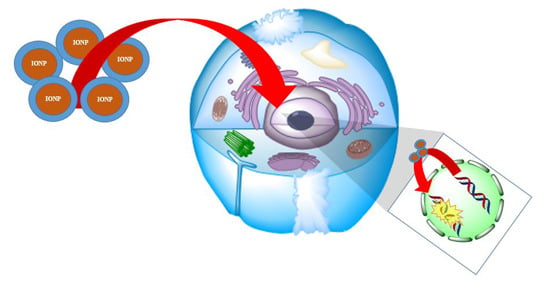
Mutagenic Effects of Iron Oxide Nanoparticles on Biological Cells
Abstract
:1. Introduction
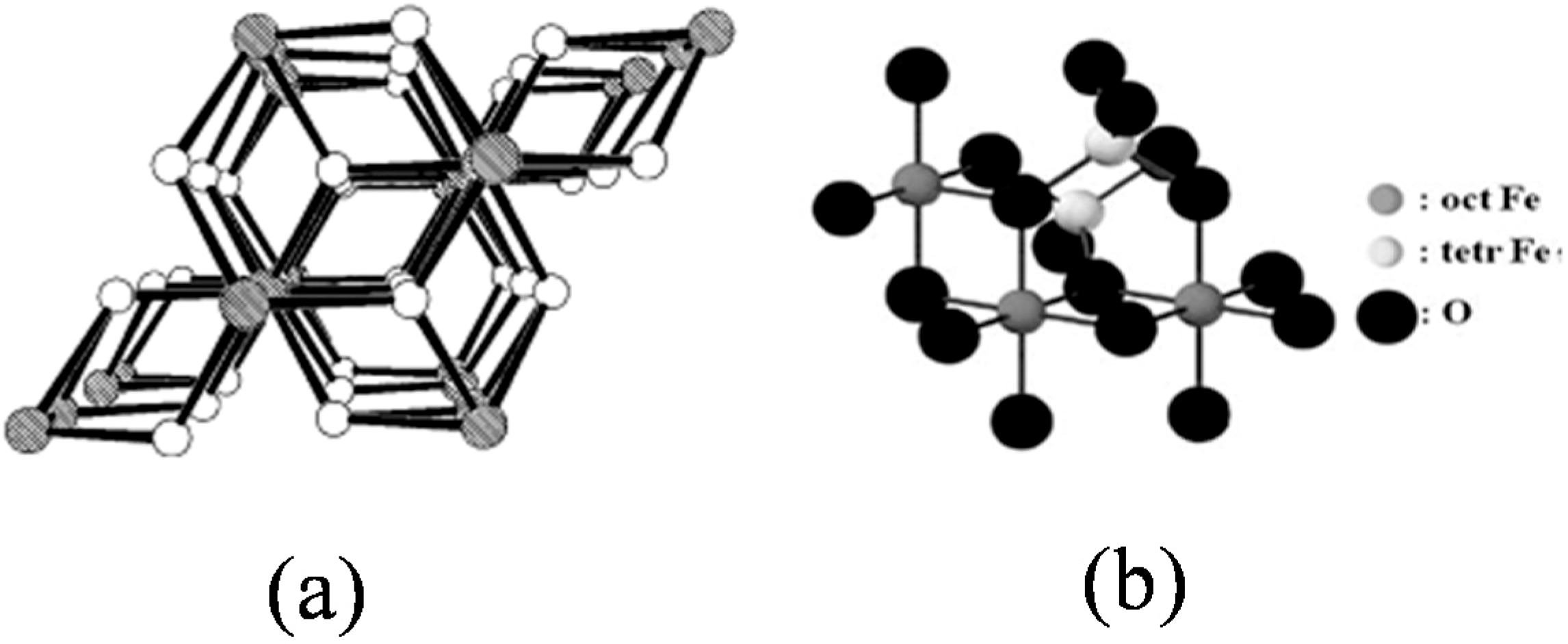
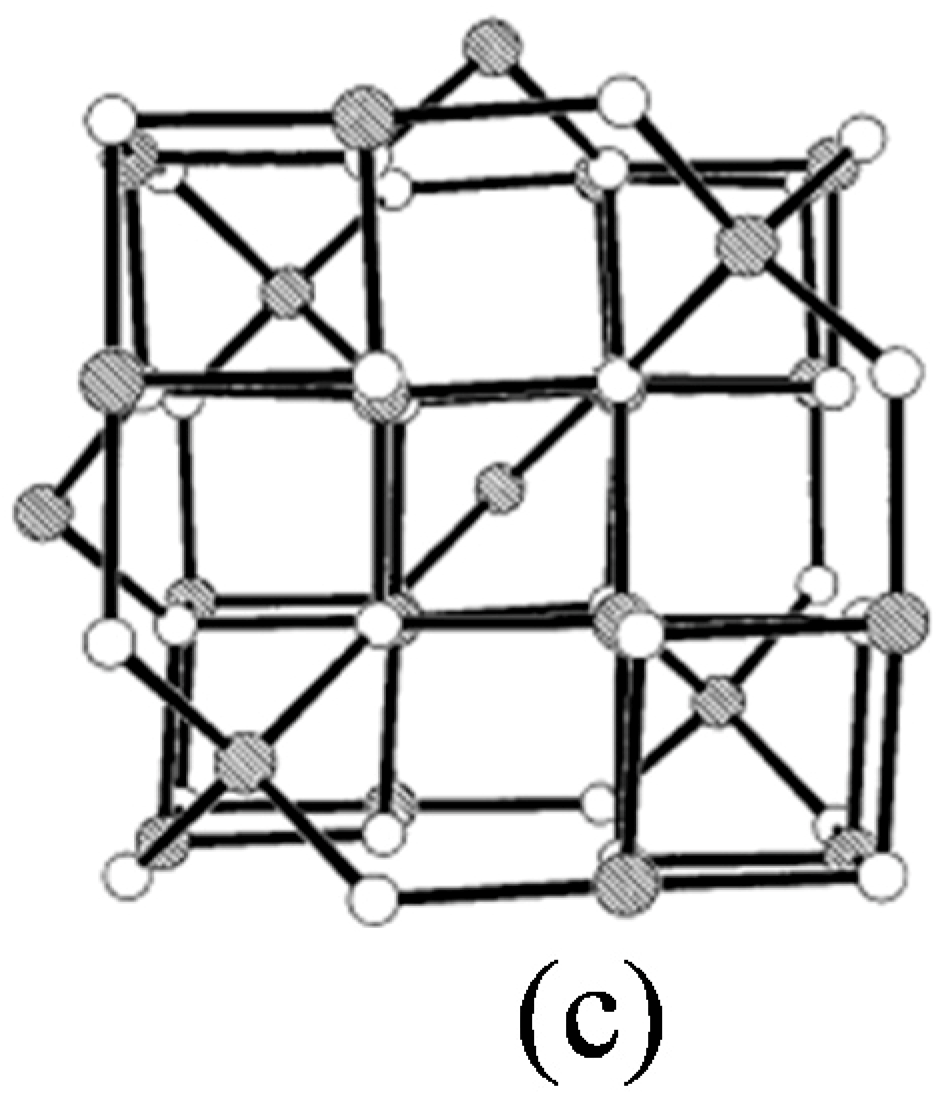
1.1. Potential Applications of Iron Oxide Nanoparticles (IONPs)
1.2. IONPs Incomplete Toxicological Profile
2. Mutagenic Impact of IONPs on Cell Lines
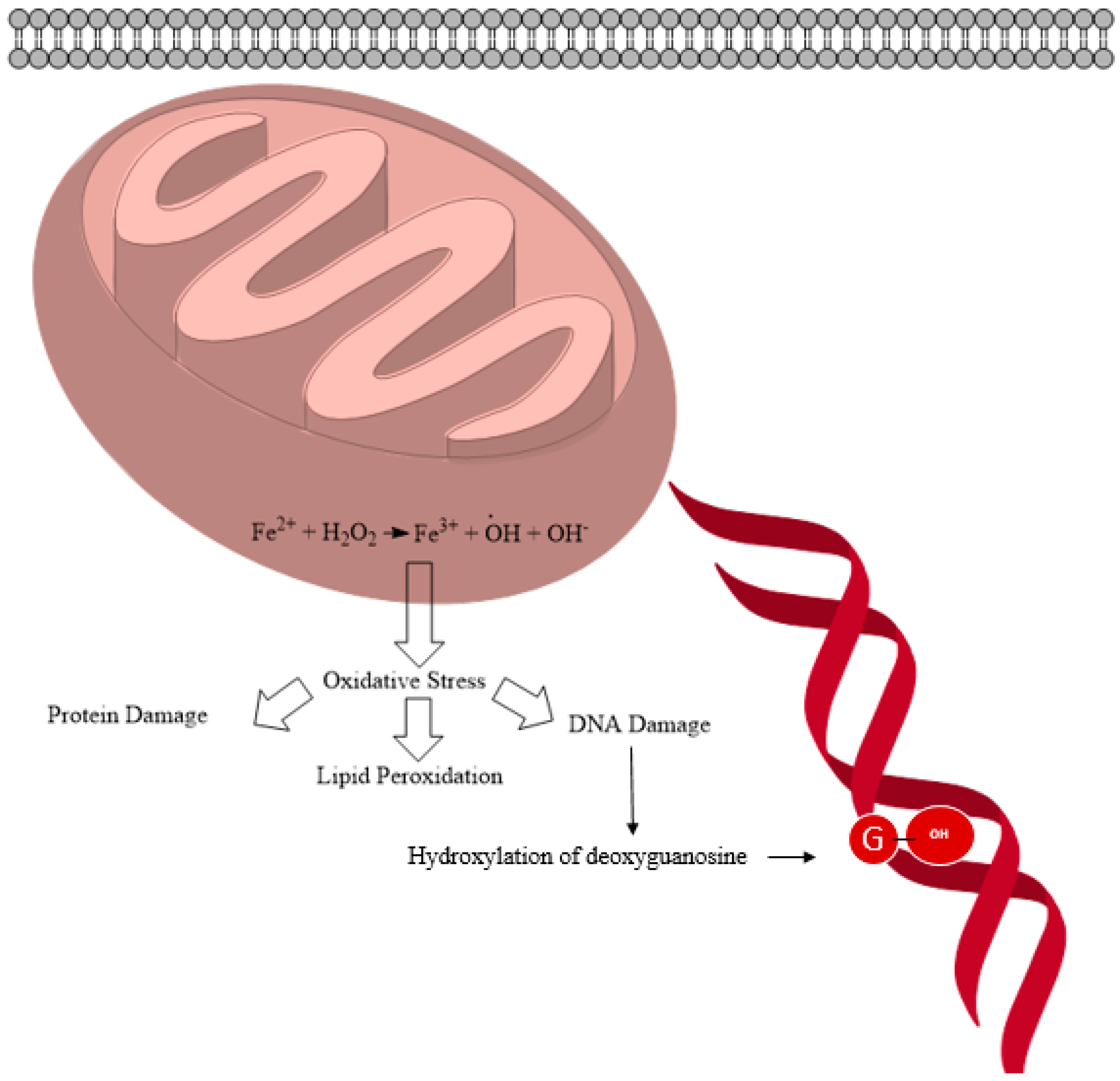
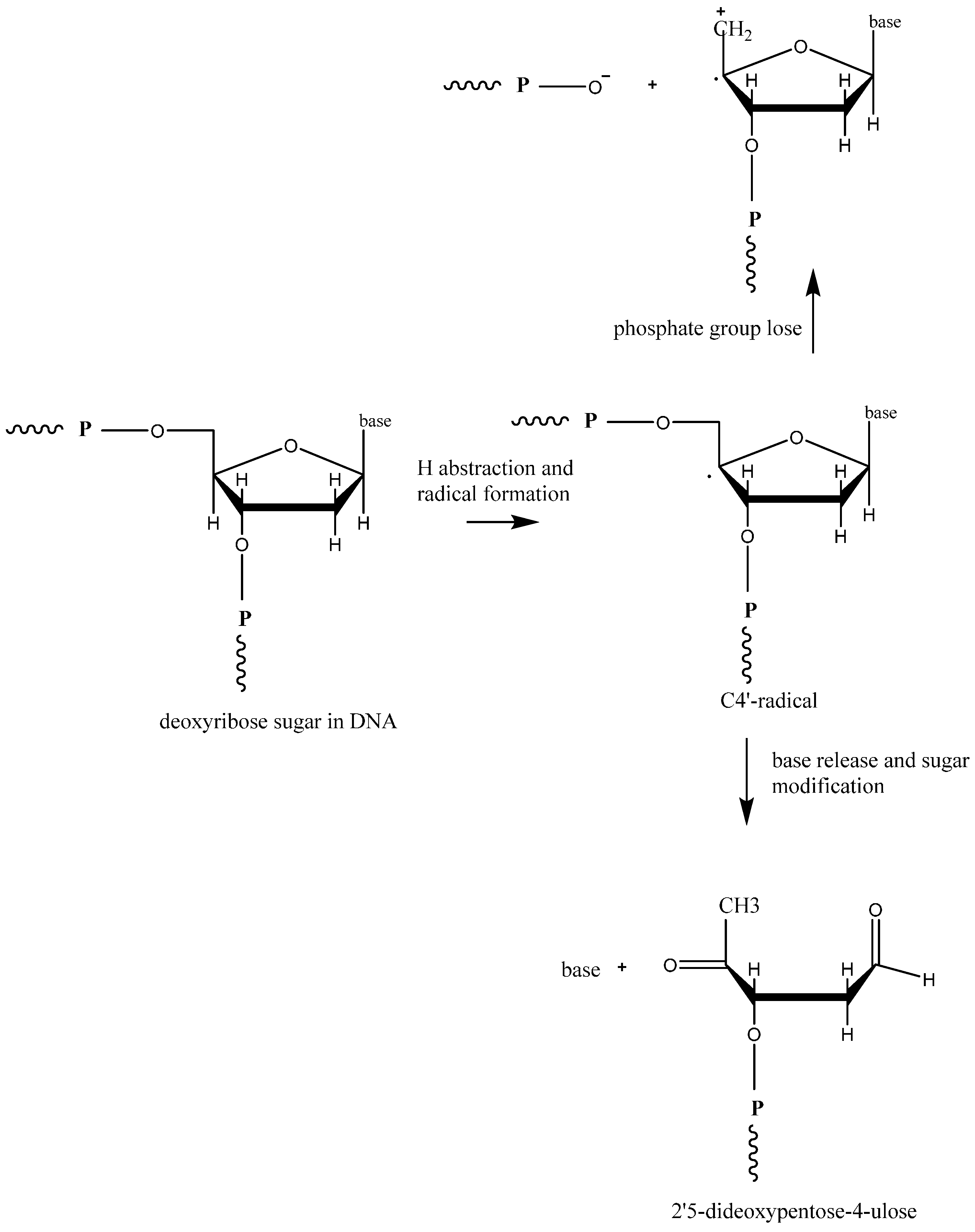
2.1. General ROS Generation and DNA Damage Mechanisms

| IONP Type and Surface Modification | Characterization and Size | Cellular Model/Organism | Impact on Cells | Ref. |
|---|---|---|---|---|
| Silica-coated, dithiocarbamate functionalized Fe3O4 NP alone and NP co-exposure with Hg | Particle sizes 100 nm (DLS) | European eel (Anguilla anguilla L.) erythrocytes | IONP-Hg complex can eliminate DNA damage which is induced by IONP or Hg alone. IONP alone is more capable of inducing mutagenicity than Hg. | [104] |
| Nanoscale and bulk materials of Fe3O4 | Nanoscale = 29.75 nm, bulk 2.15 µm (TEM) | Rat leucocytes and bone marrow cells | Showed no evidence of DNA damage by comet assay or micronucleus (MN) test for any of the tested particles. | [105] |
| Uncoated Fe3O4 (magnetite), and uncoated γ-Fe2O3 (maghemite) Dextran coated ultra-fine superparamagnetic Fe3O4 (dUSPION), dextran coated ultra-fine superparamagnetic γ-Fe2O3 (d USPION2) | Particle size = 1 nm (TEM) | Human lymphoblastoid cell line (MCL-5) | γ-Fe2O3 dUSPION2 showed significant DNA damage at a concentration of 4 µg·mL−1 and higher, having accumulated oxidative base lesions (including 8-OH-Gua). | [106] |
| Uncoated and oleic acid coated Fe3O4 (magnetite) NPs | Particle size = 9 nm | Human lymphoblastoid TK6 cells | No genotoxic effect was observed for the bare particles. However, an increased base oxidation was observed for oleic acid coated particles after 2 and 24 h of treatment. | [107] |
| Fe2O3 NPs | X-ray diffraction particle crystal diameter 31.1 nm | Human lymphoblastoid cells (TK6), Chinese hamster ovary cells (H9T3) | Iron oxide samples of 10 and 20 g/mL produced DNA tail percentages 17% and 20%, respectively, after 4 h in TK6 cells. The same two concentrations of iron oxide samples produced DNA tail percentages 33% and 48%, respectively, after 24 h in H9T3 cells. | [108] |
| Unfunctionalized Au@Fe3O4 Janus particles and functionalized particles with NH2 | Particle core sizes, gold domain 3.5 nm and iron oxide domain 16 nm (TEM) | Human microvascular endothelial cells | DNA damage was observed for unfunctionalized Janus particles, compared to NH2 functionalized particles. | [109] |
| Fe3O4 (magnetite) and Fe3O4–poly(l-lactide)–poly(ethyleneglycol)–poly(l-lactide) magnetic microspheres (Fe3O4–PLLA–PEG–PLLA MMPs) | 30 nm | Mouse fibroblast cell line (L929) Chinese hamster ovarian cell line (CHO-K1) | Fe3O4–PLLA-PEG-PLLAMMPS caused less DNA damage than Fe3O4 particles. | [110] |
| Fe3O4 (magnetite) NPs, tetraethyl orthosillicate (TEOS) coated IONP, 3-aminopropyl trimethoxy silane (APTMS) coated IONP and TEOS/APTMS coated IONP | HR-TEM bare particle size 10 ± 3 nm. TEOS coated particles 100–150 nm, APTMS coated 10 ± 4 nm, TEOS/APTMS coated 100–150 nm | Human normal fibroblast and fibrosarcoma cells | Both cell types showed a dose dependent increase in DNA tail size. Bare and TEOS coated NPs showed no extensive or dose dependent DNA damage (lower than 5% damage at 1000 g/L). APTMS and APTMS/TEOS coated NPs produced a significant dose dependent toxicity when exposed to normal cells. | [26] |
| Maghemite (γ-Fe2O3) γ-Fe2O3 coated with poly-l-lysine, d-mannose, poly(NN-dimethylacrylamide) | Number-average particle diameter γ-Fe2O3 6 nm, PLL-γ-Fe2O3 5.5 nm, mannose γ -Fe2O3 7 nm, PDMAAm-γ-Fe2O3 7.5 nm (TEM) | Human bone marrow mesenchymal stromal cells from two donors (hBMSCs-1–12 years and hBMSCs-2–54 years) | hBMSCs-2 showed more toxic effects upon exposure to IONPs than did hBMSCs-1. In hBMSCs-2, only PDMAAm-γ-Fe2O3 and γ-Fe2O3 particles increased DNA damage after 72 h of exposure to NPs. | [111] |
| Bare superparamagnetic magnetite (SPION) and poly(vinyl alcohol) PVA coated SPION | Particle size 4.5 nm (TEM) | Mouse fibroblast adhesive cells (L929) | Cells exposed to bare particles showed evidence of cytotoxicity after 24 h. No toxic effect for the coated particles was observed, even after 72 h of exposure. DNA damage is believed to be the reason behind apoptosis. | [112] |
| Bare SPION (magnetite) citrate coated, tetraethyl orthosilicate (TEOS) coated, 3-aminopropyl trimethoxy silane (APTMS) coated and TEOS/APTMS coated IONP (T-A) | Bare particle size 10 nm. Citrate coated particle 10 nm, TEOS coated particle S 100–150 nm, APTMS coated 10 nm, TEOS-APTMS coated 100–150 nm | Murine fibroblast cell line (L-929 from mouse subcutaneous connective tissue) | No extensive or dose dependent DNA damage was observed for the cells treated with bare and TEOS treated particles. SPIONS modified with APTMS and T-A showed a dose dependent mutagenicity. Cells treated with 200 ppm citrate modified SPIONS showed significant DNA damage. | [113] |
| Zero valent iron NPs (nZVI) with Na acrylic co-polymer | Particle size 50 nm (TEM) | Mytilus galloprovincialis sperm | DNA strand breakage was observed after exposure for 2 h. | [114] |
| Fe@Fe2O3 core-shell nanonecklace with MWCNT | Diameter of nanonecklace 50–150 nm (SEM) | Herring sperm DNA | DNA damage was observed by monitoring the DPV (Differential Pulse Voltammetry) response of an electrochemical indicator Co(phen)3 or Ru(NH3)63+. | [115] |
| Fe@Fe2O3 core-shell nanonecklace and Au NPs | High magnification SEM image revealed diameter 50–150 nm | Hering sperm DNA | DNA damage was detected within 5–10 min of incubation with cathodic treatment. | [116] |
| Fe3O4 (magnetite) microparticles and nanoparticles, Fe2O3 microparticles and nanoparticles | Particle sizes for Fe3O4 nano-sized 27 ± 8 nm and micro-sized 156 ± 82 nm, Fe2O3 nano-sized 35 ± 14 nm and micro-sized 147 ± 48 nm (TEM) | Syrian Hamster embryo cells | No significant DNA damage or micronucleus formation was observed in any cell samples exposed to the iron oxide NPs. | [117] |
| Fe3O4 (magnetite) NPs | Particle size 12.5 ± 4.45 nm (TEM) | Mice lung imprinting control region | Significant DNA damage in magnetite treated mice lung cells was observed. Mice treated with low dose magnetite showed a two-fold mutant frequency relative to the control. High dose treated mice showed a three-fold mutant frequency relative to the control. | [60] |
| Fe3O4 (magnetite) NPs | Average particle size 10 nm (TEM) | A549 Human lung epithelial cells | 8-OH-dG levels increased by 8- and 14-fold above the control with 10 and 100 g/L NPs, respectively. | [28] |
| Hematite (α-Fe2O3)in three sizes, Hem-nano, Hem-submicro Hem-micro | Rhombohedral hematite α-Fe2O3 (XRD) Particle sizes: nano (93 nm), sub-micro (260 nm), micro (1600 nm) from TEM | Human lung epithelial cells (A549), murine alveolar macrophages (MH-S) | No DNA damage was induced by hematite NPs. | [118] |
| Fe2O3 microparticles and nanoparticles, Fe3O4 (magnetite) microparticles and nanoparticles | Particle sizes: Fe2O3 micro (0.15–1 µm) and nano (30–60 nm) sizes, Fe3O4 micro (0.1–0.5 µm) and nano (20–40 nm) (TEM) | Human alveolar type II like epithelial cells (A549) | By the comet assay Fe2O3 and Fe3O4 caused small but significant increases in DNA damage. In terms of oxidative damage, only Fe3O4 produced significant DNA damage. The authors report that nanoparticles were higher in oxidative capacity than their micrometer sized particle counterparts. | [119] |
| Fe2O3 (hematite) NP | No information provided | human lung cells: IMR 90 (human bronchial fibroblasts) and BEAS-2B cells | After 24 h exposure to Fe2O3 NPs, IMR-90 cells showed DNA-breakage at concentrations of 10 and 50 μg/cm2; then in BEAS-2B cells DNA breakage was observed at 50 μg/cm2 Fe2O3 NPs. | [120] |
| Fe3O4 (magnetite) NPs | X-ray diffraction-crystalline 25.27 nm particles, TEM showed polygonal shaped particles with a diameter of 24.83 nm | Human skin epithelial (A431) and lung epithelial (A549) cells | A positive correlation in DNA damage and ROS generation in A431 and A549 cells was observed. | [27] |
| CuZnFe2O4, Fe3O4 (magnetite), Fe2O3 NPs | No information provided | Type II epithelial cells (A549) | By the comet assay: no DNA damage was observed for IONPs (Fe3O4, Fe2O3), but DNA damage was observed for CuZnFe2O4. In terms of oxidative damage, Fe3O4 produced oxidative DNA lesions as did CuZnFe2O4. | [63] |
| Fe3O4 (magnetite) NPs | Particle size = 8 nm (TEM) | Human hepatocyte (HL-7702 cell line) | Cells showed nuclear condensation and chromosomal DNA fragmentation after NPs exposure. | [121] |
| Fe2O3 NPs | Particle size = 50 nm | Human hepatoma Hep G2 cells | Concentration and time dependent DNA damage was observed. | [53] |
| Fe3O4 (magnetite) NPs | Particle size = 35 nm (TEM) | Mice hepatic and renal tissue | In liver tissue there was significant increase of 8-OH-dG levels for the highest dose of NPs (40 mg/kg). In kidney tissues damage was shown for a dose of 20 mg/kg NPs. A significant DPC (DNA-protein crosslinks) coefficient was observed for: (a) a dose of 40 mg/kg NPs in liver tissue and (b) a dose of 10 mg/kg NPs in kidney tissue. | [122] |
| Ultra small superparamagnetic magnetite IONPs (USPIO NPs, Fe3O4) oleic acid coated USPIO NPs | Particle sizes 8 ± 3 nm (TEM) 14–15 nm (DLS) | Human cerebral endothelial cells (HCECS) | Single and double DNA strand breaks and alkaline labile sites were detected. | [123] |
| Aminosilane-coated IONPs (AmS-IONPs) and COOH-AmS-IONPs | No information provided | Mouse brain microvessel endothelial cell line and mouse astrocytes and neurons | No toxicity was observed for any of the particles in brain endothelial cells. At high concentrations neurons displayed a toxicity to AmS-IONPs and astrocytes displayed a toxicity to COOH-AmS-IONPs. | [124] |
| Fe2O3 NPs | Particle size ranged between 19.56–48 nm (TEM) | Human breast cancer cell line (MCF-7) | Gradual nonlinear DNA damage was observed as NP dose and exposure time were increased. 60 µg/mL IONP conditions produced the most DNA damage. | [52] |
| Epidermal growth factor receptor (EGFR) targeted hybrid plasmonic core-shell iron oxide (maghemite) gold NPs (225 NP) | TEM and DLS particle size 73 ± 35 nm | Human HCC827 lung cancer cell line | An increase in DNA strand breaks for 225 NP treated cells was observed (relative to all other treatment conditions). DNA strand breaks were assessed by tracking the levels of phosphorylated H2AX expression. A slight increase in phosphorylated H2AX was also observed in cells treated with: (1) AuFe; (2) 225-Ab (antibody) alone; (3) a mixture of NP and 225-Ab relative to untreated cells. | [125,126] |
| Bare Fe3O4(magnetite)/SiO2 NPs, Fe3O4 amine-silane surface modified, Fe3O4 sulfonate-silane surface modified | TEM, DLS Fe3O4 core 12 ± 2 nm, SiO2 shell thickness 7 ± 1.5 nm, total diameter 26 ± 2.9 nm | Human cervix carcinoma cells (HeLa cells), Human lung carcinoma cells (A549) | Bare NPs increased DNA damage in terms of tail length and DNA percentage in tail relative to passivated NPs, which showed results similar to the control. | [127] |
| Fe3O4 (magnetite) NPs | No information provided | HeLa cells | After exposure of 50 µg/mL NPs, no significant change was observed in tail length. 100–200 µg/mL concentrations however, showed increased tail length and DNA percentage in the tail. | [128] |
| Fe3O4 (magnetite) and Fe2O3 NPs | Particle size < 50 nm (TEM) | Vero cell line (C1008), bacterial strains Salmonella typhimurium (TA98), S. typhimurium TA 100, TA 1535, TA 1537, and E. coli WP2uvrA | No change is observed in the number of revertant colonies in IONP treated groups or the negative control. The positive control showed mutagenicity. IONPs do not induce mutagenicity in strains S. typhimurium TA 100, TA 1535, TA 1537, and E. coli WP2uvrA. | [129] |
2.2. Vascular System and Blood Cells
2.3. Fibroblast and Stromal Cells
2.4. Reproductive Cells
2.5. Lung Cells
2.6. Liver, Kidney and Cerebral Cells
2.7. Cancerous Cells
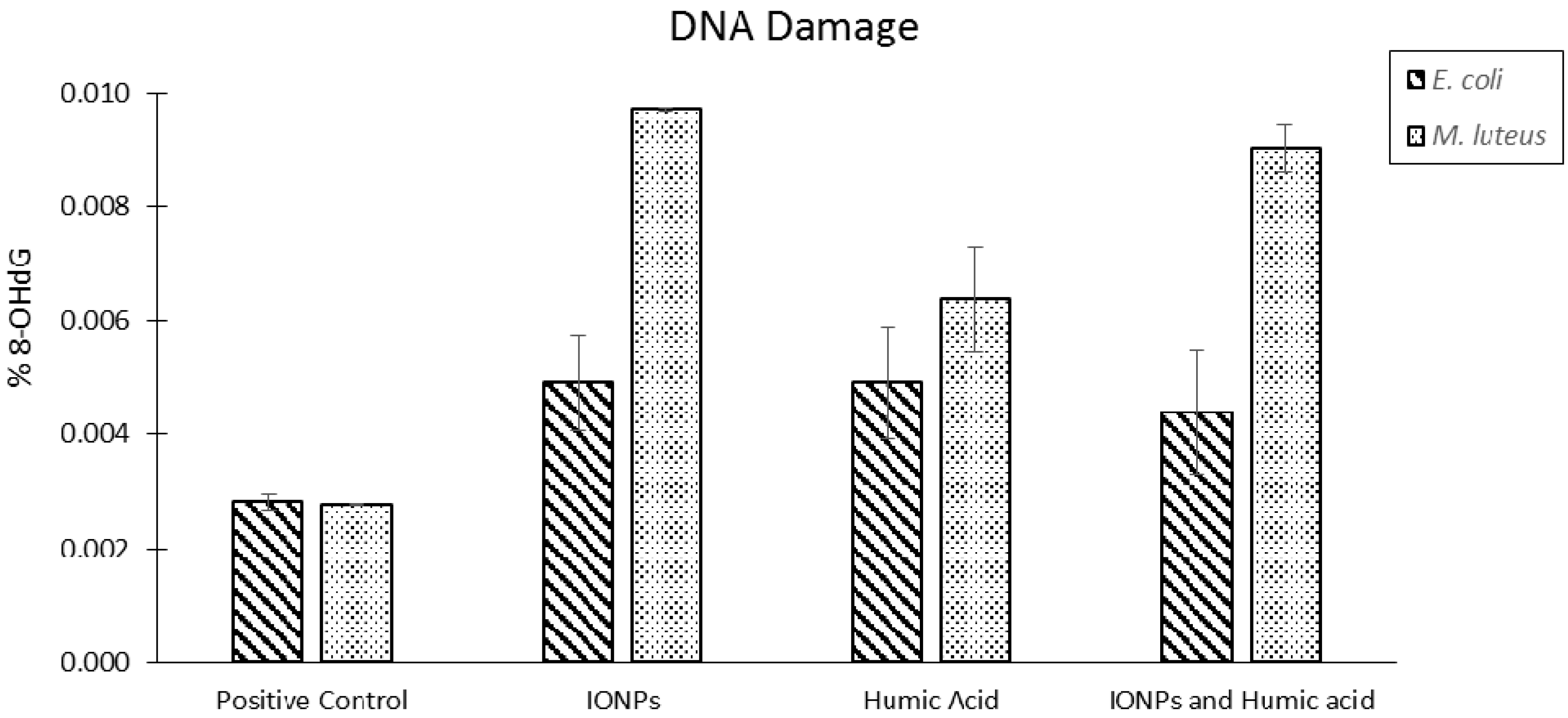
2.8. Bacterial Cells
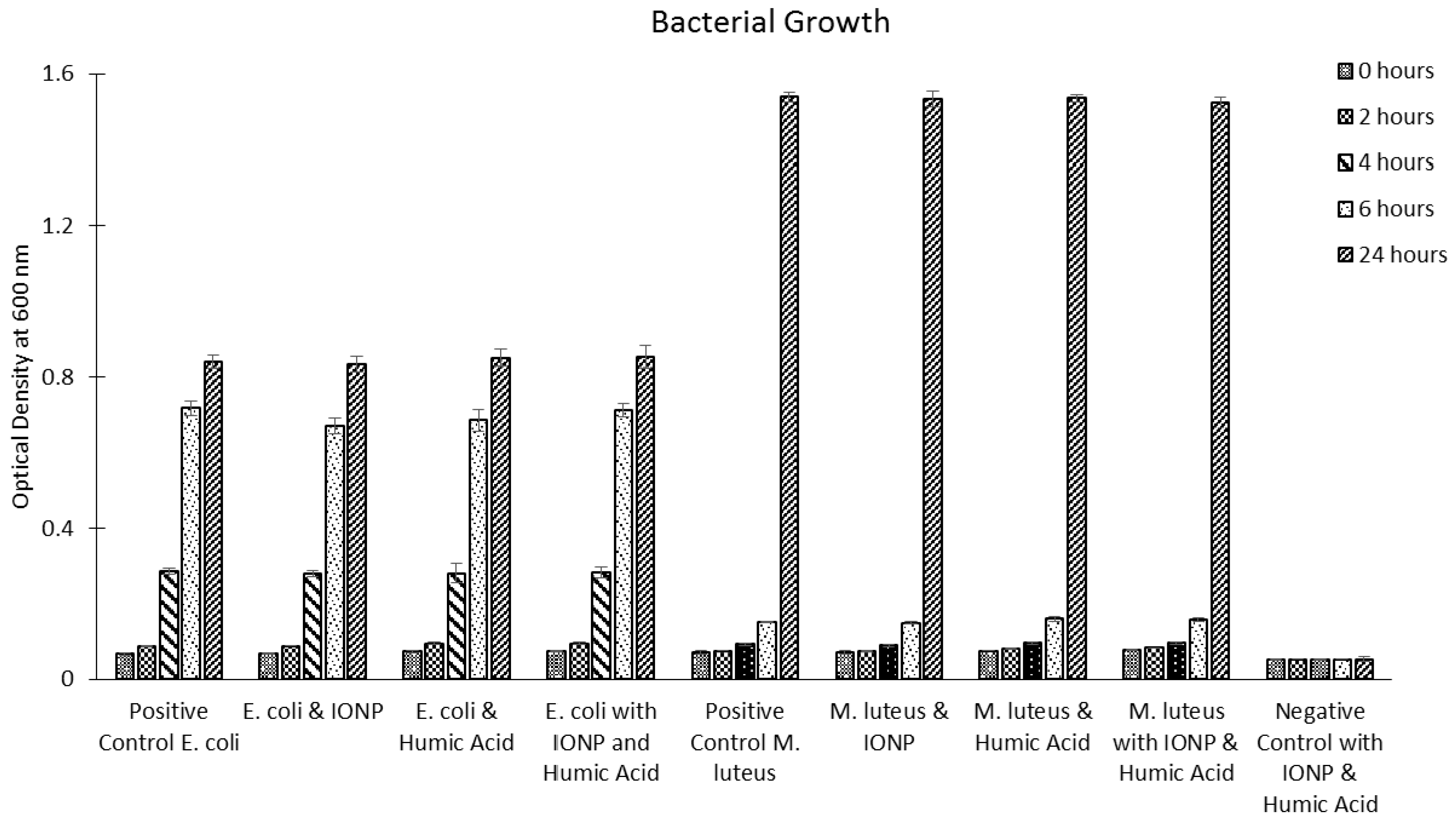
3. Summary and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yellishetty, M.; Ranjith, P.; Tharumarajah, A. Iron ore and steel production trends and material flows in the world: Is this really sustainable? Resour. Conserv. Recycl. 2010, 54, 1084–1094. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/iron_oxide/mcs-2015-fepig.pdf (accessed on 1 July 2015).
- Hilty, F.M.; Arnold, M.; Hilbe, M.; Teleki, A.; Knijnenburg, J.T.; Ehrensperger, F.; Hurrell, R.F.; Pratsinis, S.E.; Langhans, W.; Zimmermann, M.B. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat. Nanotechnol. 2010, 5, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Kapse-Mistry, S.; Govender, T.; Srivastava, R.; Yergeri, M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharmacol. 2014, 5, 2–22. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.L.; Berret, J.; Fresnais, J.; Gossuin, Y.; Sandre, O. A universal scaling law to predict the efficiency of magnetic nanoparticles as MRI T2-contrast agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Tepluchin, M.; Kureti, S.; Casapu, M.; Ogel, E.; Mangold, S.; Grunwaldt, J. Study on the hydrothermal and SO2 stability of Al2O3-supported manganese and iron oxide catalysts for lean CO oxidation. Catal. Today 2015. [Google Scholar] [CrossRef]
- Ghasemi, E.; Ziyadi, H.; Afshar, A.M.; Sillanpää, M. Iron oxide nanofibers: A new magnetic catalyst for azo dyes degradation in aqueous solution. Chem. Eng. J. 2015, 264, 146–151. [Google Scholar] [CrossRef]
- Pereira, M.; Oliveira, L.; Murad, E. Iron oxide catalysts: Fenton and Fenton-like reactions—A review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- He, F.; Li, F. Perovskite promoted iron oxide for hybrid water-splitting and syngas generation with exceptional conversion. Energy Environ. Sci. 2015, 8, 535–539. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Pereira, N.; Lima, R.; Faria, J.L.; Gomes, H.T.; Silva, A.M. Degradation of diphenhydramine by photo-Fenton using magnetically recoverable iron oxide nanoparticles as catalyst. Chem. Eng. J. 2015, 261, 45–52. [Google Scholar] [CrossRef]
- Berret, J.; Schonbeck, N.; Gazeau, F.; El Kharrat, D.; Sandre, O.; Vacher, A.; Airiau, M. Controlled clustering of superparamagnetic nanoparticles using block copolymers: Design of new contrast agents for magnetic resonance imaging. J. Am. Chem. Soc. 2006, 128, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Lodhia, J.; Mandarano, G.; Ferris, N.; Eu, P.; Cowell, S. Development and use of iron oxide nanoparticles (Part 1): Synthesis of iron oxide nanoparticles for MRI. Biomed. Imaging Interv. J. 2010, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The iron oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Nanoscale biogenic iron oxides and neurodegenerative disease. FEBS Lett. 2001, 496, 1–5. [Google Scholar] [CrossRef]
- Iglesias, O.; Labarta, A. Finite-size and surface effects in maghemite nanoparticles: Monte Carlo simulations. Phys. Rev. B 2001, 63, 184416. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, M.; Zhang, Y.; Gu, N. Synthesis of nanometer-size maghemite particles from magnetite. Colloids Surf. Physicochem. Eng. Asp. 2004, 245, 15–19. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Bødker, F.; Hansen, M.F.; Koch, C.B.; Lefmann, K.; Mørup, S. Magnetic properties of hematite nanoparticles. Phys. Rev. B 2000, 61, 6826. [Google Scholar] [CrossRef]
- Xu, S.; Habib, A.; Gee, S.; Hong, Y.; McHenry, M. Spin orientation, structure, morphology, and magnetic properties of hematite nanoparticles. J. Appl. Phys. 2015, 117, 17A315. [Google Scholar] [CrossRef]
- Zboril, R.; Mashlan, M.; Petridis, D. Iron (III) oxides from thermal processes synthesis, structural and magnetic properties, Mössbauer spectroscopy characterization, and applications. Chem. Mater. 2002, 14, 969–982. [Google Scholar] [CrossRef]
- Hasany, S.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. Nanosci. Nanotechnol. 2012, 2, 148–158. [Google Scholar] [CrossRef]
- Weissleder, R.; Stark, D.D.; Engelstad, B.L.; Bacon, B.R.; Compton, C.C.; White, D.L.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. AJR Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Lee, J.H.; Hong, S.C.; Lee, J.; Lee, J.; Han, D. Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 2013, 6, 4689–4706. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.; Ali, D.; Alarafi, S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yoneda, M.; Morohashi, A.; Hori, Y.; Okamoto, D.; Sato, A.; Kurioka, D.; Nittami, T.; Hirokawa, Y.; Shiraishi, T.; et al. Effects of Fe3O4 magnetic nanoparticles on A549 cells. Int. J. Mol. Sci. 2013, 14, 15546–15560. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Teja, A.S.; Koh, P. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Reddy, G.R.; Bhojani, M.S.; McConville, P.; Moody, J.; Moffat, B.A.; Hall, D.E.; Kim, G.; Koo, Y.E.; Woolliscroft, M.J.; Sugai, J.V.; et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 2006, 12, 6677–6686. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.D.; Khieu, D.Q.; Hoa, T.T.; Quang, D.T.; Viet, P.H.; Lam, T.D.; Hoa, N.D.; van Hieu, N. Facile synthesis of α-Fe2O3 nanoparticles for high-performance CO gas sensor. Mater. Res. Bull. 2015, 68, 302–307. [Google Scholar] [CrossRef]
- Navale, S.; Bandgar, D.; Nalage, S.; Khuspe, G.; Chougule, M.; Kolekar, Y.; Sen, S.; Patil, V. Synthesis of Fe2O3 nanoparticles for nitrogen dioxide gas sensing applications. Ceram. Int. 2013, 39, 6453–6460. [Google Scholar] [CrossRef]
- Wang, L.; Fei, T.; Lou, Z.; Zhang, T. Three-dimensional hierarchical flowerlike α-Fe2O3 nanostructures: Synthesis and ethanol-sensing properties. ACS Appl. Mater. Int. 2011, 3, 4689–4694. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zheng, Z.; Yan, B.; Zhang, J.; Gong, H.; Li, J.; Liu, C.; Shen, Z.; Yu, T. Morphology controllable synthesis of α-Fe2O3 1D nanostructures: Growth mechanism and nanodevice based on single nanowire. J. Phys. Chem. C 2008, 112, 10784–10788. [Google Scholar] [CrossRef]
- Dodi, G.; Hritcu, D.; Draganescu, D.; Popa, M.I. Iron oxide nanoparticles for magnetically assisted patterned coatings. J Magn. Magn. Mater. 2015, 388, 49–58. [Google Scholar] [CrossRef]
- Patankar, N.A. Transition between superhydrophobic states on rough surfaces. Langmuir 2004, 20, 7097–7102. [Google Scholar] [CrossRef] [PubMed]
- Patankar, N.A. Mimicking the lotus effect: Influence of double roughness structures and slender pillars. Langmuir 2004, 20, 8209–8213. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, C.; Sun, J.; Gao, L. Three-dimensional Fe2O3 nanocubes/nitrogen-doped graphene aerogels: Nucleation mechanism and lithium storage properties. Sci. Rep. 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, T.; Sirriah, S.A.; Nairat, M.; Boyacı, E.; Eroğlu, A.E.; Scott, T.B.; Hallam, K.R. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Ren, X.; Wang, P.; Yu, S. Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jang, J. Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. Chem. Commun. 2007, 41, 4230–4232. [Google Scholar] [CrossRef]
- Fresnais, J.; Yan, M.; Courtois, J.; Bostelmann, T.; Bée, A.; Berret, J. Poly(acrylic acid)-coated iron oxide nanoparticles: Quantitative evaluation of the coating properties and applications for the removal of a pollutant dye. J. Colloid Interface Sci. 2013, 395, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Zargar, B.; Parham, H.; Hatamie, A. Modified iron oxide nanoparticles as solid phase extractor for spectrophotometeric determination and separation of basic fuchsin. Talanta 2009, 77, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.; Chen, D. Fast adsorption of methylene blue on polyacrylic acid-bound iron oxide magnetic nanoparticles. Dyes Pigment. 2004, 61, 93–98. [Google Scholar] [CrossRef]
- Xue, X.; Hanna, K.; Deng, N. Fenton-like oxidation of Rhodamine B in the presence of two types of iron (II, III) oxide. J. Hazard. Mater. 2009, 166, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Sugimoto, T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J. Colloid Interface Sci. 2003, 265, 283–295. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S.; Alhader, M.S. Iron oxide nanoparticles induce oxidative stress, dna damage, and caspase activation in the human breast cancer cell line. Biol. Trace Elem. Res. 2014, 159, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, L.; Tanwir, F.; Babadi, V.Y. In vitro toxicity of iron oxide nanoparticle: Oxidative damages on Hep G2 cells. Exp. Toxicol. Pathol. 2015, 67, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Mesarosova, M.; Kozics, K.; Babelova, A.; Regendova, E.; Pastorek, M.; Vnukova, D.; Buliakova, B.; Razga, F.; Gabelova, A. The role of reactive oxygen species in the genotoxicity of surface-modified magnetite nanoparticles. Toxicol. Lett. 2014, 226, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, S.; Cai, Z. Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS ONE 2012, 7, 2012. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R. Engineered nanoparticles in consumer products: Understanding a new ingredient. Environ. Health Perspect. 2011, 119, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Chanteau, B.; Fresnais, J.; Berret, J. Electrosteric enhanced stability of functional sub-10 nm cerium and iron oxide particles in cell culture medium. Langmuir 2009, 25, 9064–9070. [Google Scholar] [CrossRef] [PubMed]
- Mélanie, A.; Rose, J.; Bottero, J.; Lowry, G.V.; Jolivet, J.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol 2009, 4, 634–641. [Google Scholar]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle–cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, Y.; Ishino, K.; Kato, T.; Goto, S.; Tada, Y.; Nakae, D.; Watanabe, M.; Wakabayashi, K. Magnetite nanoparticles induce genotoxicity in the lungs of mice via inflammatory response. Nanomaterials 2014, 4, 175–188. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moeller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Koedrith, P.; Boonprasert, R.; Kwon, J.Y.; Kim, I.; Seo, Y.R. Recent toxicological investigations of metal or metal oxide nanoparticles in mammalian models in vitro and in vivo: DNA damaging potential, and relevant physicochemical characteristics. Mol. Toxicol. 2014, 10, 107–126. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Ulrih, N.P. Multifunctional superparamagnetic iron oxide nanoparticles: Promising tools in cancer theranostics. Cancer Lett. 2013, 336, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Stroh, A.; Zimmer, C.; Gutzeit, C.; Jakstadt, M.; Marschinke, F.; Jung, T.; Pilgrimm, H.; Grune, T. Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free Radic. Biol. Med. 2004, 36, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Buratti, P.; Gammella, E.; Rybinska, I.; Cairo, G.; Recalcati, S. Recent advances in iron metabolism: Relevance for health, exercise, and performance. Med. Sci. Sports Exerc. 2015, 47, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Buyukhatipoglu, K.; Clyne, A.M. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J. Biomed. Mater. Res. A 2011, 96A, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Mesarosova, M.; Ciampor, F.; Zavisova, V.; Koneracka, M.; Ursinyova, M.; Kozics, K.; Tomasovicova, N.; Hashim, A.; Vavra, I.; Krizanova, Z.; et al. The intensity of internalization and cytotoxicity of superparamagnetic iron oxide nanoparticles with different surface modifications in human tumor and diploid lung cells. Neoplasma 2012, 59, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron-induced carcinogenesis: The role of redox regulation. J. Free Radic. Biol. Med. 1996, 20, 553–566. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Anderson, N.L.; Polanski, M.; Pieper, R.; Gatlin, T.; Tirumalai, R.S.; Conrads, T.P.; Veenstra, T.D.; Adkins, J.N.; Pounds, J.G.; Fagan, R.; et al. The human plasma proteome: A nonredundant list developed by combination of four separate sources. Mol. Cell. Proteom. 2004, 3, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Norde, W. Protein adsorption at solid surfaces: A thermodynamic approach. Pure Appl. Chem. 1994, 66, 491–496. [Google Scholar] [CrossRef]
- Lynch, I.; Cedervall, T.; Lundqvist, M.; Cabaleiro-Lago, C.; Linse, S.; Dawson, K.A. The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2007, 134, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Sund, J.; Alenius, H.; Vippola, M.; Savolainen, K.; Puustinen, A. Proteomic characterization of engineered nanomaterial–protein interactions in relation to surface reactivity. ACS Nano 2011, 5, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 2008, 101, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, V.; Graillot, A.; Vitorazi, L.; Crouzet, Q.; Marletta, G.; Loubat, C.; Berret, J. Preventing corona effects: Multiphosphonic acid poly(ethylene glycol) copolymers for stable stealth iron oxide nanoparticles. Biomacromolecules 2014, 15, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Safi, M.; Sarrouj, H.; Sandre, O.; Mignet, N.; Berret, J. Interactions between sub-10-nm iron and cerium oxide nanoparticles and 3T3 fibroblasts: The role of the coating and aggregation state. Nanotechnology 2010, 21, 145103. [Google Scholar] [CrossRef] [PubMed]
- Safi, M.; Courtois, J.; Seigneuret, M.; Conjeaud, H.; Berret, J. The effects of aggregation and protein corona on the cellular internalization of iron oxide nanoparticles. Biomaterials 2011, 32, 9353–9363. [Google Scholar] [CrossRef] [PubMed]
- Galimard, A.; Safi, M.; Ould-Moussa, N.; Montero, D.; Conjeaud, H.; Berret, J. Thirty-femtogram detection of iron in mammalian cells. Small 2012, 8, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes-Generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. Oxidative decay of DNA. J. Biol. Chem. 1997, 272, 19633–19636. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free radical-induced damage to DNA: Mechanisms and measurement 1, 2. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Von Sonntag, C. The Chemical Basis of Radiation Biology; Taylor and Francis: New York, NY, USA, 1987. [Google Scholar]
- Dizdaroglu, M. Oxidative damage to DNA in mammalian chromatin. Mutat. Res. DNAging 1992, 275, 331–342. [Google Scholar] [CrossRef]
- Breen, A.P.; Murphy, J.A. Reactions of oxyl radicals with DNA. Free Radic. Biol. Med. 1995, 18, 1033–1077. [Google Scholar] [CrossRef]
- Zhu, X.; Hondroulis, E.; Liu, W.; Li, C. Biosensing Approaches for rapid genotoxicity and cytotoxicity assays upon nanomaterial exposure. Small 2013, 9, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2ʹ-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.; Halliwell, B. Free radicals and antioxidants in the year 2000: A historical look to the future. Ann. N. Y. Acad. Sci. 2000, 899, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M.; Gajewski, E.; Reddy, P.; Margolis, S.A. Structure of a hydroxyl radical-induced DNA-protein crosslink involving thymine and tyrosine in nucleohistone. Biochemistry 1989, 28, 3625–3628. [Google Scholar] [CrossRef] [PubMed]
- Altman, S.A.; Zastawny, T.H.; Randers-Eichhorn, L.; Cacciuttolo, M.A.; Akman, S.A.; Dizdaroglu, M.; Rao, G. Formation of DNA-protein cross-links in cultured mammalian cells upon treatment with iron ions. Free Radic. Biol. Med. 1995, 19, 897–902. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Doak, S.H.; Griffiths, S.M.; Manshian, B.; Singh, N.; Williams, P.M.; Brown, A.P.; Jenkins, G.J. Confounding experimental considerations in nanogenotoxicology. Mutagenesis 2009, 24, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Collins, A. Comparison of different methods of measuring 8-oxoguanine as a marker of oxidative DNA damage. Free Radic. Res. 2000, 32, 333–341. [Google Scholar] [CrossRef]
- Mohmood, I.; Ahmad, I.; Asim, M.; Costa, L.; Lopes, C.B.; Trindade, T.; Duarte, A.C.; Pereira, E. Interference of the co-exposure of mercury with silica-coated iron oxide nanoparticles can modulate genotoxicity induced by their individual exposures—A paradox depicted in fish under in vitro conditions. Environ. Sci. Pollut. Res. Int. 2015, 22, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Rahman, M.F.; Murty, U.S.N.; Mahboob, M.; Grover, P. Comparative study of genotoxicity and tissue distribution of nano and micron sized iron oxide in rats after acute oral treatment. Toxicol. Appl. Pharmacol. 2013, 266, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.S.; Nelson, B.C.; Marquis, B.J.; Williams, P.M.; Wright, C.; Doak, S.H. DNA damaging potential of superparamagnetic iron oxide nanoparticles-role of oxidation state. Mutagenesis 2012, 27, 796–796. [Google Scholar]
- Zuzana, M.; Alessandra, R.; Lise, F.; Maria, D. Safety assessment of nanoparticles cytotoxicity and genotoxicity of metal nanoparticles in vitro. J. Biomed. Nanotechnol. 2011, 7, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Ge, J.; Cohen, J.; Pyrgiotakis, G.; Engelward, B.R.; Demokritou, P. High-throughput screening platform for engineered nanoparticle-mediated genotoxicity using comet chip technology. ACS Nano 2014, 8, 2118–2133. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, L.; Ernst, P.; Schick, I.; Koehler, O.; Oehring, H.; Tremel, W.; Hilger, I. Anti-oxidative effects and harmlessness of asymmetric Au@Fe3O4 Janus particles on human blood cells. Biomaterials 2014, 35, 6986–6997. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Lin, X.; Wang, S.; Li, L.; Liu, Y.; Ye, L.; Wang, G. Biological evaluation of Fe3O4-poly(l-lactide)-poly(ethylene glycol)-poly(l-lactide) magnetic microspheres prepared in supercritical CO2. Toxicol. Lett. 2012, 212, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Novotna, B.; Jendelova, P.; Kapcalova, M.; Rossner, P., Jr.; Turnovcova, K.; Bagryantseva, Y.; Babic, M.; Horak, D.; Sykova, E. Oxidative damage to biological macromolecules in human bone marrow mesenchymal stromal cells labeled with various types of iron oxide nanoparticles. Toxicol. Lett. 2012, 210, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Vali, H.; Imani, M.; Shokrgozar, M.A.; Azadmanesh, K.; Azari, F. Cytotoxicity and cell cycle effects of bare and poly(vinyl alcohol)-coated iron oxide nanoparticles in mouse fibroblasts. Adv. Eng. Mater. 2009, 11, B243–B250. [Google Scholar] [CrossRef]
- Hong, S.C.; Lee, J.H.; Lee, J.; Kim, H.Y.; Park, J.Y.; Cho, J.; Lee, J.; Han, D. Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups. Int. J. Nanomed. 2011, 6, 3219–3231. [Google Scholar]
- Kadar, E.; Tarran, G.A.; Jha, A.N.; Al-Subiai, S.N. Stabilization of engineered zero-valent nanoiron with Na-acrylic copolymer enhances spermiotoxicity. Environ. Sci. Technol. 2011, 45, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, K. Sensitively electrochemical detection of the DNA damage in situ by electro-Fenton reaction based on Fe@Fe2O3 core-shell nanonecklace and multi-walled carbon nanotube composite. Anal. Chim. Acta 2010, 664, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, T.; Jiao, K. Electrochemical sensing the DNA damage in situ induced by a cathodic process based on Fe@Fe2O3 core-shell nanonecklace and Au nanoparticles mimicking metal toxicity pathways in vivo. Biosens. Bioelectron. 2009, 25, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Guichard, Y.; Schmit, J.; Darne, C.; Gate, L.; Goutet, M.; Rousset, D.; Rastoix, O.; Wrobel, R.; Witschger, O.; Martin, A.; et al. Cytotoxicity and genotoxicity of nanosized and microsized titanium dioxide and iron oxide particles in syrian hamster embryo Cells. Ann. Occup. Hyg. 2012, 56, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Freyria, F.S.; Bonelli, B.; Tomatis, M.; Ghiazza, M.; Gazzano, E.; Ghigo, D.; Garrone, E.; Fubini, B. Hematite nanoparticles larger than 90 nm show no sign of toxicity in terms of lactate dehydrogenase release, nitric oxide generation, apoptosis, and comet assay in murine alveolar macrophages and human lung epithelial cells. Chem. Res. Toxicol. 2012, 25, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Moller, L. Size-dependent toxicity of metal oxide particles-A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.F.; Hoffmann, E.; Dopp, E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre Toxicol. 2009, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-L.; Zhao, S.-H.; Zhang, L.; Hu, G.-Q.; Sun, Z.-W.; Yang, W.-S. Dose-dependent cytotoxicity and oxidative stress induced by “naked” Fe3O4 Nanoparticles in human hepatocyte. Chem. Res. Chin. Univ. 2012, 28, 114–118. [Google Scholar]
- Ma, P.; Luo, Q.; Chen, J.; Gan, Y.; Du, J.; Ding, S.; Xi, Z.; Yang, X. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int. J. Nanomed. 2012, 7, 4809–4818. [Google Scholar]
- Kenzaoui, B.H.; Bernasconi, C.C.; Guney-Ayra, S.; Juillerat-Jeanneret, L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem. J. 2012, 441, 813–821. [Google Scholar]
- Sun, Z.; Yathindranath, V.; Worden, M.; Thliveris, J.A.; Chu, S.; Parkinson, F.E.; Hegmann, T.; Miller, D.W. Characterization of cellular uptake and toxicity of aminosilane-coated iron oxide nanoparticles with different charges in central nervous system-relevant cell culture models. Int. J. Nanomed. 2013, 8, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Tam, J.; Roth, J.A.; Sokolov, K.; Ramesh, R. EGFR-targeted plasmonic magnetic nanoparticles suppress lung tumor growth by abrogating G2/M cell-cycle arrest and inducing DNA damage. Int. J. Nanomed. 2014, 9, 3825–3839. [Google Scholar]
- Yokoyama, T.; Tam, J.; Kuroda, S.; Scott, A.W.; Aaron, J.; Larson, T.; Shanker, M.; Correa, A.M.; Kondo, S.; Roth, J.A. EGFR-targeted hybrid plasmonic magnetic nanoparticles synergistically induce autophagy and apoptosis in non-small cell lung cancer cells. PLoS ONE 2011, 6, e25507. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, M.A.; de Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Huang, S.; Wei, C.; Zeng, Q.; Hu, C.; Du, J.; Ding, S. Cytotoxicity Effects of Nano-Fe3O4 on HeLa Cells, Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010; pp. 1–4.
- Szalay, B.; Tatrai, E.; Nyiro, G.; Vezer, T.; Dura, G. Potential toxic effects of iron oxide nanoparticles in in vivo and in vitro experiments. J. Appl. Toxicol. 2012, 32, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Akasaka, S.; Yamamoto, K. Mutational specificity of the ferrous ion in a supF gene of Escherichia coli. Biochem. Biophys. Res. Commun. 1995, 213, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Nick, S.T.; Bolandi, A.; Obare, S.O. Interaction of engineered nickel nanoparticles with gram-positive and gram-negative bacteria. Submitted. 2015. [Google Scholar]
- Nick, S.; Bolandi, A.; Samuels, T.A.; Obare, S.O. Advances in understanding the transformation of engineered nanoparticles in the environment. Pure Appl. Chem. 2014, 86, 1129–1140. [Google Scholar] [CrossRef]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dissanayake, N.M.; Current, K.M.; Obare, S.O. Mutagenic Effects of Iron Oxide Nanoparticles on Biological Cells. Int. J. Mol. Sci. 2015, 16, 23482-23516. https://doi.org/10.3390/ijms161023482
Dissanayake NM, Current KM, Obare SO. Mutagenic Effects of Iron Oxide Nanoparticles on Biological Cells. International Journal of Molecular Sciences. 2015; 16(10):23482-23516. https://doi.org/10.3390/ijms161023482
Chicago/Turabian StyleDissanayake, Niluka M., Kelley M. Current, and Sherine O. Obare. 2015. "Mutagenic Effects of Iron Oxide Nanoparticles on Biological Cells" International Journal of Molecular Sciences 16, no. 10: 23482-23516. https://doi.org/10.3390/ijms161023482
APA StyleDissanayake, N. M., Current, K. M., & Obare, S. O. (2015). Mutagenic Effects of Iron Oxide Nanoparticles on Biological Cells. International Journal of Molecular Sciences, 16(10), 23482-23516. https://doi.org/10.3390/ijms161023482