Beclin 1 Expression is Closely Linked to Colorectal Carcinogenesis and Distant Metastasis of Colorectal Carcinoma
Abstract
:1. Introduction
2. Results and Discussion
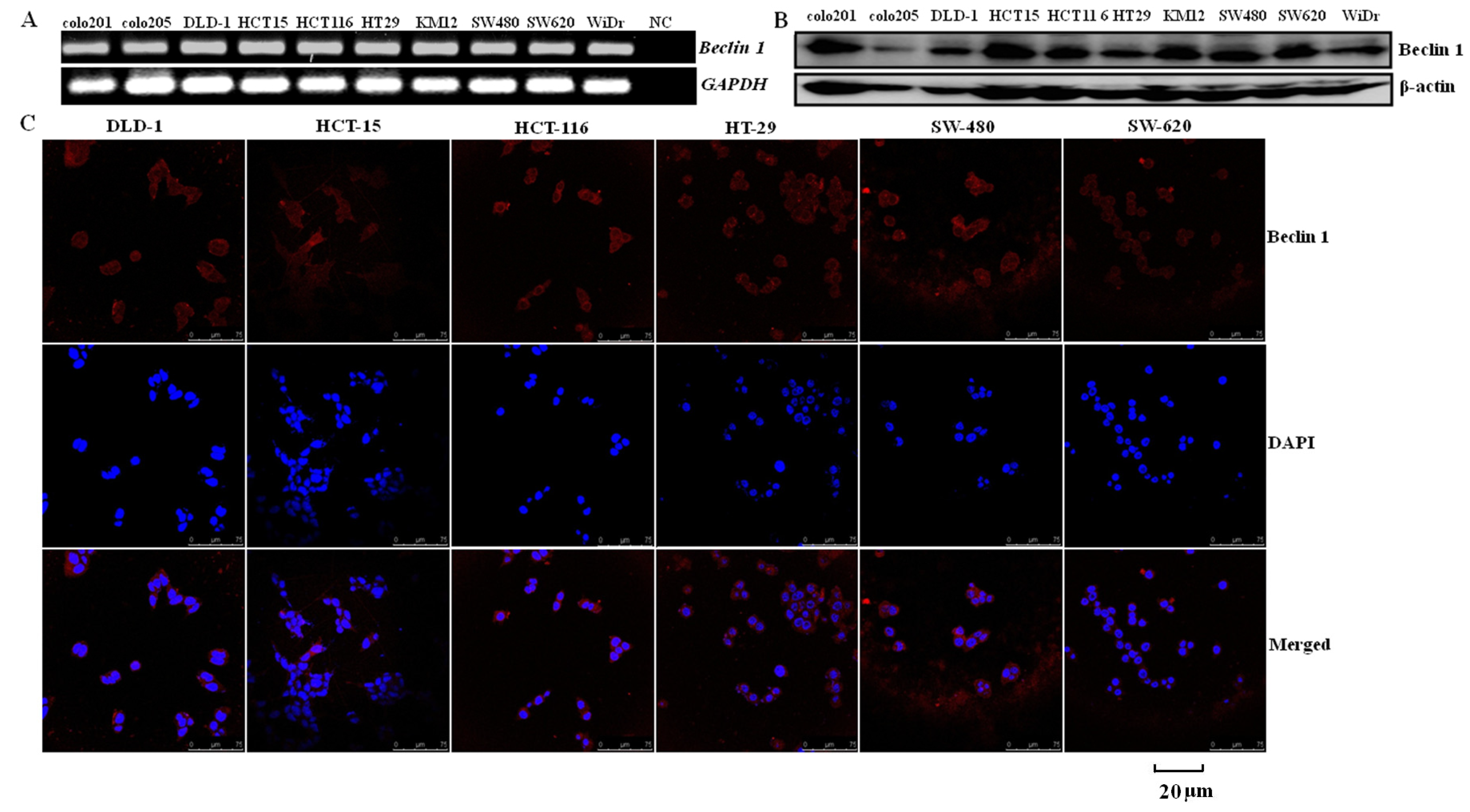
2.1. Similar Beclin 1 Expression on Colorectal Carcinoma Cell Lines
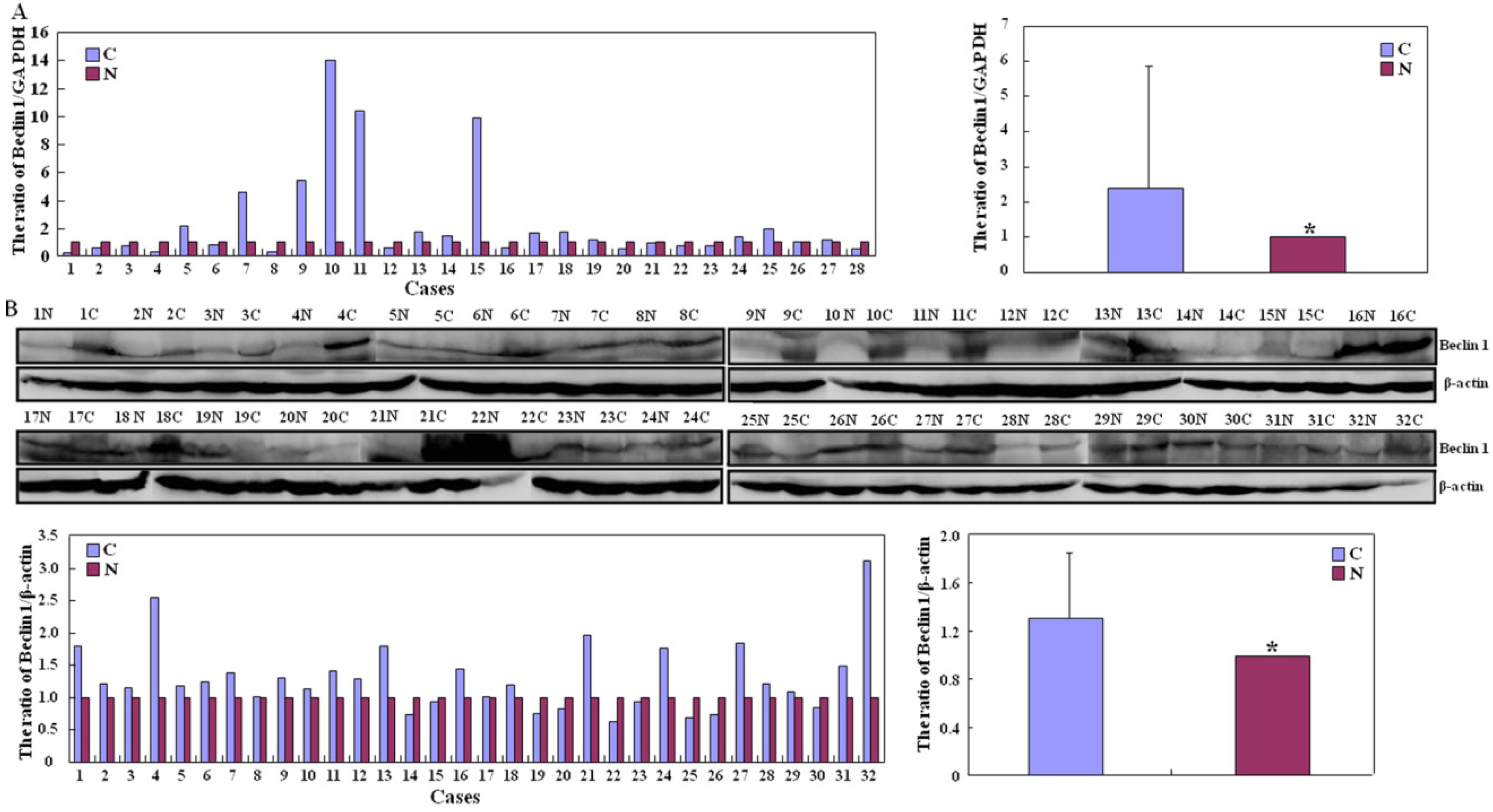
2.2. Up-Regulated Beclin 1 Expression in Colorectal Carcinogenesis
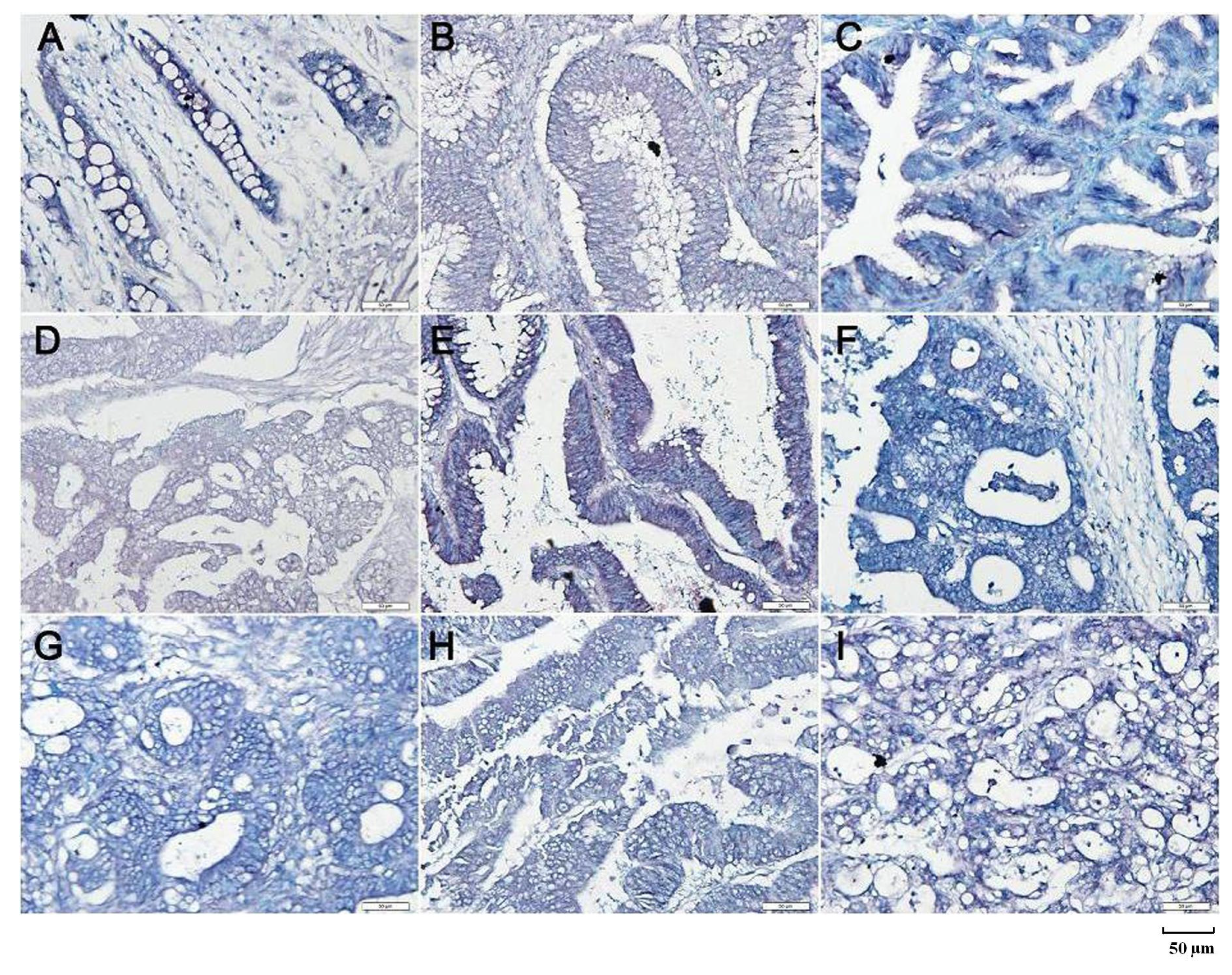
| Groups | n | Beclin 1 mRNA Expression | n | Beclin 1 Protein Expression | ||||||||
| − | + | ++ | +++ | % | − | + | ++ | +++ | % | |||
| Non-neoplastic mucosa | 74 | 39 | 24 | 9 | 2 | 47.3 * | 550 | 281 | 112 | 108 | 49 | 48.9 * |
| Adenoma | 101 | 9 | 11 | 45 | 36 | 91.1 | 139 | 5 | 29 | 61 | 44 | 96.4 |
| Primary carcinoma | 106 | 26 | 31 | 24 | 25 | 75.5 | 589 | 50 | 100 | 158 | 281 | 91.5 |
| Metastatic carcinoma in lymph node | 51 | 5 | 18 | 23 | 5 | 90.2 | 195 | 17 | 28 | 65 | 85 | 91.3 |
| Metastatic carcinoma in liver | 26 | 9 | 5 | 8 | 4 | 65.4 | 48 | 17 | 11 | 9 | 11 | 64.6 † |
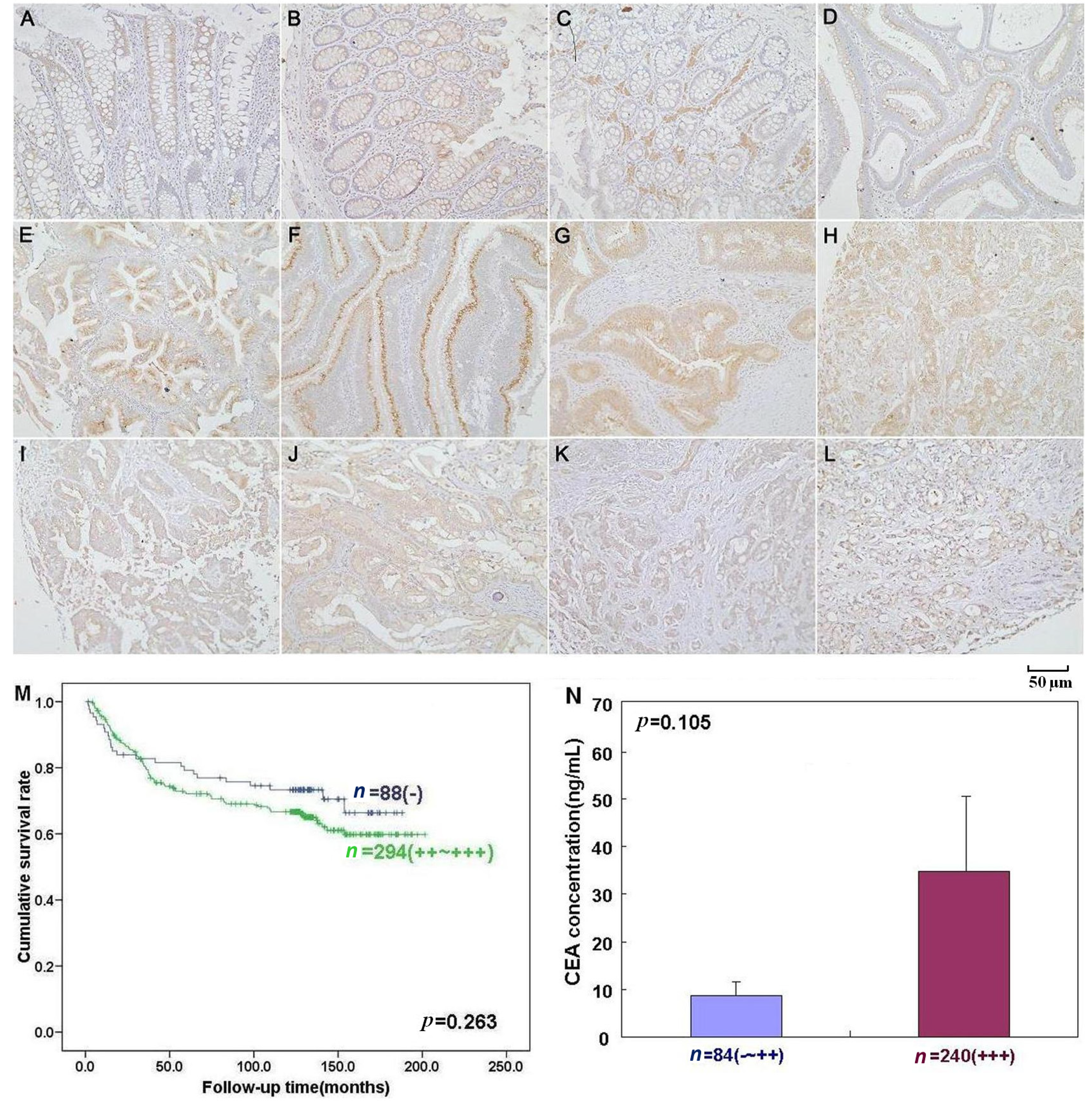
2.3. The Close Relationship between Beclin 1 Expression and Aggressiveness of Colorectal Carcinoma
| Clinicopathological Features | n | Beclin 1 Protein Expression | ||||||
| − | + | ++ | +++ | % | p Value | |||
| Age (years) | <65 | 286 | 22 | 45 | 82 | 137 | 92.3 | 0.587 |
| Sex | Male | 343 | 31 | 64 | 96 | 152 | 91.0 | 0.052 |
| Depth of invasion | Tis-T2 | 137 | 8 | 18 | 44 | 67 | 94.2 | 0.323 |
| Lymphatic invasion | − | 224 | 19 | 38 | 57 | 110 | 91.5 | 0.471 |
| Venous invasion | − | 243 | 21 | 52 | 66 | 104 | 91.4 | 0.084 |
| Lymph node metastasis | − | 306 | 24 | 54 | 86 | 142 | 92.2 | 0.719 |
| Liver metastasis | − | 528 | 42 | 88 | 137 | 261 | 92.0 | 0.003 |
| Distant metastasis | − | 523 | 42 | 87 | 136 | 258 | 92.0 | 0.017 |
| TNM staging | I–II | 243 | 14 | 40 | 67 | 122 | 94.2 | 0.822 |
| Differentiation | Well-differentiated | 260 | 21 | 46 | 78 | 115 | 91.9 | 0.433 |
| Clinicopathological Parameters | Relative Risk (95% CI) | p Value |
|---|---|---|
| Age (≥65 years) | 0.823 (0.480–1.411) | 0.478 |
| Sex (female) | 0.870 (0.535–1.412) | 0.572 |
| Depth of invasion (T2-4) | 7.086 (2.122–23.657) | 0.001 |
| Lymphatic invasion (+) | 1.391 (0.790–2.449) | 0.252 |
| Venous invasion (+) | 0.983 (0.551–1.751) | 0.953 |
| Lymph node metastasis (+) | 1.434 (0.415–4.953) | 0.569 |
| Liver metastasis (+) | 0.360 (0.069–1.867) | 0.224 |
| Distant metastasis (+) | 11.490 (2.464–53.573) | 0.002 |
| TNM staging (III–IV) | 1.991 (0.491–8.063) | 0.335 |
| Differentiation (poorly-differentiated) | 1.221 (0.820–1.819) | 0.325 |
| Beclin 1 protein expression (+++) | 1.338 (0.837–2.136) | 0.223 |
2.4. Discussion
3. Experimental Section
3.1. Cell Culture
3.2. Immunofluorescence Staining
3.3. Subjects
3.4. Pathology
3.5. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
3.6. Western Blot (WB)
3.7. Tissue Microarray (TMA) and in Situ Hybridization (ISH)
3.8. Immunohistochemistry (IHC)
3.9. Measurement of Carcinoembryonic Antigen (CEA)
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Yu, J.; Brown, K.; Levine, B. Beclin 1 contains a leucine-rich nuclear export signal that is required for its autophagy and tumor suppressor function. Cancer Res. 2001, 61, 3443–3449. [Google Scholar] [PubMed]
- Kang, R.; Livesey, K.M.; Zeh, H.J.; Loze, M.T.; Tang, D. HMGB1: A novel Beclin 1-binding protein active in autophagy. Autophagy 2010, 6, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, H.; Stenmark, H.; Platta, H.W. Ubiquitination and phosphorylation of Beclin 1 and its binding partners: Tuning class III phosphatidylinositol 3-kinase activity and tumor suppression. FEBS Lett. 2012, 586, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Xu, L.; Duan, Z.L.; Zeng, L.Q.; Yan, N.H.; Peng, Z.L. Beclin 1-mediated macroautophagy involves regulation of caspase-9 expression in cervical cancer HeLa cells. Gynecol. Oncol. 2007, 107, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Sun, Q.; Ding, W.X.; Yin, X.M.; Sobol, R.W.; Stolz, D.B.; Yu, J.; Zhang, L. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res. 2011, 71, 3625–3634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, L.; Liu, L.; Gao, P.; Tian, W.; Wang, X.; Jin, H.; Xu, H.; Chen, Q. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell 2010, 1, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Jin, S.; Yang, C.; Levine, A.J.; Heintz, N. Beclin 1,an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA 2003, 100, 15077–15082. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yu, J.; Bhagat, G.; Furuya, N.; Hibshoosh, H.; Troxel, A.; Rosen, J.; Eskelinen, E.; Mizushima, N.; Ohsumi, Y.; et al. Promotion of tumorigenesis by heterozygous disruption of the Beclin 1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Pickford, F.; Masliah, E.; Britschgi, M.; Lucin, K.; Narasimhan, R.; Jaeger, PA.; Small, S.; Spencer, B.; Rockenstein, E.; Levine, B.; et al. The autophagy-related protein Beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Investig. 2008, 118, 2190–2199. [Google Scholar] [PubMed]
- Aita, V.M.; Liang, X.H.; Murty, V.V.; Pincus, D.L.; Yu, W.; Cayanis, E.; Kalachikov, S.; Gilliam, T.C.; Levine, B. Cloning and genomic organization of Beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 1999, 59, 59–65. [Google Scholar]
- Wang, Z.H.; Peng, Z.L.; Duan, Z.L.; Liu, H. Expression and clinical significance of autophagy gene Beclin 1 in cervical squamous cell carcinoma. (in Chinese). Sichuan Da Xue Xue Bao Yi Xue Ban 2006, 37, 860–863. [Google Scholar] [PubMed]
- Shi, Y.H.; Ding, Z.B.; Zhou, J.; Qiu, S.J.; Fan, J. Prognostic significance of Beclin 1-dependent apoptotic activity in hepatocellular carcinoma. Autophagy 2009, 5, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.L.; Peng, Z.L.; Wang, Z.H. Expression and involved signal transduction pathway of autophagy gene Beclin 1 in epithelial ovarian cancer. (in Chinese). Sichuan Da Xue Xue Bao Yi Xue Ban 2007, 38, 239–242. [Google Scholar] [PubMed]
- Huang, X.; Bai, H.M.; Chen, L.; Li, B.; Lu, Y.C. Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J. Clin. Neurosci. 2010, 17, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.F.; Shao, L.J.; Wang, W.M.; Yan, X.B.; Liu, R.Y. Decreased expression of Beclin-1 and LC3 in human lung cancer. Mol. Biol. Rep. 2012, 43, 62–68. [Google Scholar]
- Park, J.M.; Huang, S.; Wu, T.T.; Foster, N.R.; Sinicrope, F.A. Prognostic impact of Beclin 1,p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol. Ther. 2013, 14, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Okura, R.; Nakamura, M. Overexpression of autophagy-related beclin-1 in cutaneous squamous cell carcinoma with lymph-node metastasis. Eur. J. Dermatol. 2011, 21, 1002–1003. [Google Scholar] [PubMed]
- Kim, H.S.; Lee, S.H.; Do, S.I.; Lim, S.J.; Park, Y.K.; Kim, Y.W. Clinicopathologic correlation of beclin-1 expression in pancreatic ductal adenocarcinoma. Pathol. Res. Pract. 2011, 207, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gou, W.F.; Zhao, S.; Xiao, L.J.; Mao, X.Y.; Xing, Y.N.; Takahashi, H.; Takano, Y.; Zheng, H.C. Beclin 1 expression is an independent prognostic factor for gastric carcinomas. Tumour Biol. 2013, 34, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, S.; Gou, W.F.; Xiao, L.J.; Takano, Y.; Zheng, H.C. Aberrant Beclin 1 expression is closely linked to carcinogenesis, differentiation, progression, and prognosis of ovarian epithelial carcinoma. Tumour Biol. 2014, 35, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.W.; Hou, Y.J.; Tan, Y.X.; Tang, L.; Pan, Y.F.; Wang, M.; Wang, H.Y. Prognostic significance of Beclin 1 in intrahepatic cholangiocellular carcinoma. Autophagy 2011, 7, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xia, S.L.; Liao, C.; Li, Y.L.; Wang, Y.F.; Li, T.P.; Zhao, M.J. Genes encoding Pir51,Beclin 1,RbAp48 and aldolase b are up or down-regulated in human primary hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 509–513. [Google Scholar] [PubMed]
- Wang, Z.H.; Xu, L.; Wang, Y.; Cao, M.Q.; Li, L.; Bai, T. Clinicopathologic correlations between human papillomavirus 16 infection and Beclin 1 expression in human cervical cancer. Int. J. Gynecol. Pathol. 2011, 30, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, Y.; Lu, C.; Zhang, L. Beclin-1 expression is a predictor of clinical outcome in patients with esophageal squamous cell carcinoma and correlated to hypoxia-inducible factor (HIF)-1alpha expression. Pathol. Oncol. Res. 2009, 15, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Pirtoli, L.; Cevenini, G.; Tini, P.; Vannini, M.; Oliveri, G.; Marsili, S.; Mourmouras, V.; Rubino, G.; Miracco, C. The prognostic role of Beclin 1 protein expression in high-grade gliomas. Autophagy 2009, 5, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Koneri, K.; Goi, T.; Hirono, Y.; Katayama, K.; Yamaguchi, A. Beclin 1 gene inhibits tumor growth in colon cancer cell lines. Anticancer Res. 2007, 27, 1453–1457. [Google Scholar] [PubMed]
- Li, B.X.; Li, C.Y.; Peng, R.Q.; Wu, X.J.; Wang, H.Y.; Wan, D.S.; Zhu, X.F.; Zhang, X.S. The expression of beclin 1 is associated with favorable prognosis in stage IIIB colon cancers. Autophagy 2009, 5, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Li, H.R.; Huang, Y.; Jiang, W.Q.; Xu, R.H.; Huang, H.Q.; Lv, Y.; Xia, Z.J.; Zhu, X.F.; Lin, T.Y.; et al. Beclin 1 expression: A predictor of prognosis in patients with extranodal natural killer T-cell lymphoma, nasal type. Autophagy 2010, 6, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.B.; Fan, X.J.; Chen, M.Y.; Xiang, J.; Huang, P.Y.; Guo, L.; Wu, X.Y.; Xu, J.; Long, Z.J.; Zhao, Y..; et al. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy 2010, 6, 395–604. [Google Scholar]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumors of the Digestive System; IARC Press: Lyon, France, 2010. [Google Scholar]
- Sobin, L.H.; Wittekind, C.H. TNM Classification of Malignant Tumours, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Kumada, T.; Tsuneyama, K.; Hatta, H.; Ishizawa, S.; Takano, Y. Improved 1-h rapid immunostaining method using intermittent microwave irradiation: practicability based on 5 years application in Toyama Medical and Pharmaceutical University Hospital. Mod. Pathol. 2004, 17, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, M.-Y.; Gou, W.-F.; Zhao, S.; Mao, X.-Y.; Zheng, Z.-H.; Takano, Y.; Zheng, H.-C. Beclin 1 Expression is Closely Linked to Colorectal Carcinogenesis and Distant Metastasis of Colorectal Carcinoma. Int. J. Mol. Sci. 2014, 15, 14372-14385. https://doi.org/10.3390/ijms150814372
Zhang M-Y, Gou W-F, Zhao S, Mao X-Y, Zheng Z-H, Takano Y, Zheng H-C. Beclin 1 Expression is Closely Linked to Colorectal Carcinogenesis and Distant Metastasis of Colorectal Carcinoma. International Journal of Molecular Sciences. 2014; 15(8):14372-14385. https://doi.org/10.3390/ijms150814372
Chicago/Turabian StyleZhang, Mei-Ying, Wen-Feng Gou, Shuang Zhao, Xiao-Yun Mao, Zhi-Hong Zheng, Yasuo Takano, and Hua-Chuan Zheng. 2014. "Beclin 1 Expression is Closely Linked to Colorectal Carcinogenesis and Distant Metastasis of Colorectal Carcinoma" International Journal of Molecular Sciences 15, no. 8: 14372-14385. https://doi.org/10.3390/ijms150814372
APA StyleZhang, M.-Y., Gou, W.-F., Zhao, S., Mao, X.-Y., Zheng, Z.-H., Takano, Y., & Zheng, H.-C. (2014). Beclin 1 Expression is Closely Linked to Colorectal Carcinogenesis and Distant Metastasis of Colorectal Carcinoma. International Journal of Molecular Sciences, 15(8), 14372-14385. https://doi.org/10.3390/ijms150814372




