Mycophenolate Antagonizes IFN-γ-Induced Catagen-Like Changes via β-Catenin Activation in Human Dermal Papilla Cells and Hair Follicles
Abstract
:1. Introduction
2. Results and Discussion
2.1. MPA Enhances the Proliferation of Human Dermal Papilla Cells (hDPCs)
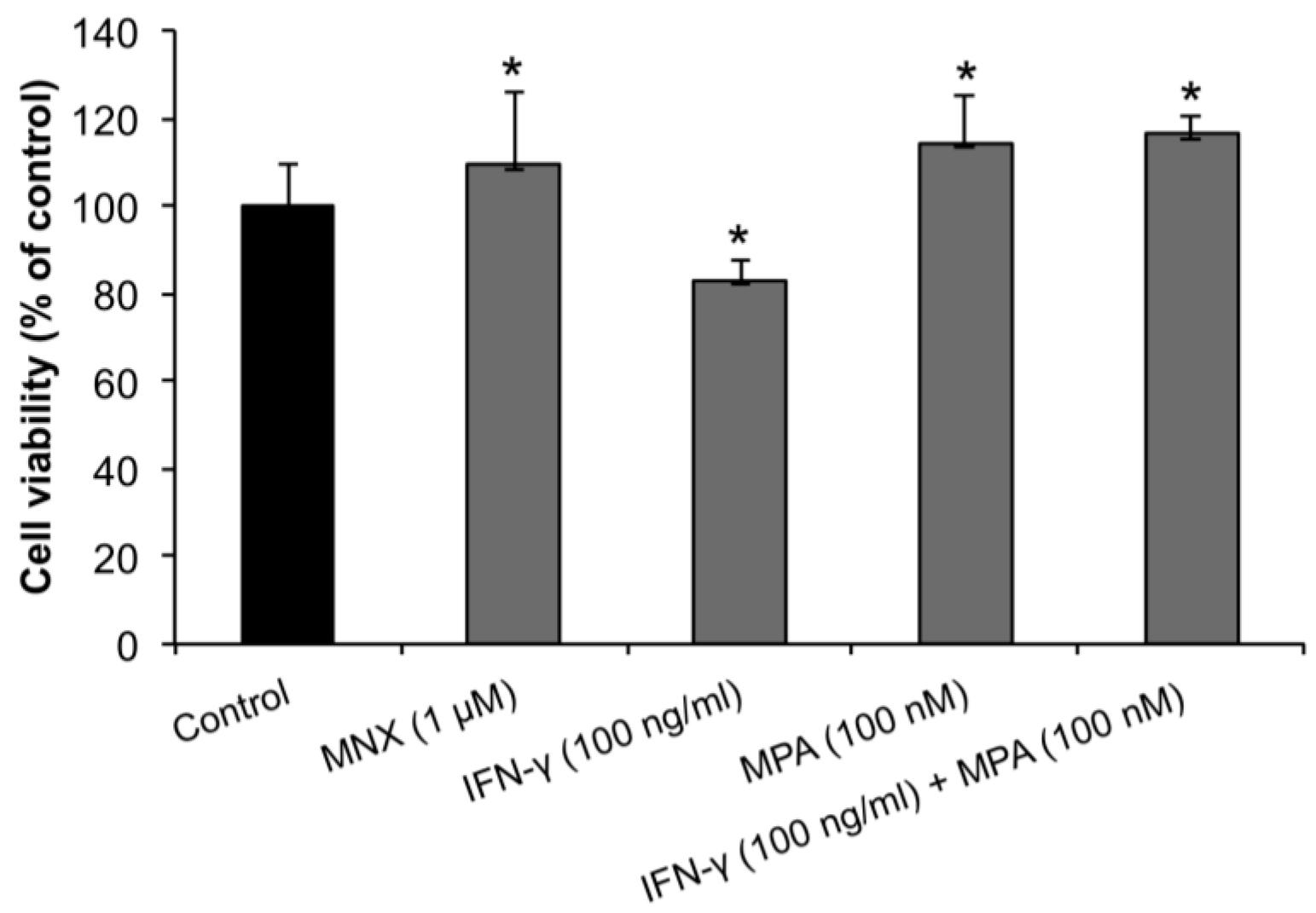
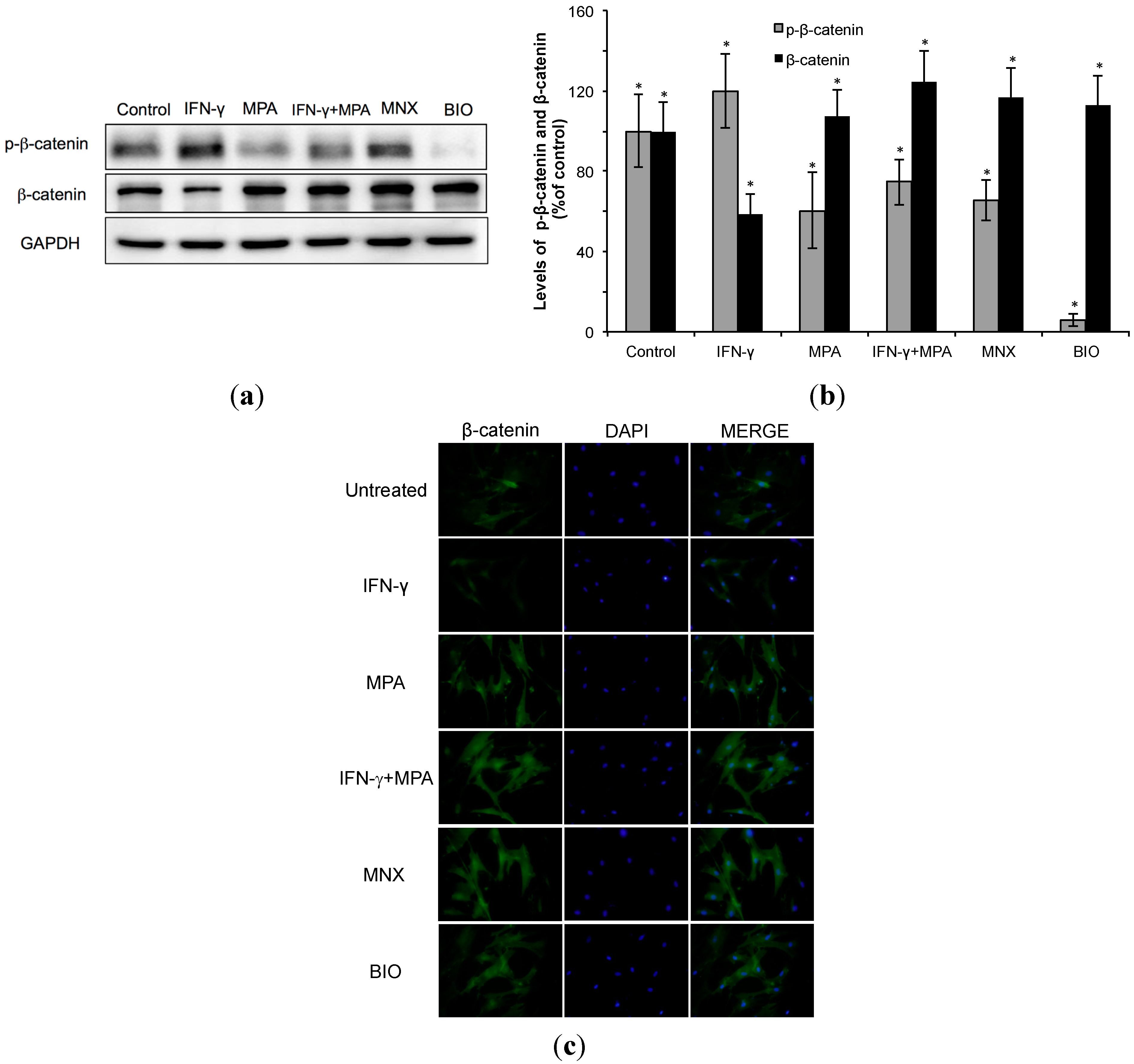
2.2. Mycophenolate (MPA) Activates the β-Catenin Pathway in Interferon (IFN)-γ-Treated hDPCs.
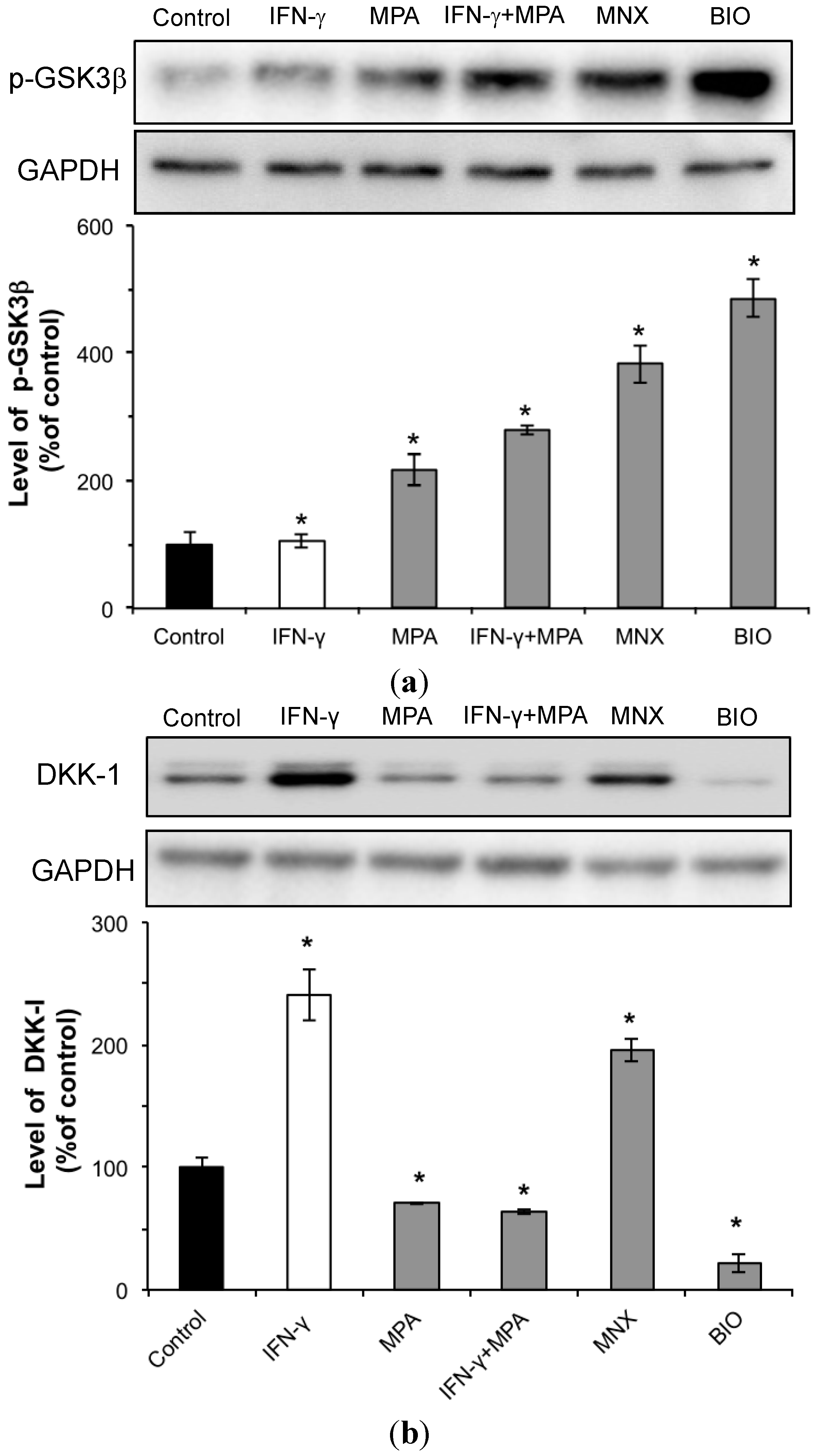
2.3. MPA Activates the β-Catenin Pathway by Inhibition of GSK3β and Reduction of DKK-1 Expression in hDPCs
2.4. MPA Regulates the Expression of β-Catenin Pathway Target Genes and Dermal Papilla (DP) Signature Genes in IFN-γ-Treated hDPCs
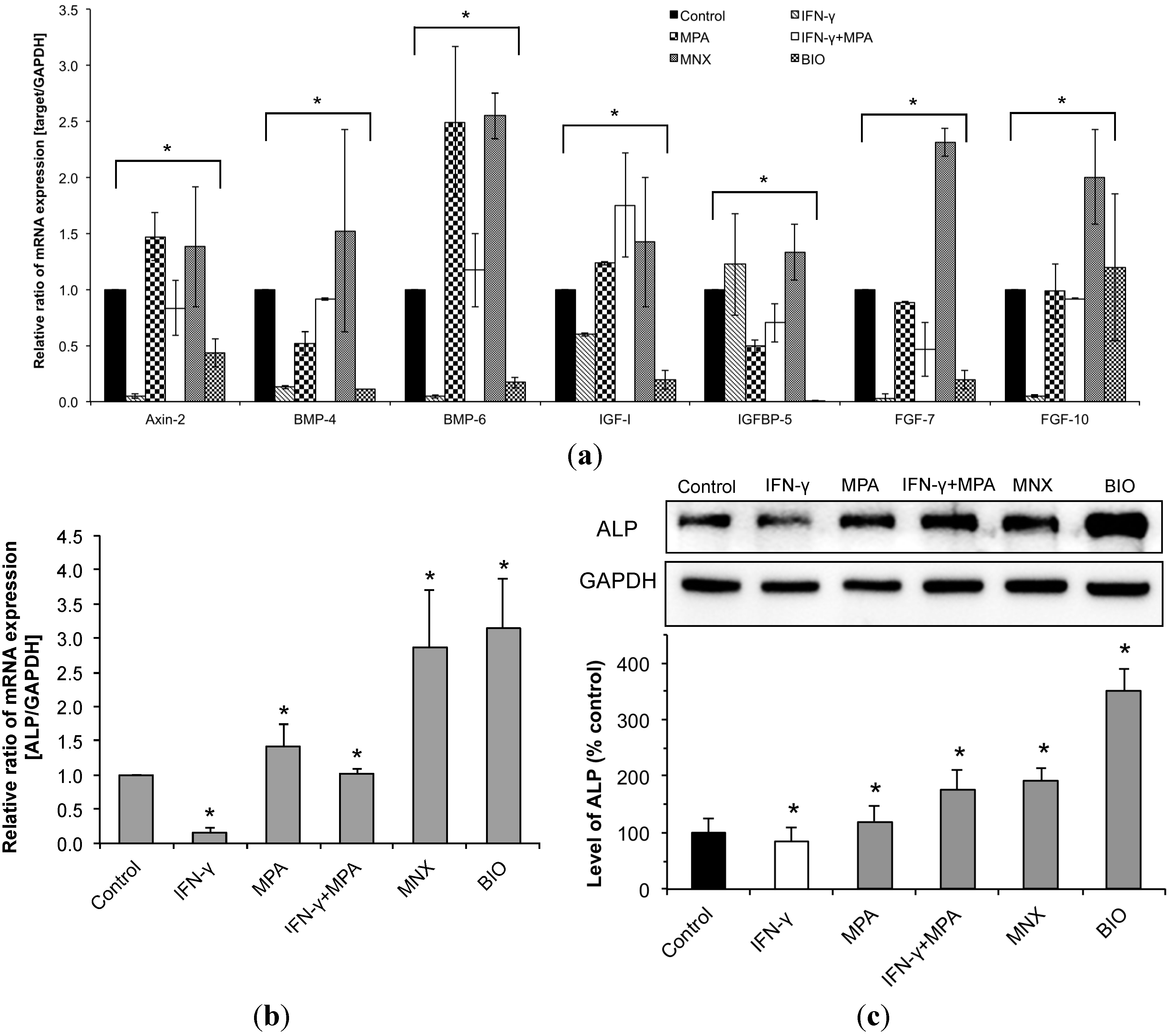
2.5. MPA Inhibits the Up-Regulation of Transforming Growth Factor (TGF)-β2 Induced by IFN-γ in hDPCs.
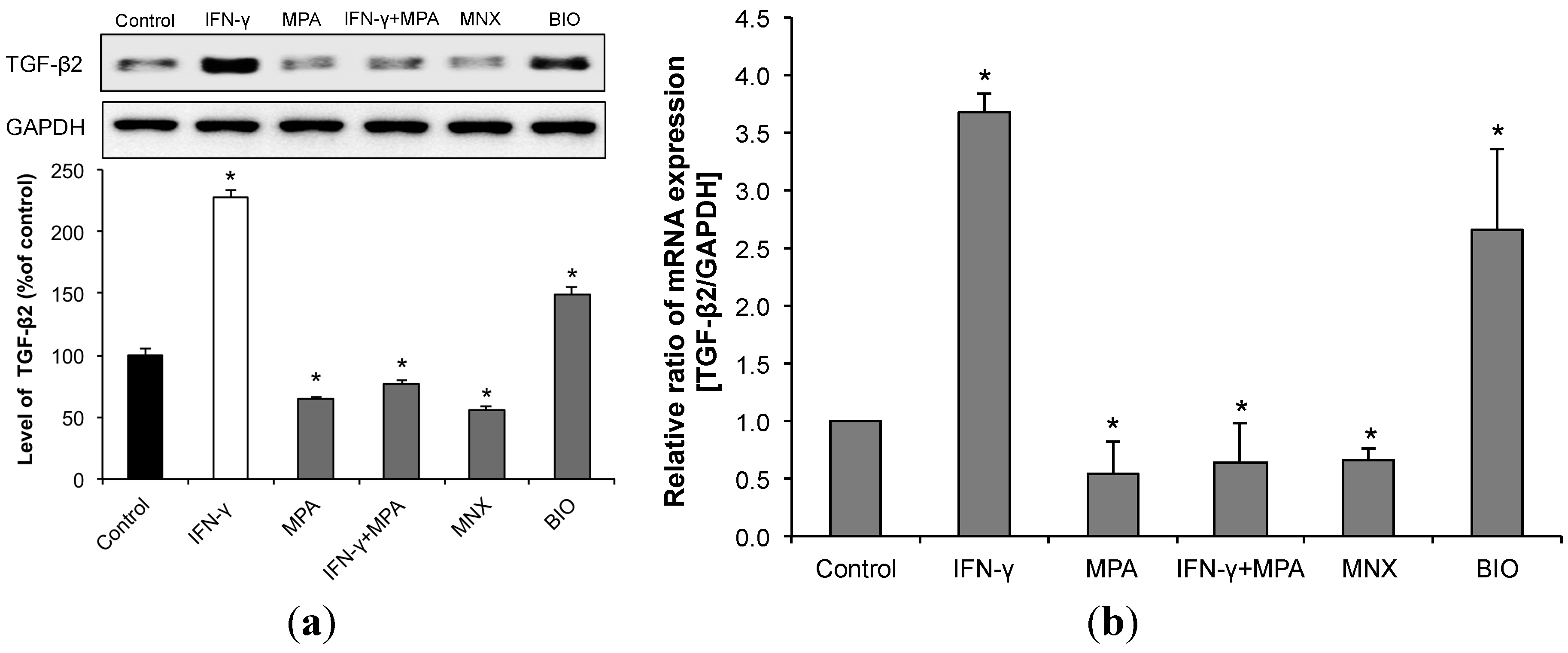
2.6. MPA Abrogates IFN-γ Inhibition of Hair Growth ex Vivo in a Culture Model
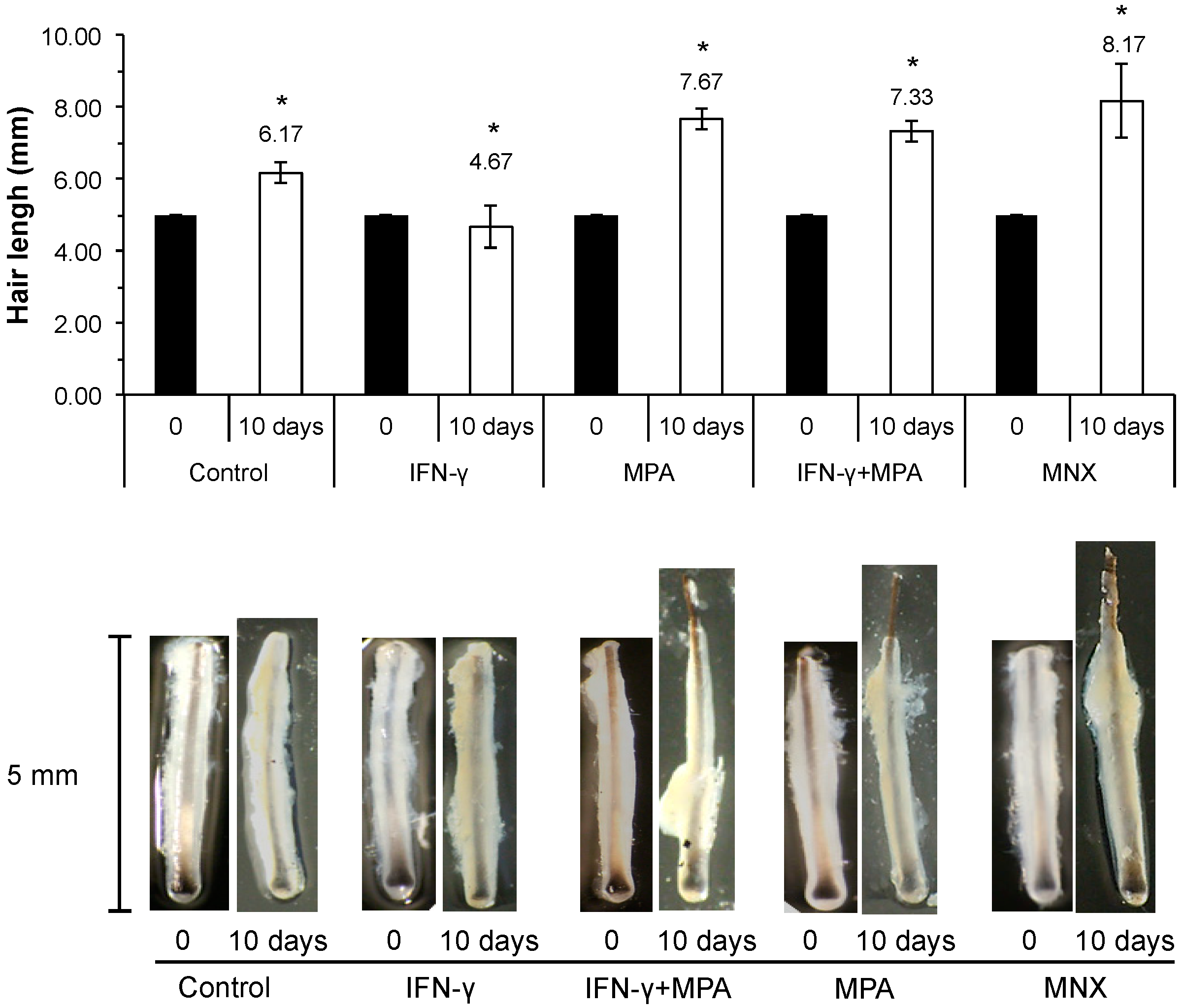
2.7. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Cultures of hDPCs
3.3. MTT Assay
3.4. Western Blot
3.5. Real-Time RT-PCR
3.6. Immunocytochemistry Assay
3.7. Hair Follicles Organ Culture and Assessment of Hair Elongation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G. Epithelial stem cells: A folliculocentric view. J. Investig. Dermatol. 2006, 126, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [PubMed]
- Yang, C.C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Millar, S.E. Towards a molecular understanding of hair loss and its treatment. Trends Mol. Med. 2001, 7, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R. The potential role of cytokines and T cells in alopecia areata. J. Investig. Dermatol. Symp. Proc. 1999, 4, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.P.; Sanders, D.; Westgate, G.E.; Kealey, T. Human hair growth in vitro: A model for the study of hair follicle biology. J. Dermatol. Sci. 1994, 7, S55–S72. [Google Scholar] [PubMed]
- Carroll, J.M.; Crompton, T.; Seery, J.P.; Watt, F.M. Transgenic mice expressing IFN-γ in the epidermis have eczema, hair hypopigmentation, and hair loss. J. Investig. Dermatol. 1997, 108, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Hirota, R.; Tajima, S.; Yoneda, Y.; Tamayama, T.; Watanabe, M.; Ueda, K.; Kubota, T.; Yoshida, R. Alopecia of IFN-γ knockout mouse as a model for disturbance of the hair cycle: A unique arrest of the hair cycle at the anagen phase accompanied by mitosis. J. Interferon Cytokine Res. 2002, 22, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ito, N.; Saathoff, M.; Bettermann, A.; Takigawa, M.; Paus, R. Interferon-γ is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br. J. Dermatol. 2005, 152, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Maretto, S.; Cordenonsi, M.; Dupont, S.; Braghetta, P.; Broccoli, V.; Hassan, A.B.; Volpin, D.; Bressan, G.M.; Piccolo, S. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA. 2003, 100, 3299–3304. [Google Scholar] [CrossRef] [PubMed]
- Ridanpaa, M.; Fodde, R.; Kielman, M. Dynamic expression and nuclear accumulation of β-catenin during the development of hair follicle-derived structures. Mech. Dev. 2001, 109, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [PubMed]
- Shimizu, H.; Morgan, B.A. Wnt signaling through the β-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J. Investig. Dermatol. 2004, 122, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. β-Catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, T.; Maruyama, T.; Sei, Y.; Adachi, K. Effects of immunosuppressive peptidyl-prolyl cis–trans isomerase (PPIase) inhibitors, cyclosporin A, FK506, ascomycin and rapamycin, on hair growth initiation in mouse: Immunosuppression is not required for new hair growth. J. Dermatol. Sci. 1995, 9, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Bottge, J.A.; Henz, B.M.; Maurer, M. Hair growth control by immunosuppression. Arch. Dermatol. Res. 1996, 288, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Rebora, A. Topical cyclosporine in alopecia areata. Arch. Dermatol. 1987, 123, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Stenn, K.S.; Link, R.E. The induction of anagen hair growth in telogen mouse skin by cyclosporine A administration. Lab. Investig. 1989, 60, 365–369. [Google Scholar] [PubMed]
- Pendry, A.; Alexander, P. Stimulation of hair growth on nude mice by cyclosporin A. In Cyclosporin A: Proceedings of An International Conference on Cyclosporin A; Elsevier Biomedial Press: Armsterdam, The Netherlands, 1981. [Google Scholar]
- Sainsbury, T.S.; Duncan, J.I.; Whiting, P.H.; Hewick, D.S.; Johnson, B.E.; Thomson, A.W.; Oliver, R.F. Differential effects of FK 506 and cyclosporine on hair regrowth in the DEBR model of alopecia areata. Transplant. Proc. 1991, 23, 3332–3334. [Google Scholar] [PubMed]
- Sawada, M.; Terada, N.; Taniguchi, H.; Tateishi, R.; Mori, Y. Cyclosporin A stimulates hair growth in nude mice. Lab. Investig. 1987, 56, 684–686. [Google Scholar] [PubMed]
- Yamamoto, S.; Kato, R. Hair growth-stimulating effects of cyclosporin A and FK506, potent immunosuppressants. J. Dermatol. Sci. 1994, 7, S47–S54. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Niida, A.; Hiroko, T.; Kasai, M.; Furukawa, Y.; Nakamura, Y.; Suzuki, Y.; Sugano, S.; Akiyama, T. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene 2004, 23, 8520–8526. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Zhang, T.; Domon, C.; Joo, C.K.; Freund, J.N.; Costantini, F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002, 22, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Peng, R. Unveiling hair follicle stem cells. Stem Cell Rev. 2010, 6, 658–664. [Google Scholar] [CrossRef] [PubMed]
- McElwee, K.J.; Kissling, S.; Wenzel, E.; Huth, A.; Hoffmann, R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J. Investig. Dermatol. 2003, 121, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Handjiski, B.; Czarnetzki, B.M.; Eichmuller, S. A murine model for inducing and manipulating hair follicle regression (catagen): Effects of dexamethasone and cyclosporin A. J. Investig. Dermatol. 1994, 103, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Handjiski, B.; Eichmuller, S.; Czarnetzki, B.M. Chemotherapy-induced alopecia in mice. Induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am. J. Pathol. 1994, 144, 719–734. [Google Scholar]
- Gafter-Gvili, A.; Sredni, B.; Gal, R.; Gafter, U.; Kalechman, Y. Cyclosporin A-induced hair growth in mice is associated with inhibition of calcineurin-dependent activation of NFAT in follicular keratinocytes. Am. J. Physiol. Cell Physiol. 2003, 284, C1593–C1603. [Google Scholar] [CrossRef] [PubMed]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.M.; Verstuyf, A.; Jensen, K.B.; Watt, F.M. Differential sensitivity of epidermal cell subpopulations to β-catenin-induced ectopic hair follicle formation. Dev. Biol. 2010, 343, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhang, K.; Ye, J.X.; Lian, X.H.; Yang, T. Wnt10b promotes growth of hair follicles via a canonical Wnt signaling pathway. Clin. Exp. Dermatol. 2011, 36, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Van Mater, D.; Kolligs, F.T.; Dlugosz, A.A.; Fearon, E.R. Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003, 17, 1219–1224. [Google Scholar]
- Foitzik, K.; Lindner, G.; Mueller-Roever, S.; Maurer, M.; Botchkareva, N.; Botchkarev, V.; Handjiski, B.; Metz, M.; Hibino, T.; Soma, T.; et al. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J. 2000, 14, 752–760. [Google Scholar] [PubMed]
- Hibino, T.; Nishiyama, T. Role of TGF-β2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Fukuzato, Y.; Nakajima, T.; Yoshikawa, K.; Itami, S. Androgen-inducible TGF-β1 from balding dermal papilla cells inhibits epithelial cell growth: A clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002, 16, 1967–1969. [Google Scholar] [PubMed]
- Soma, T.; Tsuji, Y.; Hibino, T. Involvement of transforming growth factor-β2 in catagen induction during the human hair cycle. J. Investig. Dermatol. 2002, 118, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Denda, S.; Soma, T.; Raftery, L.; Momoi, T.; Hibino, T. A potential suppressor of TGF-β delays catagen progression in hair follicles. J. Investig. Dermatol. Symp. Proc. 2003, 8, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Fujiwara, S.; Shirakata, Y.; Hashimoto, K.; Kishimoto, J. Hair-inducing ability of human dermal papilla cells cultured under Wnt/β-catenin signaling activation. Exp. Dermatol. 2012, 21, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Kurosaka, A. Inhibition of glycogen synthase kinase-3 enhances the expression of alkaline phosphatase and insulin-like growth factor-1 in human primary dermal papilla cell culture and maintains mouse hair bulbs in organ culture. Arch. Dermatol. Res. 2009, 301, 357–365. [Google Scholar] [PubMed]
- DasGupta, R.; Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999, 126, 4557–4568. [Google Scholar] [PubMed]
- Jo, S.J.; Choi, S.J.; Yoon, S.Y.; Lee, J.Y.; Park, W.S.; Park, P.J.; Kim, K.H.; Eun, H.C.; Kwon, O. Valproic acid promotes human hair growth in in vitro culture model. J. Dermatol. Sci. 2013, 72, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.S.; Min do, S.; Kim, H.Y.; Choi, K.Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Wiltse, J. Mode of action: Inhibition of histone deacetylase, altering WNT-dependent gene expression, and regulation of β-catenin—Developmental effects of valproic acid. Crit. Rev. Toxicol. 2005, 35, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [PubMed]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dickkopf 1 promotes regression of hair follicles. J. Investig. Dermatol. 2012, 132, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Twentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.P.; Green, M.R.; Kealey, T. Human hair growth in vitro. J. Cell. Sci. 1990, 97, 463–471. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ryu, S.; Lee, Y.; Hyun, M.Y.; Choi, S.Y.; Jeong, K.H.; Park, Y.M.; Kang, H.; Park, K.Y.; Armstrong, C.A.; Johnson, A.; et al. Mycophenolate Antagonizes IFN-γ-Induced Catagen-Like Changes via β-Catenin Activation in Human Dermal Papilla Cells and Hair Follicles. Int. J. Mol. Sci. 2014, 15, 16800-16815. https://doi.org/10.3390/ijms150916800
Ryu S, Lee Y, Hyun MY, Choi SY, Jeong KH, Park YM, Kang H, Park KY, Armstrong CA, Johnson A, et al. Mycophenolate Antagonizes IFN-γ-Induced Catagen-Like Changes via β-Catenin Activation in Human Dermal Papilla Cells and Hair Follicles. International Journal of Molecular Sciences. 2014; 15(9):16800-16815. https://doi.org/10.3390/ijms150916800
Chicago/Turabian StyleRyu, Sunhyo, Yonghee Lee, Moo Yeol Hyun, Sun Young Choi, Kwan Ho Jeong, Young Min Park, Hoon Kang, Kui Young Park, Cheryl A. Armstrong, Andrew Johnson, and et al. 2014. "Mycophenolate Antagonizes IFN-γ-Induced Catagen-Like Changes via β-Catenin Activation in Human Dermal Papilla Cells and Hair Follicles" International Journal of Molecular Sciences 15, no. 9: 16800-16815. https://doi.org/10.3390/ijms150916800
APA StyleRyu, S., Lee, Y., Hyun, M. Y., Choi, S. Y., Jeong, K. H., Park, Y. M., Kang, H., Park, K. Y., Armstrong, C. A., Johnson, A., Song, P. I., & Kim, B. J. (2014). Mycophenolate Antagonizes IFN-γ-Induced Catagen-Like Changes via β-Catenin Activation in Human Dermal Papilla Cells and Hair Follicles. International Journal of Molecular Sciences, 15(9), 16800-16815. https://doi.org/10.3390/ijms150916800





