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Provost, E.B.; Chaumont, A.; Kicinski, M.; Cox, B.; Fierens, F.; Bernard, A.; Nawrot, T.S. Serum levels of club cell secretory protein (Clara) and short- and long-term exposure to particulate air pollution in adolescents. Environ. Int. 2014, 68, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Pieters, N.; Plusquin, M.; Cox, B.; Kicinski, M.; Vangronsveld, J.; Nawrot, T.S. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: A meta-analysis. Heart 2012, 98, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide—Global update 2005—Summary of Risk Assessment; World Health Organization (WHO): Geneva, Switzerland, 2005. [Google Scholar]
- Silva, R.A.; West, J.J.; Zhang, Y.; Anenberg, S.C.; Lamarque, J.F.; Shindell, D.T.; Collins, W.J.; Dalsoren, S.; Faluvegi, G.; Folberth, G. Global premature mortality due to anthropogenic outdoor air pollution and the contribution of past climate change. Environ. Res. Lett. 2013, 8, 034005. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussel, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Kiruri, L.W.; Khachatryan, L.; Dellinger, B.; Lomnicki, S. Effect of copper oxide concentration on the formation and persistency of environmentally persistent free radicals (EPFRs) in particulates. Envrion. Sci. Technol. 2014, 48, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.A.; Hebert, V.Y.; Thibeaux, T.M.; Orchard, M.A.; Hasan, F.; Cormier, S.; Thevenot, P.T.; Lomnicki, S.M.; Varner, K.J.; Dellinger, B.; et al. Model combustion-generated particulate matter containing persistant free radicals redox cycle to produce reactive oxygen species. Chem. Res. Toxicol. 2013, 26, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, L.; Vejerano, E.; Lomnicki, S.; Dellinger, B. Environmentally persistent free radicals (EPFRs). 1. Generation of reactive oxygen species in aqueous solutions. Environ. Sci. Technol. 2011, 45, 8559–8566. [Google Scholar] [CrossRef] [PubMed]
- Popek, R.; Gawronska, H.; Wrochna, M.; Gawronski, S.W.; Saebo, A. Paticulate matter on foliage of 13 woody species: Deposition on surfaces and phytostabilization in waxes—A 3-year study. Int. J. Phytoremediat. 2012, 15, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Saebo, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate mattter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427–428, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Dzierzanowski, K.; Popek, R.; Gawronska, H.; Saebo, A.; Gawronski, S.W. Deposition of particulate matter of different size fractions on leaf surface and waxes of urban forest species. Int. J. Phytoremediat. 2011, 13, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Beckett, K.P.; Freer-Smith, P.; Taylor, G. Effective tree species for local air quality management. J. Arboric. 2000, 26, 12–19. [Google Scholar]
- Beckett, K.P.; Freer-Smith, P.; Taylor, G. Urban woodlands: Their role in reducing the effects of particulate pollution. Environ. Pollut. 1998, 99, 347–360. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Gawroński, S.W. The level of particulate matter on foliage depends on the distance from the source of emission. Int. J. Phytoremediat. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C.; Hoehn, R.E.; Walton, J.T.; Bond, J. A ground-based method of assessing urban forest structure and ecosystem services. Arboricult. Urban For. 2008, 34, 347–358. [Google Scholar]
- Yang, J.; McBride, J.; Zhou, J.; Sun, Z. The urban forest in Beijing and its role in air pollution reduction. Urban For. Urban Green 2005, 3, 65–68. [Google Scholar] [CrossRef]
- Yin, S.; Shen, Z.; Zhou, P.; Zou, X.; Che, S.; Wang, W. Quantifying air pollution attenuation within urban parks: An experimental approach in Shangai, China. Environ. Pollut. 2011, 159, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.G.; Bealey, W.J.; Fowler, D.; Dragosits, U.; Skiba, U.; Smith, R.I.; Donovan, R.G.; Brett, H.E.; Hewitt, C.N.; Nemitz, E. Quantifying the effect of urban tree planting on concentrations and depositions of PM10 in two UK conurbations. Atmos. Environ. 2007, 41, 8455–8467. [Google Scholar] [CrossRef]
- Nowak, D.J. Air pollution removal by Chicago’s urban forest. Available online: http://www.nrs.fs.fed.us/pubs/gtr/gtr_ne186.pdf (accessed on 22 October 2015).
- Freer-Smith, P.H.; El-Khatib, A.A.; Taylor, G. Capture of particulate pollution by trees: A comparison of species typical of semi-arid areas (Ficus nitidaand Eucalyptus globulus) with European and north American species. Water Air Soil Pollut. 2003, 155, 173–87. [Google Scholar] [CrossRef]
- Fowler, D.; Cape, J.N.; Unsworth, M.H. Deposition of atmospheric pollutants on forests. Philos. Trans. R. Soc. Lond. 1989, 324, 247–265. [Google Scholar] [CrossRef]
- Weber, F.; Kowarik, I.; Säumel, I. Herbaceous plants as filters: Immobilization of particulates along urban street corridors. Environ. Pollut. 2014, 186, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, H.; Blumenstock, M.; McLachan, M.S. Retention and mobility of atmospheric particle-associated organic pollutant PCDD/Fs and PAHs in maize leaves. New Phytol. 2000, 148, 473–480. [Google Scholar]
- Wang, L.; Gong, H.; Liao, W.; Wang, Z. Accumulation of particles on the surface of leaves during leaf expansion. Sci. Total Environ. 2015, 532, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Przybysz, A.; Sæbø, A.; Hanslin, H.M.; Gawronski, S.W. Accumulation of particulate matter and trace elements on vegetation as affected by pollution level, rainfall and the passage of time. Sci. Total Environ. 2014, 481, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Freer-Smith, P.H.; Becket, K.P.; Taylor, G. Deposition velocities to Sorbus aria, Acer campestre, Populus deltoids x trichocarpa “Beaupre”, Pinus nigra and x Cupressocyparis leylandii for coarse, fine and ultra-fine particles in the urban environment. Environ. Pollut. 2005, 133, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Heerden van, P.D.R.; Krüger, G.H.J.; Kilbourn, L.M. Dynamic responses of photosystem II in the Namib Desert shrub, Zygophyllum prismatocarpum, during and after foliar deposition of limestone dust. Environ. Pollut. 2007, 146, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, M.; Pavlíková, D.; Zemanová, V.; Hnilička, F.; Urbanová, V.; Száková, I. Trace elements present in airborne particulate matter—Stressors of plant metabolism. Ecotoxicol. Environ. Safe 2012, 79, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Mai, B.; Meng, X.; Bi, X.; Sheng, G.; Fu, J.; Peng, P. Particle-bound polychlorinated dibenzo-p-dioxins and dibenzofurans in the atmosphere of Guangzhou, China. Atmos. Environ. 2006, 40, 96–108. [Google Scholar] [CrossRef]
- Przybysz, A.; Popek, R.; Gawronska, H.; Grab, K.; Loskot, K.; Wrochna, M.; Gawronski, S.W. Efficiency of photosynthetic apparatus of plants grown in sites differing in level of particulate matter. Acta Sci. Pol. Hortorum Cultus. 2014, 13, 17–30. [Google Scholar]
- Vardaka, E.; Cook, C.M.; Lanaras, T.; Sgardelis, S.P.; Pantis, J.D. Effect of dust from a limestone quarry on the photosynthesis of Quercus coccifera, an evergreen schlerophyllous shrub. Bull Environ. Contam. Toxicol. 1995, 54, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, D.V. Effects of particulates (dust) on cotton growth, photosynthesis, and respiration. Agron. J. 1986, 76, 1078–1081. [Google Scholar] [CrossRef]
- Takagi, M.; Gyokusen, K. Light and atmospheric pollution affect photosynthesis of street trees in urban environments. Urban For. Urban Green. 2004, 2, 167–171. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, X.; Li, L.; Li, Z.; Luo, Y. Rhizobia and their bio-partners as novel drivers for functional remediation in contaminated soils. Front. Plant Sci. 2015, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, E. Regulation of root growth by plant hormones—Roles for auxin and gibberellin. Crit. Rev. Plant Scis. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Saleem, M.; Arshad, M.; Hussain, S.; Bhatti, A.S. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotechnol. 2007, 34, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Hontzeas, N.; Hontzeas, C.E.; Glick, B.R. Reaction mechanisms of the bacterial enzyme 1-aminocyclopropane-1-carboxylate deaminase. Biotechnol. Adv. 2006, 24, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Ryu, C.-M. Bacterial endophytes as elicitors of induced systemic resistance. In Soil Biology Volume 9: Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin, Germany, 2006; pp. 33–52. [Google Scholar]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microb. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sun, M.Z.; Zhang, C.; Xin, Y. Antioxidant properties of Lactobacillus and its protecting effect to oxidative stress Caco-2 cells. J. Anim. Plant Sci. 2014, 24, 1766–1771. [Google Scholar]
- Van Sluys, M.A.; Monteiro-Vitorello, C.B.; Camargo, L.E.; Menck, C.F.; da Silva, A.C.; Ferro, J.A.; Oliveira, M.C.; Setubal, J.C.; Kitajima, J.P.; Simpson, A.J. Comparative genome analysis of plant-associated bacteria. Annu. Rev. Phytopathol. 2002, 40, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 6, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Directive 1999/13/EC. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01999L0013–20101201&qid=1406008935749&from=ES (accessed on 31 August 2015).
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of biogenic volatile organic compounds (BVOC) emitted by urban trees on ozone concentration in cities: A review. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Arneth, A.; Harisson, S.; Zaehle, S.; Tsigaridis, K.; Menon, S.; Bartlein, P.J.; Feichter, J.; Korhola, A.; Kulmala, M.; O’Donnell, D.; et al. Terrestrial biogeochemical feedbacks in the climate system. Nat. Geosci. 2010, 3, 525–532. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The model of emissions of gases and aerosols from nature version 2.1. (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T.; Uhde, E. Organic Indoor Air Pollutants: Occurrence-Measurement-Evaluation, 2nd ed.; Wiley: New York, NY, USA, 2009; pp. 1–464. [Google Scholar]
- Yu, C.; Crump, D. A review of the emission of VOCs from polymeric materials used in buildings. Build. Environ. 1998, 33, 357–374. [Google Scholar] [CrossRef]
- Harrison, S.P.; Morfopoulos, C.; Dani, K.G.S.; Prentice, I.C.; Arneth, A.; Atwell, B.J.; Barkley, M.P.; Leishman, M.R.; Loreto, F.; Medlyn, B.E.; et al. Volatile isoprenoid emissions from plastid to planet. New Phytol. 2012, 197, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bluyssen, P.M.; Janssen, S.; van den Brink, L.H.; de Kluizenaar, Y. Assessment of wellbeing in an indoor office environment. Build. Environ. 2011, 46, 2632–2640. [Google Scholar] [CrossRef]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for indoor air quality: Selected pollutants. In World Health Organization, Regional Office for Europe, Copenhagen; World Health Organization (WHO): Geneva, Switzerland, 2010. [Google Scholar]
- Mendes, A.; Pereira, C.; Mendes, D.; Aguiar, L.; Neves, P.; Silva, S.; Batterman, S.; Teixeira, J.P. Indoor air quality and thermal comfort-results of a pilot study in eldery care centers in Portugal. J. Toxicol. Environ. Health A 2013, 76, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P. Trends in Europe to reduce the indoor air pollution of VOCs. Indoor Air 2003, 13, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Mizukoshi, A.; Yangisawa, Y. Identification of responsible volatile chemicals that induce hypersensitive reactions to multiple chemical sensitivity patients. J. Expo. Anal. Environ. Epidemiol. 2004, 14, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O. Human health effects of environmental pollutants: New insights. Environ. Monit. Assesmt. 1998, 53, 245–258. [Google Scholar] [CrossRef]
- Kostiainen, R. Volatile organic compounds in the indoor air of normal and sick houses. Atmos. Environ. 1995, 29, 693–702. [Google Scholar] [CrossRef]
- Karl, T.; Harley, P.; Emmons, L.; Thornton, B.; Guenther, A.; Basu, C.; Turnipseed, A.; Jardine, K. Efficient atmospheric cleansing of oxidized organic trace gases by vegetation. Science 2010, 330, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Pennisi, S.V.; Son, K.C.; Kays, S.J. Screening indoor plants for volatile organic pollutant removal efficiency. Horscience 2009, 44, 1377–1381. [Google Scholar]
- Kim, K.J.; Kil, M.J.; Song, J.S.; Yoo, E.H. Efficiency of volatile formaldehyde removal by indoor plants: Contribution of aerial plant parts versus the root zone. Hortscience 2008, 133, 521–526. [Google Scholar]
- Agrawal, M.; Singh, B.; Rajput, M.; Marshall, F.; Bell, J.N. Effect of air pollution on peri-urban agriculture: A case study. Environ. Pollut. 2003, 126, 323–329. [Google Scholar] [CrossRef]
- Giese, M.; Baue-Doranth, U.; Langebartels, C.; Sandermann, H., Jr. Detoxification of formaldehyde by the spider plant (Chlorophytum comosum L.) and by soybean (Glycine max L.) cell-suspension cultures. Plant Physiol. 1994, 104, 1301–1309. [Google Scholar] [PubMed]
- Wolverton, B.C.; Wolverton, J.D. Plants and soil microorganisms: Removal of formaldehyde, ethylbenzene, and ammonia from the indoor environment. J. Miss Acad. Sci. 1993, 38, 11–15. [Google Scholar]
- Dela Cruz, M.; Christensen, J.H.; Thomsen, J.D.; Müller, R. Can ornamental potted plants remove volatile organic compounds from indoor air?—A review. Envrion. Sci. Pollut. Res. 2014, 21, 13909–13928. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ge, Y.; Gu, B.; Min, Y.; Tani, A.; Chang, J. Role of management strategies and environmental factors in determining the emission of biogenic volatile organic compounds from urban greenspaces. Environ. Sci. Technol. 2014, 48, 6237–6246. [Google Scholar] [CrossRef] [PubMed]
- Treesubsuntorn, C.; Thiravetyan, P. Removal of benzene from indoor air by Dracaena sanderiana: Effect of wax and stomata. Atmos. Environ. 2012, 57, 317–321. [Google Scholar] [CrossRef]
- Yoo, M.H.; Kwon, Y.J.; Son, K.C.; Kays, S.J. Efficacy of indoor plants for the removal of single and mixed volatile organic pollutants and physiological effects of the volatiles on the plants. J. Am. Soc. Horticult. Sci. 2006, 131, 452–458. [Google Scholar]
- Winter, K.; Holtum, J.A.M. Facultative crassulacean acid metabolism (CAM) plants: Powerful tools for unravelling the functional elements of CAM photosynthesis. J. Exp. Bot. 2014, 65, 3425–3441. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A. Crassulacean acid metabolism and fitness under water deficit stress: If not for carbon gain, what is facultative CAM good for? Ann. Bot. 2009, 103, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Sriprapat, W.; Thiravertyan, P. Phytoremediation of BTEX from indoor air by Zamioculcas zamiifolia. Water Air Soil Pollut. 2013, 224, 1482. [Google Scholar] [CrossRef]
- Ugrekhelidze, D.; Korte, F.; Kvesitadze, G. Uptake and transformation of benzene and toluene by plant leaves. Ecotoxicol. Environ. Saf. 1997, 37, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.H. Air Pollution and Forests; Springer: New York, NY, USA, 1990. [Google Scholar]
- Su, Y.H.; Liang, Y.C. The foliar uptake and downward translocation of trichloroethylene and 1,2,3-trichlorobenzene in air-plant-water systems. J. Hazard Mater. 2013, 252–253, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.; Mackay, D.; Tam, D.; Shiu, W.Y. Chemicals by plants: A review of processes, correlations and models. Chemosphere 1990, 21, 297–331. [Google Scholar] [CrossRef]
- Ryan, J.A.; Bell, R.M.; Davidson, J.M.; O’Connor, G.A. Plant uptake of non-ionic organic chemicals from soils. Chemosphere 1988, 17, 2299–2323. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Berti, W.B. Remediation of contaminated soils with green plants: An overview. In Vitro Cell. Dev. Biol. 1993, 29, 207–212. [Google Scholar] [CrossRef]
- Trapp, S.; Köhler, A.; Larsen, L.C.; Zambrano, K.C.; Karlson, U. Phytotoxicity of fresh and weathered diesel and gasoline to willow and poplar trees. J. Soils Sediments 2001, 1, 71–76. [Google Scholar] [CrossRef]
- Schmitz, H.; Hilgers, U.; Weidner, M. Assimilation and metabolism of formaldehyde by leaves appear unlikely to be of value for indoor air purification. New Phytol. 2000, 147, 307–315. [Google Scholar] [CrossRef]
- Korte, F.; Kvesitadze, G.; Ugrekhelidze, D.; Gordeziani, M.; Khatisashvili, G.; Buadze, O.; Zaalishvili, G.; Coulston, F. Organic toxicants and plants. Ecotoxicol. Environ. Saf. 2000, 47, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Burken, J.G. Uptake and metabolism of organic compounds: Green-liver model. In Phytoremediation: Transformation and Control of Contaminants; McCutcheon, S.C., Schnoor, J.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 59–84. [Google Scholar]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Voriskova, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Baldotto, L.E.B.; Olivares, F.L. Phylloepiphytic interaction between bacteria and different plant species in a tropical agricultural system. Can. J. Microbiol. 2008, 54, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E.; Brandl, M.T. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Knief, C.; Delmotte, N.; Chaffron, S.; Stark, M.; Innerebner, G.; Wassman, R.; von Mering, C.; Vorholt, J.A. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J. 2012, 6, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Ruppel, S. Progress in cultivation-independent phyllosphere microbiology. FEMS Microbiol. Ecol. 2014, 87, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Imran, A.; Khan, Q.M.; Afzal, M. Plant-bacteria partnerships for the remediation of persistent organic pollutants. Environ. Sci. Pollut. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, M.; Dowling, D. Plant-associated bacterial degradation of toxic organic compounds in soil. Int. J. Environ. Res. Public Health 2009, 6, 2226–2247. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; van der Lelie, D.; Artois, T.; Smeets, K.; Taghavi, S.; Newman, L.; Carleer, R.; Vangronsveld, J. Bioaugmentation with engineered endophytic bacteria improves contaminant fate in phytoremediation. Environ. Sci. Technol. 2009, 43, 9413–9418. [Google Scholar] [CrossRef] [PubMed]
- Barac, T.; Taghavi, S.; Borremans, B.; Provoost, A.; Oeyen, L.; Colpaert, J.V.; Vangronsveld, J.; van der Lelie, D. Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat. Biotechnol. 2004, 22, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Orwell, R.L.; Wood, R.L.; Terran, J.; Torpy, F.; Burchett, M.D. Removal of Benzene by the indoor plant/substrate microcosm and implication to air quality. Water Air Soil Pollut. 2004, 157, 193–207. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, Z.; Ling, W.; Huang, J. Arbuscular mycorrhizal fungal hyphae contribute to the uptake of polycyclic aromatic hydrocarbons by plant roots. Bioresour. Technol. 2010, 101, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, F.; Nasseri, S.; Mesdaghinia, A.; Nabizadeh, R.; Zafari, D.; Khodakaramian, G.; Chehregani, A. Phytoremediation of petroleum-polluted soils: Application of Polygonum aviculare and its root-associated (penetrated) fungal strains for bioremediation of petroleum-polluted soils. Ecotoxicol. Environ. Saf. 2010, 73, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Bouwer, E.J.; Zehnder, A.J.B. Bioremediation of organic compounds-putting microbial metabolisms to work. Trends Biotechnol. 1993, 11, 360–367. [Google Scholar] [CrossRef]
- Xu, A.J.; Wu, M.; He, Y.Y. Toluene biofiltration enhanced by ryegrass. Bull Environ. Contam. Toxicol. 2013, 90, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Qin, N.; Wang, J.G.; Tong, H. Formaldehyde biofiltration as affected by spider plant. Bioresour. Technol. 2010, 101, 6930–6934. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A.; Orwell, R.L.; Tarran, J.; Torpy, F.; Burchett, M. Potted-plant/growth media interactions and capacity for removal of volatiles from indoor air. J. Hortic. Sci. Biotechnol. 2002, 77, 120–129. [Google Scholar]
- Ramos, J.L.; Molina, L.; Segura, A. Removal of organic toxic chemicals in the rhizosphere and phyllosphere of plants. Microb. Biotechnol. 2009, 2, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, H.; Al-Mailem, D.; Dashti, N.; Hakam, L.; Eliyas, M.; Radwan, S. The abundant occurance of hydrocarbon-utilizing bacteria in the phyllospheres of cultivated and wild plants in Kuwait. Int. Biodeterior. Biodegrad. 2012, 73, 73–79. [Google Scholar] [CrossRef]
- Al-Awadhi, H.; El-Nemr, I.; Mahmoud, H.; Sorkhoh, N.A.; Radwan, S.S. Plant-associated bacteria as tools for phytoremediation of oily nitrogen-poor soils. Int. J. Phytoremediat. 2009, 11, 11–27. [Google Scholar] [CrossRef]
- Al-Mailem, D.M.; Sorkhoh, N.A.; Marafie, M.; Al-Awadhi, H.; Eliyas, M.; Radwan, S.S. Oil phytoremediation potential of hypersaline coast of the Arabian Gulf using rhizosphere technology. Bioresour. Technol. 2010, 101, 5786–5792. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Halverson, L.J.; Beattie, G.A. Identification and genetic characterization of phenol-degrading bacteria from leaf microbial communities. Microb. Ecol. 2009, 57, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Halverson, L.J.; Beattie, G.A. Bacterial degradation of airborne phenol in the phyllosphere. Environ. Microbiol. 2007, 2, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Yutthammo, C.; Thongthammachat, N.; Pinphanickakarn, P.; Luepromchai, E. Diversity and activity of PAH-degrading bacteria in the phyllosphere of ornamental plants. Microb. Ecol. 2010, 59, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Sorkhoh, N.; Salamah, S.; Eliyas, M.; Radwan, S. The potential of epiphytic hydrocarbon-utilizing bacteria on legume leaves for attenuation of atmospheric hydrocarbon pollutants. J. Environ. Manag. 2012, 93, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Al-Awadhi, H.; Dashti, N.; Khanafer, M.; El-Nemr, I.; Sorkhoh, N.; Radwan, S.S. Bioremediation of atmospheric hydrocarbons via bacteria naturally associated with leaves of higher plants. Int. J. Phytoremediat. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ilori, M.O.; Ezeani, C.J.; Amund, O.O.; Omoijiahina, S.A.; Adebusoye, S.A. Occurrence and growth potential of hydrocarbon degrading bacteria on the phyllosphere of some tropical plants. Afr. J. Biotechnol. 2006, 5, 542–545. [Google Scholar]
- Sorkhoh, N.A.; Al-Mailem, D.M.; Ali, N.; Al-Awadhi, H.; Salamah, S.; Eliyas, M.; Radwan, S.S. Bioremediation of volatile oil hydrocarbons by epiphytic bacteria associated with American grass (Cynodon sp.) and broad bean (Vicia faba) leaves. Int. Biodeterior. Biodegrad. 2011, 65, 797–802. [Google Scholar] [CrossRef]
- Waight, K.; Pinayakong, O.; Luepromchai, E. Degradation of phenanthrene on plant leaves by phyllosphere bacteria. J. Gen. Appl. Microbiol. 2007, 3, 265–272. [Google Scholar] [CrossRef]
- De Kempeneer, L.; Sercu, B.; Vanbrabant, W.; van Langenhove, H.; Verstraete, W. Bioaugmentation of the phyllosphere for the removal of toluene from indoor air. Appl. Microbiol. Technol. 2004, 64, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, H.; Sato, I.; Sakakibara, M.; Yurimoto, H.; Sakai, Y. Distribution of methanothrophs in the phyllosphere. Biosci. Biotechnol. Biochem. 2012, 76, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Cho, J.K.; Choi, H.; Chu, H.; Lian, S.; Cho, W.K. Bacterial communities in the phylloplane of Prunus species. J. Basis Microb. 2015, 55, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Nadalig, T.; Farhan, U.I.; Haque, M.; Roselli, S.; Schaller, H.; Bringel, F.; Vuilleumier, S. Detection and isolation of chloromethane-degrading bacteria from Arabidopsis thaliana phyllosphere and characterization of chloromethane utilization genes. FEMS Microbiol. Ecol. 2011, 77, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Scheublin, T.R.; Deusch, S.; Moreno-Forero, S.K.; Müller, J.A.; van der Meer, J.R.; Leveau, J.H. Transcriptional profiling of Gram-positive Arthrobacter in the phyllosphere: Induction of pollutant degradation genes by natural plant phenolic compunds. Environ. Microbiol. 2014, 16, 2212–2225. [Google Scholar] [CrossRef] [PubMed]
- Scheublin, T.R.; Leveau, J.H. Isolation of Arthrobacter species from the phyllosphere and demonstration of their epiphytic fitness. Microbiologyopen 2013, 2, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, I.F.; Ion, B. The effects of air pollutants on vegetation and the role of vegetation in reducing atmospheric pollution. Available online: http://cdn.intechopen.com/pdfs-wm/18642.pdf (accessed on 22 October 2015).
- NRC (National Research Council). Advancing the Science of Climate Change; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Frumkin, H.; Hess, J.; Luber, G.; Malilay, J.; McGeehin, M. Climate change: The public health response. Am. J. Public Health 2008, 98, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Horii, C.V.; Munger, J.W.; Wofsy, S.C.; Zahniser, M.; Nelson, D.; McManus, J.B. Fluxes of nitrogen oxides over a temperate deciduous forest. J. Geophys. Res. 2004, 109, D08305. [Google Scholar] [CrossRef]
- Parrish, D.D.; Zhu, T. Clean air for megacities. Science 2009, 326, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Flechard, C.; Skiba, U.; Coyle, M.; Cape, J.N. The atmospheric budget of oxidized nitrogen and its role in ozone formation and deposition. New Phytol. 1998, 139, 11–23. [Google Scholar] [CrossRef]
- Samoli, E.; Aga, E.; Touloumi, G.; Nisiotis, K.; Forsberg, B.; Lefranc, A.; Pekkanen, J.; Wojtyniak, B.; Schindler, C.; Niciu, E.; et al. Short-term effects of nitrogen dioxide on mortality: An analysis within the APHEA project. Eur. Respir. J. 2006, 27, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.D.; Tran, H.; Levy, J.I. The health benefits of reduced tropospheric ozone in California. J. Air Waste Manag. Assoc. 2006, 56, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Vagaggini, B.; Taccola, M.; Cianchetti, S.; Carnevali, S.; Bartoli, M.L.; Bacci, E.; Dente, F.L.; Di Franco, A.; Giannini, D.; Paggiaro, P.I. Ozone exposure increases eosinophilic airway response induced by previous allergen challenge. Am. J. Respir. Crit. Care Med. 2002, 166, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Hernandez, O.; Garcia-Reynoso, J.A.; Ojeda-Ramirez, M.M.; Suarez-Lastra, M. Retrospective health impact assessment for ozone pollution in Mexico city from 1991 to 2011. Atmosfera 2014, 27, 261–271. [Google Scholar] [CrossRef]
- Nowak, D.J.; Civerloo, K.L.; Rao, S.T.; Sistla, G.; Luley, C.J.; Crane, D.E. A modeling study of the impact of urban trees on ozone. Atmos. Environ. 2000, 34, 1610–1613. [Google Scholar] [CrossRef]
- Taha, H. Modeling impacts of increased urban vegetation on ozone air quality in the South Coast Air Basin. Atmos. Environ. 1996, 30, 3423–3430. [Google Scholar] [CrossRef]
- Cardelino, C.A.; Chameides, W.L. Natural hydrocarbons, urbanization, and urban ozone. J. Geophys. Res. 1990, 95, 13971–13979. [Google Scholar] [CrossRef]
- Bytnerowicz, A.; Fenn, M.E.; Miller, P.R.; Arbaugh, M.J. Wet and dry pollutant deposition to the mixed conifer forest. In Oxidant Air Pollution Impacts in the Montane Forests of Southern California: A Case Study of the San Bernardino Mountains; Miller, P.R., McBride, J.R., Eds.; Springer: New York, NY, USA, 1999; pp. 235–369. [Google Scholar]
- Sinha, R.K.; Singh, S. Plants combating air pollution. In Green Plants and Pollution: Nature’s Technology for Abating and Combating Environmental Pollution (Air, Water and Soil Pollution Science and Technology); Sinha, R.K., Ed.; Nova Science Publishers: Palo Alto, CA, USA, 2010. [Google Scholar]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Sedjo, R.; Sohngen, B. Carbon sequestration in forests and soils. Annu. Rev. Resour. Econ. 2012, 4, 127–144. [Google Scholar] [CrossRef]
- Scheller, R.M.; van Tuyl, S.; Clark, K.L.; Hom, J.; La Puma, I. Carbon sequestration in New Jersey Pine Barrens under different scenarios of fire management. Ecosystems 2011, 14, 987–1004. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems: A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Vallano, D.; Sparks, J. Foliar δ15N values as indicators of foliar uptake of atmospheric nitrogen pollution. In Stable Isotopes as Indicators of Ecological Change; Dawson, T.E., Siegwolf, R.T.W., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2007; pp. 93–109. [Google Scholar]
- Takahashi, M.; Kondo, K.; Morikawa, H. Assimilation of nitrogen dioxide in selected plant taxa. Acta Biotechnol. 2003, 23, 241–247. [Google Scholar] [CrossRef]
- Segschneider, H.; Wildt, J.; Forstel, H. Uptake of 15NO2 by sunflower (Helianthus anuus) during exposures in light and darkness: Quantities, relationship to stomatal aperture and incorporation into different nitrogen pools within the plant. New Phytol. 1995, 131, 109–119. [Google Scholar] [CrossRef]
- Welburn, A. Atmospheric nitrogenous compounds and ozone—Is NOx fixation by plants a possible solution? New Phytol. 1998, 139, 5–9. [Google Scholar] [CrossRef]
- Welburn, A. Why are atmospheric oxides of nitrogen usually phytotoxic and not alternative fertilizers? New Phytol. 1990, 115, 395–429. [Google Scholar] [CrossRef]
- Gebler, A.; Rienks, M.; Rennenberg, H. Stomatal uptake and cuticular adsorption contribute to dry deposition of NH3 and NO2 to needles of adult spruce (Picea abies) trees. New Phytol. 2002, 156, 179–194. [Google Scholar]
- Theone, B.; Schroder, P.; Papen, H.; Egger, A.; Rennenberg, H. Absorption of atmospheric NO2 by spruce (Picea abies L. Karst.) trees. I. NO2 influx and its correlation with nitrate reduction. New Phytol. 1991, 117, 575–585. [Google Scholar]
- Altimir, N.; Kolari, P.; Tuovinen, J.P.; Vesala, T.; Bäck, J.; Suni, T.; Kulmala, M.; Hari, P. Foliage surface ozone deposition: A role for surface moisture? Biogeosciences 2006, 3, 1–20. [Google Scholar] [CrossRef]
- Loreto, F.; Fares, S. Is ozone flux inside leaves only a damage indicator? Clues from volatile isoprenoid studies. Plant Physiol. 2007, 143, 1096–1100. [Google Scholar] [PubMed]
- Fares, S.; Park, J.-H.; Ormeno, E.; Gentner, D.R.; McKay, M. Ozone uptake by citrus trees exposed to a range of ozone concentrations. Atmos. Environ. 2010, 44, 3404–3412. [Google Scholar] [CrossRef]
- Pell, E.J.; Schlagnhaufer, C.D.; Arteca, R.N. Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant. 1997, 100, 264–273. [Google Scholar] [CrossRef]
- Oksanen, E.; Häikiö1, E.; Sober, J.; Karnosky, D.F. Ozone-induced H2O2 accumulation in field-grown aspen and birch is linked to foliar ultrastructure and peroxisomal activity. New Phytol. 2004, 161, 791–799. [Google Scholar]
- Zheng, Y.; Shimizu, H.; Barnes, J.D. Limitations to CO2 assimilation in ozone-exposed leaves of Plantago major. New Phytol. 2002, 155, 67–68. [Google Scholar] [CrossRef]
- Sandermann, H.; Welburn, A.R.; Heah, R.L. Forest Decline and Ozone: A Comparison of Controlled Chamber Experiments and Field Experiments; Springer Verlag: Berlin, Germany, 1997; pp. 1–400. [Google Scholar]
- Darrall, N.M. The effect of air pollutants on physiological processes in plants. Plant Cell Environ. 1989, 12, 1–30. [Google Scholar] [CrossRef]
- Vollenweider, P.; Gunthardt-Goerg, M.S. Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ. Pollut. 2005, 137, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Papen, H.; Gebler, A.; Zumbusch, E.; Rennenberg, H. Chemolithoautotrophic nitrifiers in the phyllosphere of a spruce ecosystem receiving high atmospheric nitrogen input. Curr. Microbiol. 2002, 44, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.A.; Hungate, B.A. Mycorrhizal controls on belowground litter quality. Ecology 2003, 84, 2302–2312. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Lesaulnier, C.; Papamichail, D.; McCorkle, S.; Olivier, B.; Skiena, S.; Taghavi, S.; Zak, D.; van der Lelie, D. Elevated atmospheric CO2 affects soil microbial diversity associated with trembling aspen. Environ. Microbiol. 2008, 10, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Wolch, J.R.; Byrne, J.; Newell, J.P. Urban green space, public health, and environmental justice: The challenge of making cities “just green enough”. Landsc. Urban Plan 2014, 123, 234–244. [Google Scholar] [CrossRef]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Haslmayr, H.P.; Meibner, S.; Langella, F.; Baumgarten, A.; Geletneky, J. Establishing best practice for microbially aided phytoremediation. Environ. Sci. Pollut. Res. 2014, 21, 6765–6774. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.S.; Castro, P.M.L.; Dodd, J.C.; Vosátka, M. Different native arbuscular mycorrhizal fungi influence the coexistence of two plant species in a highly alkaline anthropogenic sediment. Plant Soil 2006, 287, 209–221. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Prieto-Fernandez, A.; Kidd, P.S.; Weyens, N.; Rodriguez-Garrido, B.; Touceda-Gonzalez, M.; Acea, M.J.; Vangronsveld, J. Improving performance of Cytisus striatus on substrates contaminated with hexachlorocyclohexane (HCH) isomers using bacterial inoculants: Developing a phytoremediation strategy. Plant Soil 2013, 362, 247–260. [Google Scholar] [CrossRef]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Ofek-Lalzar, M.; Sela, N.; Goldman-Voronov, M.; Green, S.J.; Hadar, Y.; Minz, D. Niche and host-associated functional signatures of the root surface microbiome. Nat. Commun. 2014, 5, 4950. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Law, A.D.; Moe, L.A. Nicotiana roots recruit rare rhizosphere taxa as major root-inhabiting microbes. Microb. Ecol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Pomerantz, M.; Taha, H. Cool surfaces and shade trees to reduce energy use and improve air quality in urban areas. Sol. Energy 2001, 70, 295–310. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Kenbner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistance of soil organic matter as an ecosystem property. Nature 2011, 40, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mabry, K.E.; Barret, G.W. Effects of corridors on home range sizes and interpatch movements of three small mammal species. Landsc. Ecol. 2002, 17, 629–636. [Google Scholar] [CrossRef]
- Seok, Y.H.; Jian, K.; Sung, C.M. Acoustic effects of green roof systems on a low-profiled structure at street level. Build. Environ. 2012, 50, 44–55. [Google Scholar]
- Mentens, J.; Raes, D.; Hermy, M. Green roofs as a tool for solving the rainwater runoff problem in the urbanized 21st century? Landsc. Urban Plan 2006, 77, 217–226. [Google Scholar] [CrossRef]
- EEA. “Urban Adaptation to Climate Change in Europe”—Challenges and Opportunities for Cities Together with Supportive National and European Policies; EEA Report 2/2012; EEA: Copenhagen, Denmark, 2012; p. 143. [Google Scholar]
- Barton, J.; Pretty, J. What is the best dose of nature and green exercise for improving mental health? A multi-study analysis. Environ. Sci. Technol. 2010, 44, 3947–3955. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Aspinal, P.; Montarzino, A. The childhood factor: Adult visits to green places and the significance of childhood experiences. Environ. Behav. 2008, 40, 111–143. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Evolutionary ecology of plant–microbe interactions: Soil microbial structure alters selection on plant traits. New Phytol. 2011, 192, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.A.; Lennon, J.T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. 2012, 109, 14058–14062. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Moe, L.A. Multitrophic microbial interactions for eco-and agro-biotechnological processes: Theory and practice. Trends Biotechnol. 2014, 32, 529–537. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weyens, N.; Thijs, S.; Popek, R.; Witters, N.; Przybysz, A.; Espenshade, J.; Gawronska, H.; Vangronsveld, J.; Gawronski, S.W. The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants. Int. J. Mol. Sci. 2015, 16, 25576-25604. https://doi.org/10.3390/ijms161025576
Weyens N, Thijs S, Popek R, Witters N, Przybysz A, Espenshade J, Gawronska H, Vangronsveld J, Gawronski SW. The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants. International Journal of Molecular Sciences. 2015; 16(10):25576-25604. https://doi.org/10.3390/ijms161025576
Chicago/Turabian StyleWeyens, Nele, Sofie Thijs, Robert Popek, Nele Witters, Arkadiusz Przybysz, Jordan Espenshade, Helena Gawronska, Jaco Vangronsveld, and Stanislaw W. Gawronski. 2015. "The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants" International Journal of Molecular Sciences 16, no. 10: 25576-25604. https://doi.org/10.3390/ijms161025576
APA StyleWeyens, N., Thijs, S., Popek, R., Witters, N., Przybysz, A., Espenshade, J., Gawronska, H., Vangronsveld, J., & Gawronski, S. W. (2015). The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants. International Journal of Molecular Sciences, 16(10), 25576-25604. https://doi.org/10.3390/ijms161025576