Insights on Antioxidant Assays for Biological Samples Based on the Reduction of Copper Complexes—The Importance of Analytical Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Complexing Agent Selection
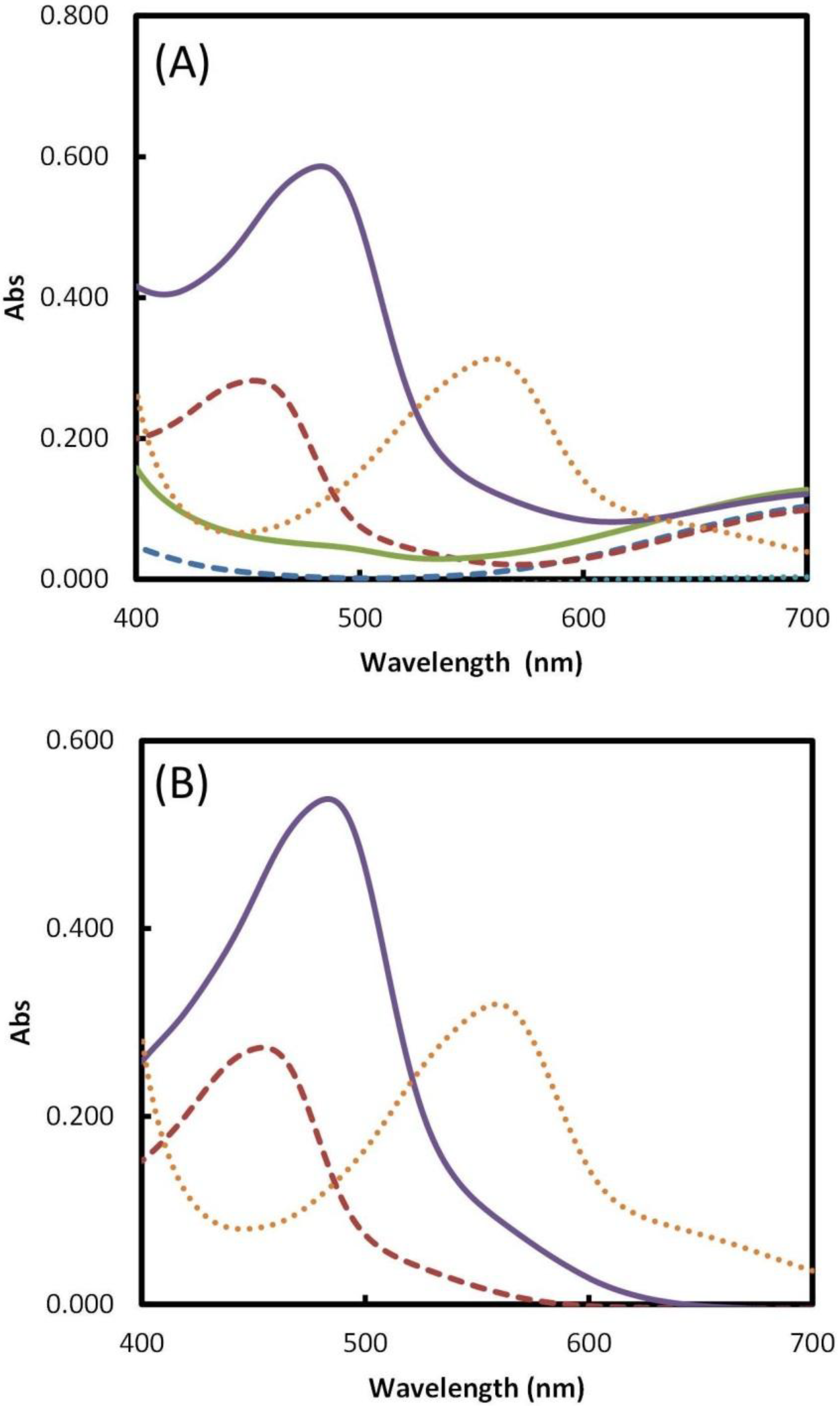
2.2. Reagents Addition Order

2.3. Trolox Equivalent Value
| TE | Ascorbic Acid | Trolox | Uric Acid | Glutathione |
|---|---|---|---|---|
| TE(BCS) | 1.15 ± 0.02 | 1.00 ± 0.01 | 1.90 ± 0.02 | 0.62 ± 0.02 |
| TE(NC) | 0.90 ± 0.03 | 1.00 ± 0.01 | 1.96 ± 0.02 | 0.54 ± 0.01 |
| TE(BCA) | 1.03 ± 0.03 | 1.00 ± 0.04 | 2.46 ± 0.09 | 0.57 ± 0.02 |
| [AA] µM | [AU] µM | [GSH] µM | TE Expected | TE Found Average ± SD | RD% a |
|---|---|---|---|---|---|
| 10 | 10 | 10 | 0.037 | 0.038 ± 0.000 | 3.0 |
| 10 | 10 | 50 | 0.065 | 0.066 ± 0.000 | 1.7 |
| 10 | 50 | 10 | 0.111 | 0.112 ± 0.001 | 0.5 |
| 10 | 50 | 50 | 0.139 | 0.142 ± 0.001 | 1.8 |
| 50 | 50 | 10 | 0.157 | 0.158 ± 0.004 | 0.9 |
| 50 | 50 | 50 | 0.185 | 0.188 ± 0.001 | 1.9 |
| 50 | 10 | 10 | 0.082 | 0.085 ± 0.003 | 2.7 |
| 50 | 10 | 50 | 0.110 | 0.114 ± 0.001 | 2.9 |
2.4. TE of Biological Antioxidants in Mixtures
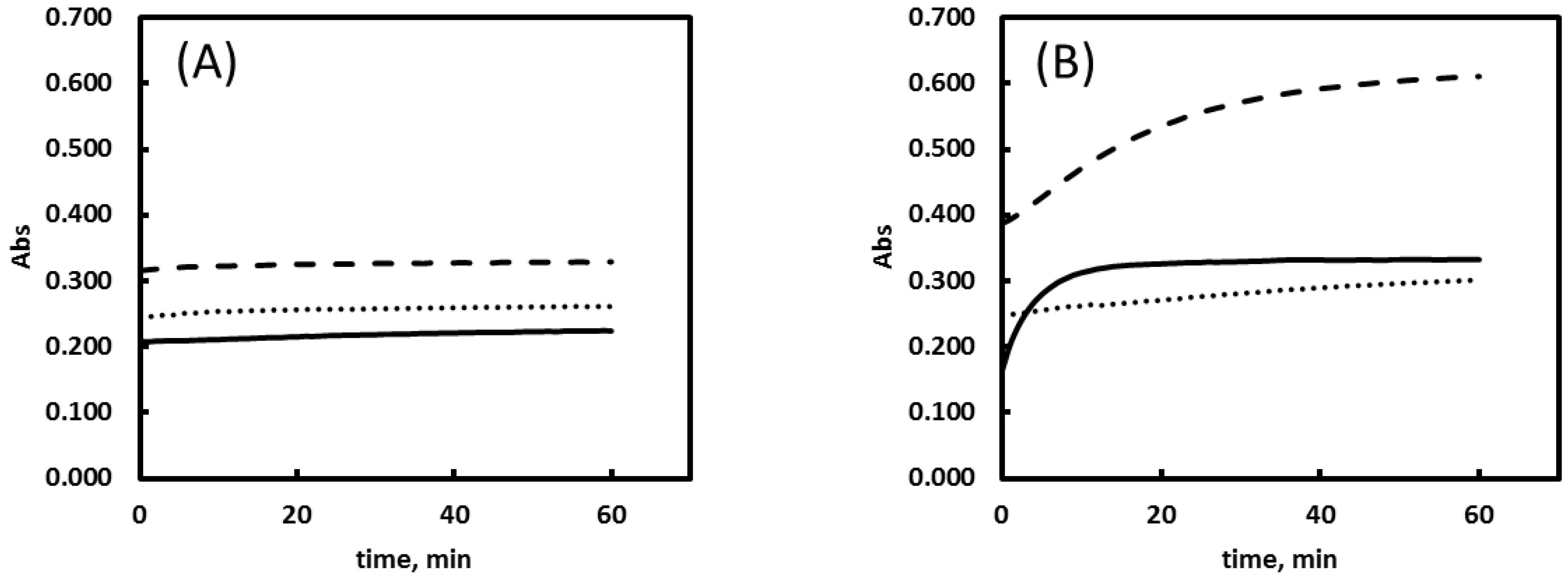
2.5. Buffer Solutions
| Buffer | Ascorbic Acid | Trolox | Uric Acid | Glutathione |
|---|---|---|---|---|
| Ammonium acetate, pH 7.0 | 1.148 ± 0.024 | 1.000 ± 0.008 | 1.899 ± 0.013 | 0.624 ± 0.018 |
| Tris-glycine urea, pH 7.0 | 0.813 ± 0.040 | 1.000 ± 0.050 | 0.983 ± 0.049 | 0.552 ± 0.028 |
| PBS, pH 7.4 | 0.991 ± 0.044 | 1.000 ± 0.050 | 0.766 ± 0.038 | 0.668 ± 0.033 |
2.6. Sample Analysis and Validation
| Nominal Conc. (µM) | Ascorbic Acid (µM) | Uric Acid (µM) | Glutathione (µM) | Trolox (µM) |
|---|---|---|---|---|
| 10 | 10.0 ± 0.7 | 10.0 ± 0.6 | 9.9 ± 0.5 | 10.5 ± 0.6 |
| 20 | 20.3 ± 0.9 | 20.0 ± 0.7 | 21.8 ± 0.7 | 20.2 ± 0.7 |
| 40 | 39.8 ± 2.0 | 39.6 ± 1.7 | 42.0 ± 0.9 | 40.6 ± 0.7 |
| 60 | 59.3 ± 2.6 | 60.3 ± 1.9 | 61.0 ± 1.3 | 61.0 ± 1.2 |
| 100 | 99.9 ± 2.8 | 100 ± 1.5 | 97.9 ± 1.9 | 100.9 ± 1.1 |
| Sample | Absorbance | TEAC (µM) | Recovery% | ||
|---|---|---|---|---|---|
| Added Uric Acid Concentration | Trolox Equivalent (µM) | ||||
| 0 µM | 25 µM | 0 µM | 44.1 µM | ||
| S1 | 0.260 ± 0.002 | 0.524 ± 0.006 | 25.7 ± 0.2 | 66.7 ± 0.8 | 93.0 |
| S2 | 0.271 ± 0.004 | 0.518 ± 0.006 | 27.4 ± 0.4 | 66.1 ± 0.7 | 87.9 |
| S3 | 0.235 ± 0.005 | 0.493 ± 0.004 | 21.6 ± 0.4 | 62.2 ± 0.5 | 92.0 |
| S4 | 0.265 ± 0.003 | 0.511 ± 0.005 | 26.4 ± 0.3 | 65.1 ± 0.6 | 87.7 |
| S5 | 0.275 ± 0.004 | 0.520 ± 0.005 | 27.9 ± 0.4 | 66.5 ± 0.7 | 87.6 |
3. Experimental Section
3.1. Materials
3.2. Instrumentation
3.3. Validation and Sample Analysis
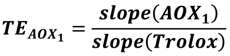
4. Conclusions
- An additive response should be provided for the targeted group of antioxidants in the applied concentration range, because as long as the additivity is verified, the nature of the used Cu complexing agent does not seem to influence the results;
- Additive response is provided as long as the oxidant complex is maintained in molar excess considering the electron transfer processes;
- Selection of the buffering system affects more the TE values that the complexing agent used, and this is related to the electron transfer mechanisms of the antioxidants;
- The selection of buffer solution is also important to avoid measurement artefacts due to protein precipitation in biological samples.
Acknowledgments
Author Contributions
Supplementary Files
Supplementary File 1Supplementary Information
Conflicts of Interest
References
- Azzi, A.; Davies, K.J.A.; Kelly, F. Free radical biology—Terminology and critical thinking. FEBS Lett. 2004, 558, 3–6. [Google Scholar] [CrossRef]
- Dilis, V.; Trichopoulou, A. Assessment of antioxidants in foods and biological samples: A short critique. Int. J. Food Sci. Nutr. 2010, 61, 441–448. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar]
- Bartosz, G. Total antioxidant capacity. In Advances in Clinical Chemistry; Herbert, E., Spiegel, G.N., Kwang-Jen, H., Eds.; Elsevier: Waltham, MA, USA, 2003; Volume 37, pp. 219–292. [Google Scholar]
- Jansen, E.; Ruskovska, T. Comparative analysis of serum (anti)oxidative status parameters in healthy persons. Int. J. Mol. Sci. 2013, 14, 6106–6115. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Rorabacher, D.B. Electron transfer by copper centers. Chem. Rev. 2004, 104, 651–697. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Yin, J.-J.; Fu, P.P.; Lutterodt, H.; Zhou, Y.-T.; Antholine, W.E.; Wamer, W. Dual role of selected antioxidants found in dietary supplements: Crossover between anti- and pro-oxidant activities in the presence of copper. J. Agric. Food Chem. 2012, 60, 2554–2561. [Google Scholar] [CrossRef]
- Chobot, V.; Kubicova, L.; Bachmann, G.; Hadacek, F. Versatile redox chemistry complicates antioxidant capacity assessment: Flavonoids as milieu-dependent anti- and pro-oxidants. Int. J. Mol. Sci. 2013, 14, 11830–11841. [Google Scholar] [CrossRef]
- Kondakçi, E.; Özyürek, M.; Güçlü, K.; Apak, R. Novel pro-oxidant activity assay for polyphenols, vitamins C and E using a modified CUPRAC method. Talanta 2013, 115, 583–589. [Google Scholar] [CrossRef]
- Bakir, T.; Yildoǧan Beker, B.; Sönmezoǧlu, I.; Imer, F.; Apak, R. Antioxidant and prooxidant effects of α-tocopherol in a linoleic acid-copper(II)-ascorbate system. Eur. J. Lipid Sci. Technol. 2013, 115, 372–376. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Evaluation of the copper(II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: The CUPRAC–BCS assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Maia, M.A.; Reis, S.; Segundo, M.A. Rapid assessment of endpoint antioxidant capacity of red wines through microchemical methods using a kinetic matching approach. Talanta 2012, 97, 473–483. [Google Scholar] [CrossRef]
- Ribeiro, J.P.N.; Magalhães, L.M.; Reis, S.; Lima, J.L.F.C.; Segundo, M.A. High-throughput total cupric ion reducing antioxidant capacity of biological samples determined using flow injection analysis and microplate-based methods. Anal. Sci. 2011, 27, 483–488. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Troncoso, A.M.; García-Parrilla, M.C. The antioxidant activity of wines determined by the ABTS+ method: Influence of sample dilution and time. Talanta 2004, 64, 501–509. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Differences in responsivity of original cupric reducing antioxidant capacity and cupric-bathocuproine sulfonate assays to antioxidant compounds. Anal. Biochem. 2012, 423, 36–38. [Google Scholar] [CrossRef]
- Lima, M.J.R.; Tóth, I.V.; Rangel, A.O.S.S. A new approach for the sequential injection spectrophotometric determination of the total antioxidant activity. Talanta 2005, 68, 207–213. [Google Scholar] [CrossRef]
- Ding, X.; Xie, H.; Kang, Y.J. The significance of copper chelators in clinical and experimental application. J. Nutr. Biochem. 2011, 22, 301–310. [Google Scholar] [CrossRef]
- Brenner, A.J.; Harris, E.D. A quantitative test for copper using bicinchoninic acid. Anal. Biochem. 1995, 226, 80–84. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Hawkins, C.J.; Perrin, D.D. Oxidation-reduction potentials of metal complexes in water. Part II. Copper complexes with 2,9-dimethyl- and 2-chloro-1,10-phenanthroline. J. Chem. Soc. 1963, 1963, 3097–3099. [Google Scholar]
- Zhou, L.; Singleton, C.; le Brun, N.E. High Cu(I) and low proton affinities of the cxxc motif of bacillus subtilis copz. Biochem. J. 2008, 413, 459–465. [Google Scholar] [CrossRef]
- Huang, X.; Cuajungco, M.P.; Atwood, C.S.; Hartshorn, M.A.; Tyndall, J.D.A.; Hanson, G.R.; Stokes, K.C.; Leopold, M.; Multhaup, G.; Goldstein, L.E.; et al. Cu(II) potentiation of Alzheimer aβ neurotoxicity: Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999, 274, 37111–37116. [Google Scholar] [CrossRef]
- Zhou, F.; Millhauser, G.L. The rich electrochemistry and redox reactions of the copper sites in the cellular prion protein. Coord. Chem. Rev. 2012, 256, 2285–2296. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçǧ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar]
- Gosmaro, F.; Bagnati, M.; Berto, S.; Bellomo, G.; Prenesti, E. Measurement of total antioxidant capacity of human plasma: Setting and validation of the CUPRAC-BCS method on routine apparatus Advia 2400. Talanta 2013, 115, 526–532. [Google Scholar] [CrossRef]
- Çekiç, S.D.; Kara, N.; Tütem, E.; Başkan, K.S.; Apak, R. Protein-incorporated serum total antioxidant capacity measurement by a modified CUPRAC (cupric reducing antioxidant capacity) method. Anal. Lett. 2012, 45, 754–763. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Bektaşoǧlu, B.; Bener, M. Cupric ion reducing antioxidant capacity assay for antioxidants in human serum and for hydroxyl radical scavengers. Methods Mol. Biol. 2010, 594, 215–239. [Google Scholar] [CrossRef]
- Robinson, K.M.; Morré, J.T.; Beckman, J.S. Triuret: A novel product of peroxynitrite-mediated oxidation of urate. Arch. Biochem. Biophys. 2004, 423, 213–217. [Google Scholar] [CrossRef]
- Çekiç, S.D.; Başkan, K.S.; Tütem, E.; Apak, R. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta 2009, 79, 344–351. [Google Scholar] [CrossRef]
- Khalil, M.M.; Radalla, A.M.; Mohamed, A.G. Potentiometric investigation on complexation of divalent transition metal ions with some zwitterionic buffers and triazoles. J. Chem. Eng. Data 2009, 54, 3261–3272. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 5th ed.; Pearson: Harlow, UK, 2005. [Google Scholar]
- DeSilva, B.; Garofolo, F.; Rocci, M.; Martinez, S.; Dumont, I.; Landry, F.; Dicaire, C.; Szekely-Klepser, G.; Weiner, R.; Arnold, M.; et al. 2012 white paper on recent issues in bioanalysis and alignment of multiple guidelines. Bioanalysis 2012, 4, 2213–2226. [Google Scholar] [CrossRef]
- European Medical Agency. Guideline on Bioanalytical Method Validation; European Medical Agency (EMA), Committee for Medicinal Products for Human Use (CHMP): London, UK, 2011. [Google Scholar]
- Viswanathan, C.T.; Bansal, S.; Booth, B.; DeStefano, A.J.; Rose, M.J.; Sailstad, J.; Shah, V.P.; Skelly, J.P.; Swann, P.G.; Weiner, R. Quantitative bioanalytical methods validation and implementation: Best practices for chromatographic and ligand binding assays. Pharm. Res. 2007, 24, 1962–1973. [Google Scholar] [CrossRef]
- Smith, G. Review of the 2008 european medicines agency concept paper on bioanalytical method validation. Bioanalysis 2009, 1, 877–881. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marques, S.S.; Magalhães, L.M.; Tóth, I.V.; Segundo, M.A. Insights on Antioxidant Assays for Biological Samples Based on the Reduction of Copper Complexes—The Importance of Analytical Conditions. Int. J. Mol. Sci. 2014, 15, 11387-11402. https://doi.org/10.3390/ijms150711387
Marques SS, Magalhães LM, Tóth IV, Segundo MA. Insights on Antioxidant Assays for Biological Samples Based on the Reduction of Copper Complexes—The Importance of Analytical Conditions. International Journal of Molecular Sciences. 2014; 15(7):11387-11402. https://doi.org/10.3390/ijms150711387
Chicago/Turabian StyleMarques, Sara S., Luís M. Magalhães, Ildikó V. Tóth, and Marcela A. Segundo. 2014. "Insights on Antioxidant Assays for Biological Samples Based on the Reduction of Copper Complexes—The Importance of Analytical Conditions" International Journal of Molecular Sciences 15, no. 7: 11387-11402. https://doi.org/10.3390/ijms150711387
APA StyleMarques, S. S., Magalhães, L. M., Tóth, I. V., & Segundo, M. A. (2014). Insights on Antioxidant Assays for Biological Samples Based on the Reduction of Copper Complexes—The Importance of Analytical Conditions. International Journal of Molecular Sciences, 15(7), 11387-11402. https://doi.org/10.3390/ijms150711387