Impact of Serum Chemerin Levels on Liver Functional Reserves and Platelet Counts in Patients with Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Patient Baseline Characteristics and Laboratory Data
| Variables | n = 44 |
|---|---|
| Sex (male/female) | 29/15 |
| Age (years) | 71 (50–82) |
| Etiology (B/C/B + C/other) | 4/38/1/1 |
| BMI (kg/m2) | 22.5 (15.6–33.5) |
| Child-Pugh score (5/6/7/8) | 9/22/7/6 |
| ALB (g/dL) | 3.5 (2.6–4.5) |
| ALT (IU/L) | 46 (12–146) |
| T-Bil (mg/dL) | 1.0 (0.5–3.7) |
| PLT (×104/μL) | 9.5 (3.6–18.8) |
| PT (%) | 70 (50–100) |
| FPG (mg/dL) | 100 (74–224) |
| HbA1c (%) | 5.0 (3.6–9.4) |
| AFP (ng/dL) | 32.5 (1.7–16931) |
| PIVKA-II (mAU/mL) | 26.5 (5–1860) |
| Tumor size (cm) | 1.9 (1.0–15.3) |
| Tumor number (1/2/3/4/5) | 30/9/2/2/1 |
| Stage (I/II/III) | 17/21/6 |
| Initial treatment (resection/RFA/TACE + RFA) | 1/38/5 |
| Chemerin (ng/mL) | 130.5 (80–312) |
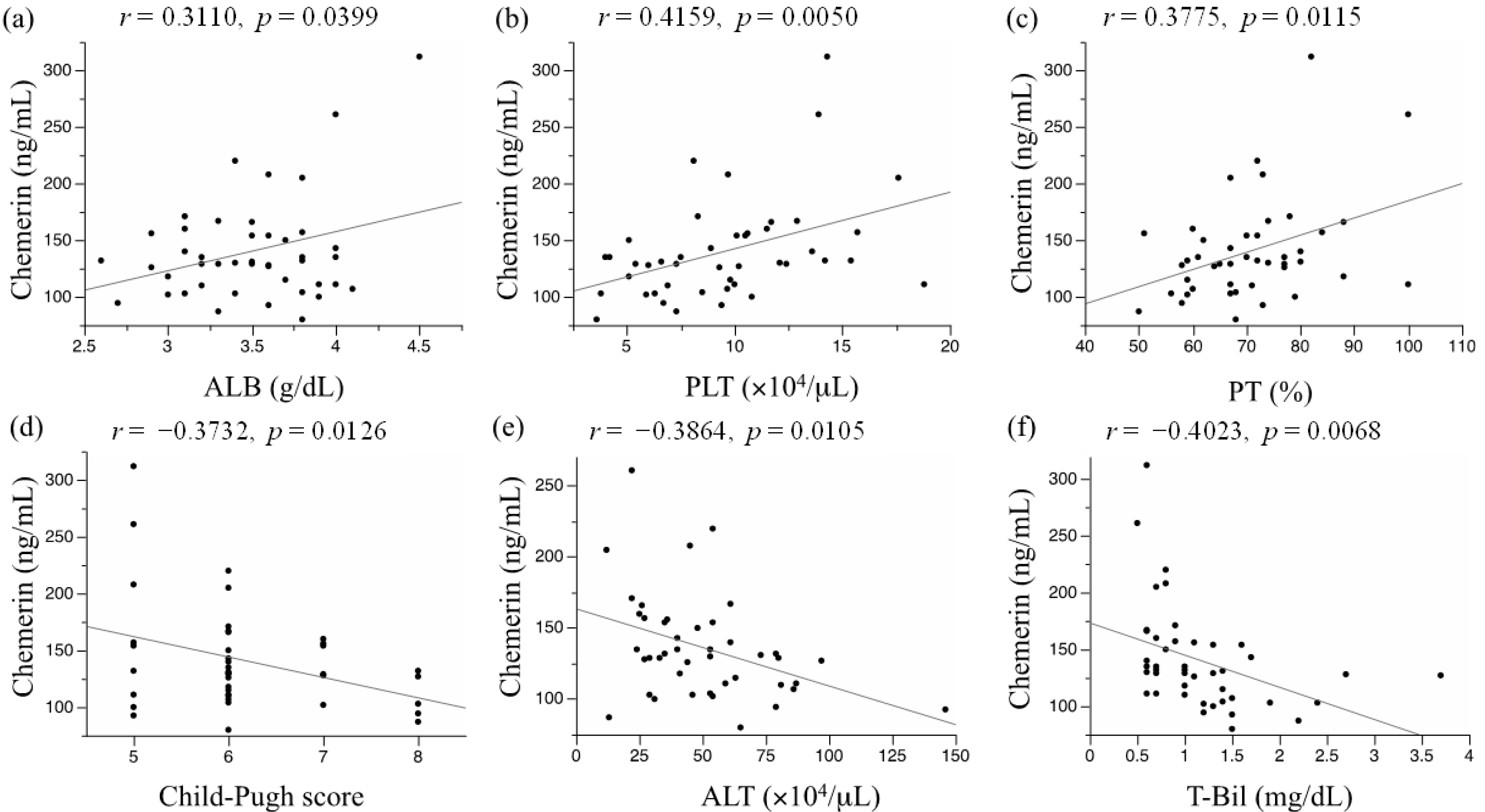
2.2. Association of the Serum Chemerin Level with Liver Functional Reserve and Other Clinical Indexes
| Parameters | Pearson’s Correlation Coefficient | p Value |
|---|---|---|
| Age | 0.2294 | 0.1389 |
| BMI | 0.2040 | 0.1895 |
| Child-Pugh score | −0.3732 | 0.0126 * |
| ALB (g/dL) | 0.3110 | 0.0399 * |
| ALT (IU/L) | −0.3864 | 0.0105 * |
| T-Bil (mg/dL) | −0.4023 | 0.0068 *,† |
| PLT (×104/μL) | 0.4159 | 0.0050 *,† |
| PT (%) | 0.3775 | 0.0115 * |
| FPG (mg/dL) | −0.1145 | 0.4761 |
| HbA1c (%) | −0.0509 | 0.7750 |
| FIRI (mg/dL) | −0.2217 | 0.2226 |
| HOMA-IR | −0.1093 | 0.4963 |
| AFP (ng/dL) | −0.1764 | 0.2698 |
| PIVKA-II (mAU/mL) | 0.0493 | 0.7689 |
| Tumor number | −0.1100 | 0.4773 |
| Tumor size (cm) | −0.0510 | 0.7423 |
| d-ROM (Carr U) | −0.0427 | 0.7830 |
| BAP (μmol/L) | −0.3591 | 0.2782 |
| Leptin (ng/mL) | 0.0805 | 0.6032 |
| Visfatin (ng/mL) | −0.0181 | 0.9074 |
| Resistin (ng/mL) | 0.0832 | 0.5913 |
| Vaspin (ng/mL) | −0.1180 | 0.4457 |
2.3. Impact of the Serum Chemerin Levels on Recurrence-Free Survival and Overall Survival in Patients with Hepatocellular Carcinoma (HCC)

2.4. Discussion
3. Materials and Methods
3.1. Patients and Measurement of Serum Chemerin Concentration
3.2. Treatment and Follow-Up Strategy

3.3. Statistical Analysis
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 2006, 35, 204–214. [Google Scholar]
- Sun, B.; Karin, M. Obesity, inflammation, and liver cancer. J. Hepatol. 2012, 56, 704–713. [Google Scholar]
- Shimizu, M.; Tanaka, T.; Moriwaki, H. Obesity and hepatocellular carcinoma: Targeting obesity-related inflammation for chemoprevention of liver carcinogenesis. Semin. Immunopathol. 2013, 35, 191–202. [Google Scholar]
- Imai, K.; Takai, K.; Nishigaki, Y.; Shimizu, S.; Naiki, T.; Hayashi, H.; Uematsu, T.; Sugihara, J.; Tomita, E.; Shimizu, M.; et al. Insulin resistance raises the risk for recurrence of stage I hepatocellular carcinoma after curative radiofrequency ablation in hepatitis C virus-positive patients: A prospective, case series study. Hepatol. Res. 2010, 40, 376–382. [Google Scholar]
- Suzuki, Y.; Imai, K.; Takai, K.; Hanai, T.; Hayashi, H.; Naiki, T.; Nishigaki, Y.; Tomita, E.; Shimizu, M.; Moriwaki, H. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: A prospective case series study using the d-ROM test. J. Cancer Res. Clin. Oncol. 2013, 139, 845–852. [Google Scholar]
- Watanabe, N.; Takai, K.; Imai, K.; Shimizu, M.; Naiki, T.; Nagaki, M.; Moriwaki, H. Increased levels of serum leptin are a risk factor for the recurrence of stage I/II hepatocellular carcinoma after curative treatment. J. Clin. Biochem. Nutr. 2011, 49, 153–158. [Google Scholar]
- Roh, S.G.; Song, S.H.; Choi, K.C.; Katoh, K.; Wittamer, V.; Parmentier, M.; Sasaki, S. Chemerin—A new adipokine that modulates adipogenesis via its own receptor. Biochem. Biophys. Res. Commun. 2007, 362, 1013–1018. [Google Scholar]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar]
- Dong, B.; Ji, W.; Zhang, Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern. Med. 2011, 50, 1093–1097. [Google Scholar]
- Min, J.L.; Nicholson, G.; Halgrimsdottir, I.; Almstrup, K.; Petri, A.; Barrett, A.; Travers, M.; Rayner, N.W.; Mägi, R.; Pettersson, F.H.; et al. Coexpression network analysis in abdominal and gluteal adipose tissue reveals regulatory genetic loci for metabolic syndrome and related phenotypes. PLoS Genet. 2012, 8, e1002505. [Google Scholar]
- Sell, H.; Divoux, A.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Bedossa, P.; Tordjman, J.; Eckel, J.; Clément, K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 2010, 95, 2892–2896. [Google Scholar]
- Yilmaz, Y.; Yonal, O.; Kurt, R.; Alahdab, Y.O.; Eren, F.; Ozdogan, O.; Celikel, C.A.; Imeryuz, N.; Kalayci, C.; Avsar, E. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand. J. Gastroenterol. 2011, 46, 91–97. [Google Scholar]
- Saremi, A.; Shavandi, N.; Parastesh, M.; Daneshmand, H. Twelve-week aerobic training decreases chemerin level and improves cardiometabolic risk factors in overweight and obese men. Asian J. Sports Med. 2010, 1, 151–158. [Google Scholar]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar]
- Parolini, S.; Santoro, A.; Marcenaro, E.; Luini, W.; Massardi, L.; Facchetti, F.; Communi, D.; Parmentier, M.; Majorana, A.; Sironi, M.; et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007, 109, 3625–3632. [Google Scholar]
- Vermi, W.; Riboldi, E.; Wittamer, V.; Gentili, F.; Luini, W.; Marrelli, S.; Vecchi, A.; Franssen, J.D.; Communi, D.; Massardi, L.; et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J. Exp. Med. 2005, 201, 509–515. [Google Scholar]
- Gu, P.; Jiang, W.; Lu, B.; Shi, Z. Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin. Exp. Hypertens. 2013. [Google Scholar] [CrossRef]
- Kukla, M.; Zwirska-Korczala, K.; Gabriel, A.; Waluga, M.; Warakomska, I.; Szczygiel, B.; Berdowska, A.; Mazur, W.; Wozniak-Grygiel, E.; Kryczka, W. Chemerin, vaspin and insulin resistance in chronic hepatitis C. J. Viral Hepat. 2010, 17, 661–667. [Google Scholar]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar]
- Krautbauer, S.; Wanninger, J.; Eisinger, K.; Hader, Y.; Beck, M.; Kopp, A.; Schmid, A.; Weiss, T.S.; Dorn, C.; Buechler, C. Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver. Exp. Mol. Pathol. 2013, 95, 199–205. [Google Scholar]
- Takahashi, M.; Takahashi, Y.; Takahashi, K.; Zolotaryov, F.N.; Hong, K.S.; Kitazawa, R.; Iida, K.; Okimura, Y.; Kaji, H.; Kitazawa, S.; et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008, 582, 573–578. [Google Scholar]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Filarsky, M.; Bauer, S.; Wiest, R.; Farkas, S.; Scherer, M.N.; Schäffler, A.; Aslanidis, C.; et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin. Endocrinol. 2010, 72, 342–348. [Google Scholar]
- Tonjes, A.; Fasshauer, M.; Kratzsch, J.; Stumvoll, M.; Bluher, M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS One 2010, 5, e13911. [Google Scholar]
- Kukla, M.; Zwirska-Korczala, K.; Hartleb, M.; Waluga, M.; Chwist, A.; Kajor, M.; Kajor, M.C.; Berdowska, A.; Wozniak-Grygiel, E.; Buldak, R. Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2010, 45, 235–242. [Google Scholar]
- Afdhal, N.; McHutchison, J.; Brown, R.; Jacobson, I.; Manns, M.; Poordad, F.; Weksler, B.; Esteban, R. Thrombocytopenia associated with chronic liver disease. J. Hepatol. 2008, 48, 1000–1007. [Google Scholar]
- Chou, R.; Wasson, N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: A systematic review. Ann. Intern. Med. 2013, 158, 807–820. [Google Scholar]
- Ninomiya, S.; Shimizu, M.; Imai, K.; Takai, K.; Shiraki, M.; Hara, T.; Tsurumi, H.; Ishizaki, S.; Moriwaki, H. Possible role of visfatin in hepatoma progression and the effects of branched-chain amino acids on visfatin-induced proliferation in human hepatoma cells. Cancer Prev. Res. 2011, 4, 2092–2100. [Google Scholar]
- Ernst, M.C.; Haidl, I.D.; Zuniga, L.A.; Dranse, H.J.; Rourke, J.L.; Zabel, B.A.; Butcher, E.C.; Sinal, C.J. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 2012, 153, 672–682. [Google Scholar]
- Takahashi, M.; Okimura, Y.; Iguchi, G.; Nishizawa, H.; Yamamoto, M.; Suda, K.; Kitazawa, R.; Fujimoto, W.; Takahashi, K.; Zolotaryov, F.N.; et al. Chemerin regulates beta-cell function in mice. Sci. Rep. 2011, 1, 123. [Google Scholar]
- Kralisch, S.; Weise, S.; Sommer, G.; Lipfert, J.; Lossner, U.; Bluher, M.; Stumvoll, M.; Fasshauer, M. Interleukin-1β induces the novel adipokine chemerin in adipocytes in vitro. Regul. Pept. 2009, 154, 102–106. [Google Scholar]
- Kingston, M.E.; Ali, M.A.; Atiyeh, M.; Donnelly, R.J. Diabetes mellitus in chronic active hepatitis and cirrhosis. Gastroenterology 1984, 87, 688–694. [Google Scholar]
- Wang, C.; Wu, W.K.; Liu, X.; To, K.F.; Chen, G.G.; Yu, J.; Ng, E.K. Increased serum chemerin level promotes cellular invasiveness in gastric cancer: A clinical and experimental study. Peptides 2013, 51, 131–138. [Google Scholar]
- Wang, N.; Wang, Q.J.; Feng, Y.Y.; Shang, W.; Cai, M. Overexpression of chemerin was associated with tumor angiogenesis and poor clinical outcome in squamous cell carcinoma of the oral tongue. Clin. Oral Investig. 2013, 18, 997–1004. [Google Scholar]
- Lin, W.; Chen, Y.L.; Jiang, L.; Chen, J.K. Reduced expression of chemerin is associated with a poor prognosis and a lowed infiltration of both dendritic cells and natural killer cells in human hepatocellular carcinoma. Clin. Lab. 2011, 57, 879–885. [Google Scholar]
- Yamaguchi, Y.; Du, X.Y.; Zhao, L.; Morser, J.; Leung, L.L. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastoma. J. Biol. Chem. 2011, 286, 39510–39519. [Google Scholar]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005, 280, 34661–34666. [Google Scholar]
- Clinical Practice Guidelines for hepatocellular carcinoma—The Japan Society of Hepatology 2009 update. Hepatol. Res. 2010, 40, 2–144.
- Hayashi, M.; Matsui, O.; Ueda, K.; Kawamori, Y.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Takashima, T.; Nonomura, A.; Nakanuma, Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: Evaluation by CT during intraarterial injection of contrast medium. Am. J. Roentgenol. 1999, 172, 969–976. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Imai, K.; Takai, K.; Hanai, T.; Shiraki, M.; Suzuki, Y.; Hayashi, H.; Naiki, T.; Nishigaki, Y.; Tomita, E.; Shimizu, M.; et al. Impact of Serum Chemerin Levels on Liver Functional Reserves and Platelet Counts in Patients with Hepatocellular Carcinoma. Int. J. Mol. Sci. 2014, 15, 11294-11306. https://doi.org/10.3390/ijms150711294
Imai K, Takai K, Hanai T, Shiraki M, Suzuki Y, Hayashi H, Naiki T, Nishigaki Y, Tomita E, Shimizu M, et al. Impact of Serum Chemerin Levels on Liver Functional Reserves and Platelet Counts in Patients with Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2014; 15(7):11294-11306. https://doi.org/10.3390/ijms150711294
Chicago/Turabian StyleImai, Kenji, Koji Takai, Tatsunori Hanai, Makoto Shiraki, Yusuke Suzuki, Hideki Hayashi, Takafumi Naiki, Youichi Nishigaki, Eiichi Tomita, Masahito Shimizu, and et al. 2014. "Impact of Serum Chemerin Levels on Liver Functional Reserves and Platelet Counts in Patients with Hepatocellular Carcinoma" International Journal of Molecular Sciences 15, no. 7: 11294-11306. https://doi.org/10.3390/ijms150711294
APA StyleImai, K., Takai, K., Hanai, T., Shiraki, M., Suzuki, Y., Hayashi, H., Naiki, T., Nishigaki, Y., Tomita, E., Shimizu, M., & Moriwaki, H. (2014). Impact of Serum Chemerin Levels on Liver Functional Reserves and Platelet Counts in Patients with Hepatocellular Carcinoma. International Journal of Molecular Sciences, 15(7), 11294-11306. https://doi.org/10.3390/ijms150711294






