Surface-Deacetylated Chitin Nano-Fiber/Hyaluronic Acid Composites as Potential Antioxidative Compounds for Use in Extended-Release Matrix Tablets
Abstract
:1. Introduction
2. Results and Discussion
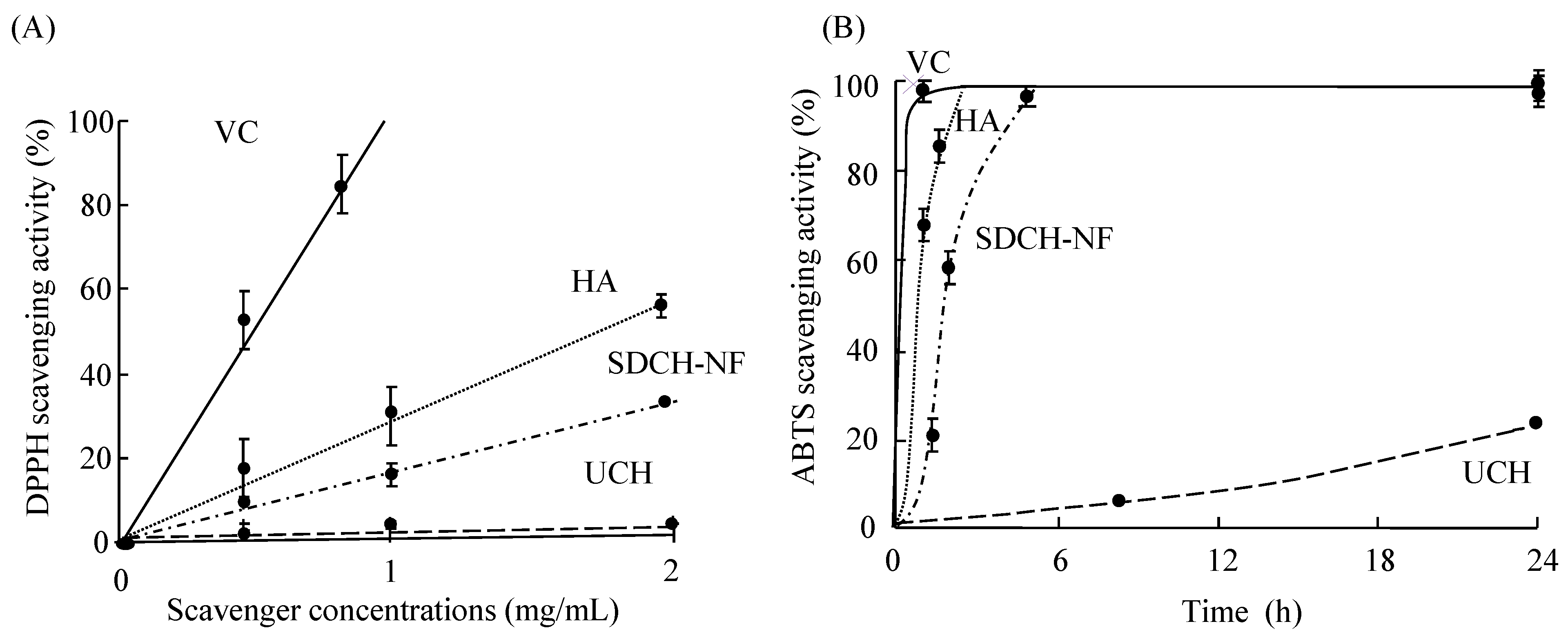
2.1. Scavenging Activity of UCH, SDCH-NF, and HA on DPPH and ABTS Radicals
 ), hyaluronic acid (HA) (
), hyaluronic acid (HA) (  ) surface-deacetylated chitin nano-fiber (SDCH-NF) (
) surface-deacetylated chitin nano-fiber (SDCH-NF) (  ), and untreated chitin (UCH) (
), and untreated chitin (UCH) (  ).
).
 ), hyaluronic acid (HA) (
), hyaluronic acid (HA) (  ) surface-deacetylated chitin nano-fiber (SDCH-NF) (
) surface-deacetylated chitin nano-fiber (SDCH-NF) (  ), and untreated chitin (UCH) (
), and untreated chitin (UCH) (  ).
).
2.2. Characterization of SDCH-NF/HA Composite
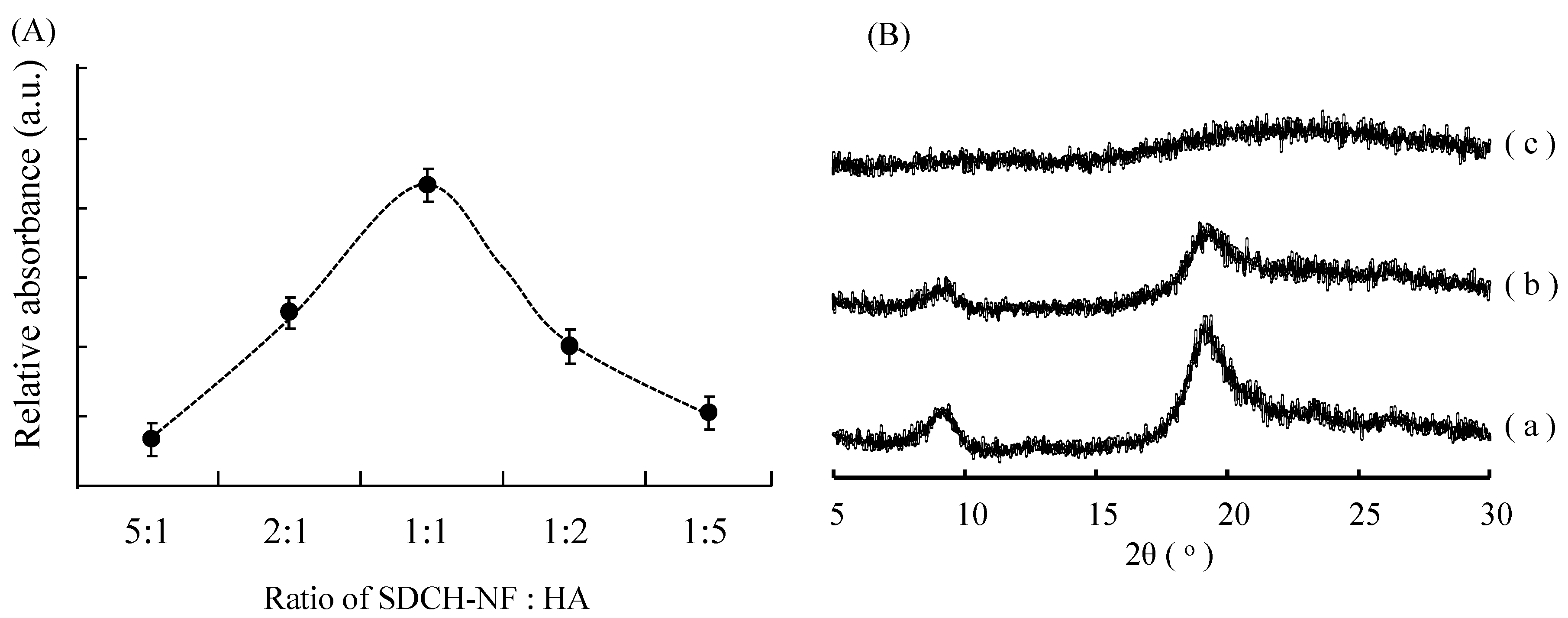
2.3. In Vitro Release Study of Famotidine (FMT) and Scavenging Activity of SDCH-NF/HA/FMT Tablet

2.4. Surface Structure of Tablet before and after Release Study of UCH/FMT, SDCH-NF/FMT, and SDCH-NF/HA/FMT Tablet

3. Experimental Section
3.1. Reagents
3.2. Preparations of SDCH-NF
3.3. Preparation of SDCH-NF/HA Tablets Containing Famotidine (FMT)
3.4. Scavenging Activity of UCH, SDCH-NF, and HA on DPPH and ABTS Radicals
3.5. Turbidity Measurements
3.6. X-ray Diffraction (XRD)
3.7. Dissolution of Famotidine from Tablets
3.8. Scanning Electron Microscopy (SEM)
3.9. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liew, C.V.; Chan, L.W.; Ching, L.; Heng, P.W. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int. J. Pharm. 2006, 309, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Tanaka, Y.; Yakou, S.; Takayama, K. In vivo drug release from hydrophilic dextran tablets capable of forming polyion complex. J. Control. Release 2006, 114, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.A.; Tadros, M.I. Design and in vitro evaluation of interpolymer complex bound metformin sustained release tablet. Eur. J. Pharm. Biopharm. 2008, 69, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Hiorth, M.; Versland, T.; Heikkilä, J.; Tho, I.; Sande, S.A. Immersion coating of pellet cores consisting of chitosan and calcium intended for colon drug delivery. Int. J. Pharm. 2006, 308, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yu, G.; Hirata, T.; Terao, J.; Lin, H. Antioxidative activities of several marine polysaccharides evaluated in suspension and organic solvents. Biosci. Biotechnol. Biochem. 1998, 62, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Anraku, M.; Kabashima, M.; Namura, H.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Furutani, N.; Tomida, H. Antioxidant protection of human serum albumin by chitosan. Int. J. Biol. Macromol. 2008, 43, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Fujii, T.; Furutani, N.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Tomida, H. Antioxidant effects of a dietary supplement: Reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem. Toxicol. 2009, 47, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control. Release 2011, 155, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitin nanostructures in living organisms. In Chitin: Formation and Diagenesis; Gupta, N., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 34, pp. 1–34. [Google Scholar]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Motylenko, M.V.; Beyer, J.; Makarova, A.A.; Stocker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.V.; Bazhenov, V.V.; et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.I.; Heitmann, J. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Soares, J.R.; Dins, T.C.P.; Cunha, A.P.; Almeida, L.M. Antioxidant activities of some extracts of Thymus zygis. Free Radic. Res. 1997, 26, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activity of chitooligosaccharides by electron spin resonance spectrometry. J. Agric. Food Chem. 2003, 51, 4624–4627. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Hiraga, A.; Iohara, D.; Pipkin, J.D.; Uekama, K.; Hirayama, F. Slow-release of famotidine from tablets consisting of chitosan/sulfobutyl ether β-cyclodextrin composites. Int. J. Pharm. 2015, 487, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Gong, S. Novel thiolated carboxymethyl chitosan-g-β-cyclodextrin as mucoadhesive hydrophobic drug delivery carriers. Carbohydr. Polym. 2008, 73, 117–125. [Google Scholar] [CrossRef]
- Anraku, M.; Iohara, D.; Hiraga, A.; Uekama, K.; Ifuku, S.; Pipkin, J.D.; Hirayama, F. Formation of elastic gels from deacetylated chitin nanofibers reinforced with sulfobutyl ether β-cyclodextrin. Chem. Lett. 2015, 44, 285–287. [Google Scholar] [CrossRef]
- Nellore, R.V.; Rekhi, G.S.; Hussain, A.S.; Tillman, L.G.; Augsburger, L.L. Development of metoprolol tartrate extended-release matrix tablet formulation for regulatory policy consideration. J. Control. Release 1998, 50, 247–256. [Google Scholar] [CrossRef]
- Kranz, H.; Guthmann, C.; Wagner, T.; Lipp, R.; Reiinhard, J. Development of a single unit extended release formulation for ZK 811 752, a weakly basic drug. Eur. J. Pharm. Sci. 2005, 26, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, W.; Lu, Y.; Wang, Z. Chitosan semi-interpenetrating polymer network. J. Appl. Polym. Sci. 1997, 65, 1445–1450. [Google Scholar] [CrossRef]
- Zhong, Z.; Guo, Q. Interpolymer complexes and miscible blends of poly (N-vinyl-2-pyrrolidone) with novolac resin and the effect of crosslinking on related behavior. Polym. Int. 1996, 41, 315–322. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Kuan, C.Y.; Lee, S.T.; Lu, K.T.; Jang, S.F.J. Chitosan–Polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. I. Effect of phosphorous polyelectrolyte complex and enzymatic hydrolysis of polymer. J. Appl. Polym. Sci. 1999, 74, 1868–1879. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Obinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Wakuda, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Beneficial and preventive effect of chitin nanofibrils in a dextran sulfate sodium-induced acute ulcerative colitis model. Carbohydr. Polym. 2012, 87, 1399–1403. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. α-Chitin nanofibrils improve inflammatory and fibrosis responses in mice with inflammatory bowel disease. Carbohydr. Polym. 2012, 90, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. A comparative study analysis of α-chitin and β-chitin nanofibrils by using an inflammatory-bowel disease mouse model. J. Chitin Chitosan Sci. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Azuma, K.; Nagae, T.; Nagai, T.; Izawa, H.; Morimoto, M.; Murahata, Y.; Osaki, T.; Tsuka, T.; Imagawa, T.; Ito, N.; et al. Effects of surface-deacetylated chitin nanofibers in an experimental model of hypercholesterolemia. Int. J. Mol. Sci. 2015, 16, 17445–17455. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y.; et al. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Nishihara, M.; Shimizu, H.; Itoh, Y.; Takashima, O.; Osaki, T.; Itoh, N.; Imagawa, T.; Murahata, Y.; Tsuka, T.; et al. Biological adhesive based on carboxymethyl chitin derivatives and chitin nanofibers. Biomaterials 2015, 42, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Kogure, K.; Goto, S.; Abe, K.; Ohiwa, C.; Akasu, M.; Terada, H. Potent antiperoxidation activity of the bisbenzylisoquinoline alkaloid cepharanthine: The amine moiety is responsible for its pH-dependent radical scavenge activity. Biochim. Biophys. Acta 1990, 1426, 133–142. [Google Scholar] [CrossRef]
- Leelarungrayub, N.; Rattanapanone, V.; Chanarat, N.; Gebicki, J.M. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition 2006, 22, 266–274. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anraku, M.; Tabuchi, R.; Ifuku, S.; Ishiguro, T.; Iohara, D.; Hirayama, F. Surface-Deacetylated Chitin Nano-Fiber/Hyaluronic Acid Composites as Potential Antioxidative Compounds for Use in Extended-Release Matrix Tablets. Int. J. Mol. Sci. 2015, 16, 24707-24717. https://doi.org/10.3390/ijms161024707
Anraku M, Tabuchi R, Ifuku S, Ishiguro T, Iohara D, Hirayama F. Surface-Deacetylated Chitin Nano-Fiber/Hyaluronic Acid Composites as Potential Antioxidative Compounds for Use in Extended-Release Matrix Tablets. International Journal of Molecular Sciences. 2015; 16(10):24707-24717. https://doi.org/10.3390/ijms161024707
Chicago/Turabian StyleAnraku, Makoto, Ryo Tabuchi, Shinsuke Ifuku, Takako Ishiguro, Daisuke Iohara, and Fumitoshi Hirayama. 2015. "Surface-Deacetylated Chitin Nano-Fiber/Hyaluronic Acid Composites as Potential Antioxidative Compounds for Use in Extended-Release Matrix Tablets" International Journal of Molecular Sciences 16, no. 10: 24707-24717. https://doi.org/10.3390/ijms161024707
APA StyleAnraku, M., Tabuchi, R., Ifuku, S., Ishiguro, T., Iohara, D., & Hirayama, F. (2015). Surface-Deacetylated Chitin Nano-Fiber/Hyaluronic Acid Composites as Potential Antioxidative Compounds for Use in Extended-Release Matrix Tablets. International Journal of Molecular Sciences, 16(10), 24707-24717. https://doi.org/10.3390/ijms161024707






