Quality Control and Stability Studies with the Monoclonal Antibody, Trastuzumab: Application of 1D- vs. 2D-Gel Electrophoresis
Abstract
:1. Introduction
2. Results and Discussion
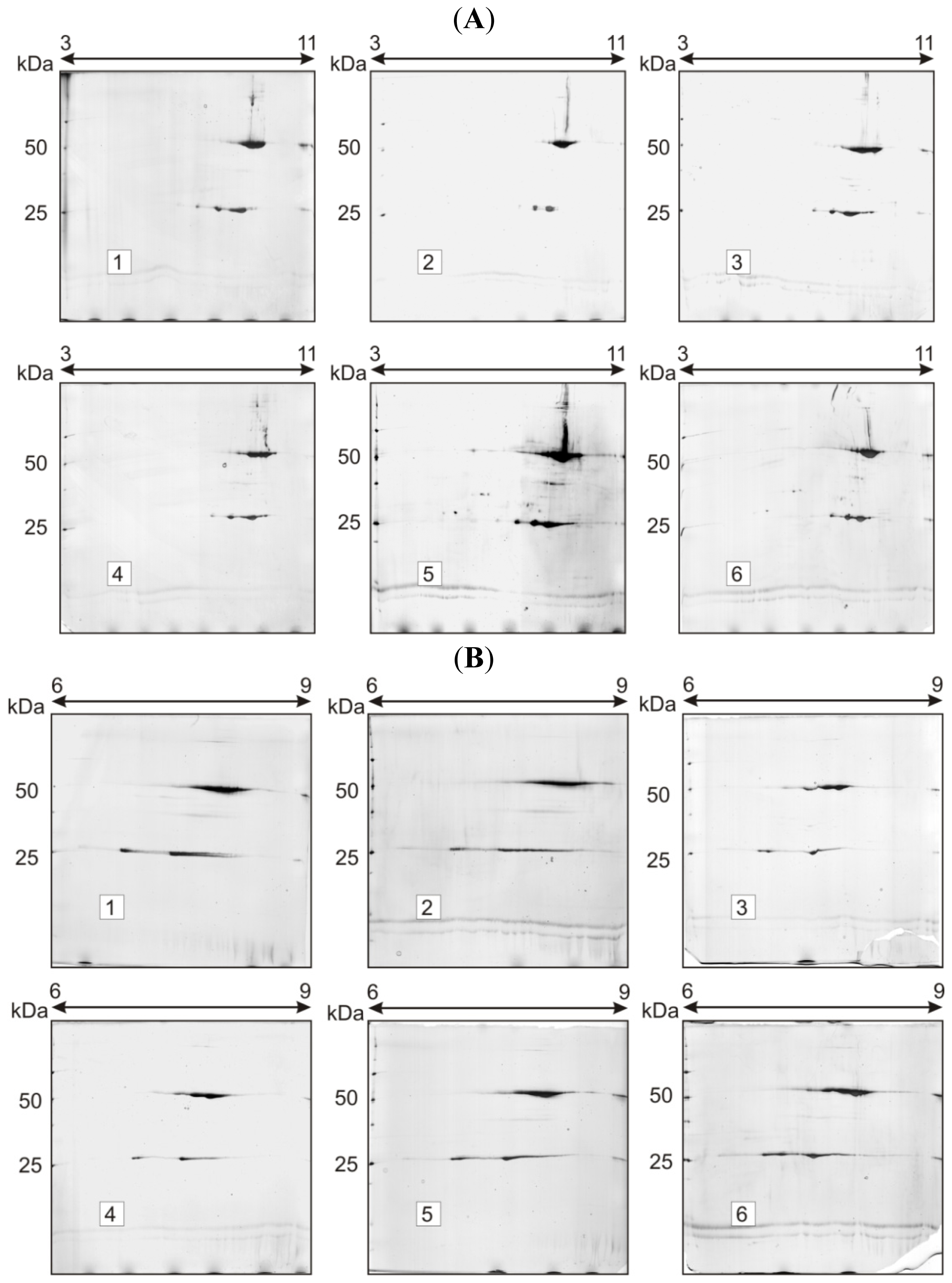
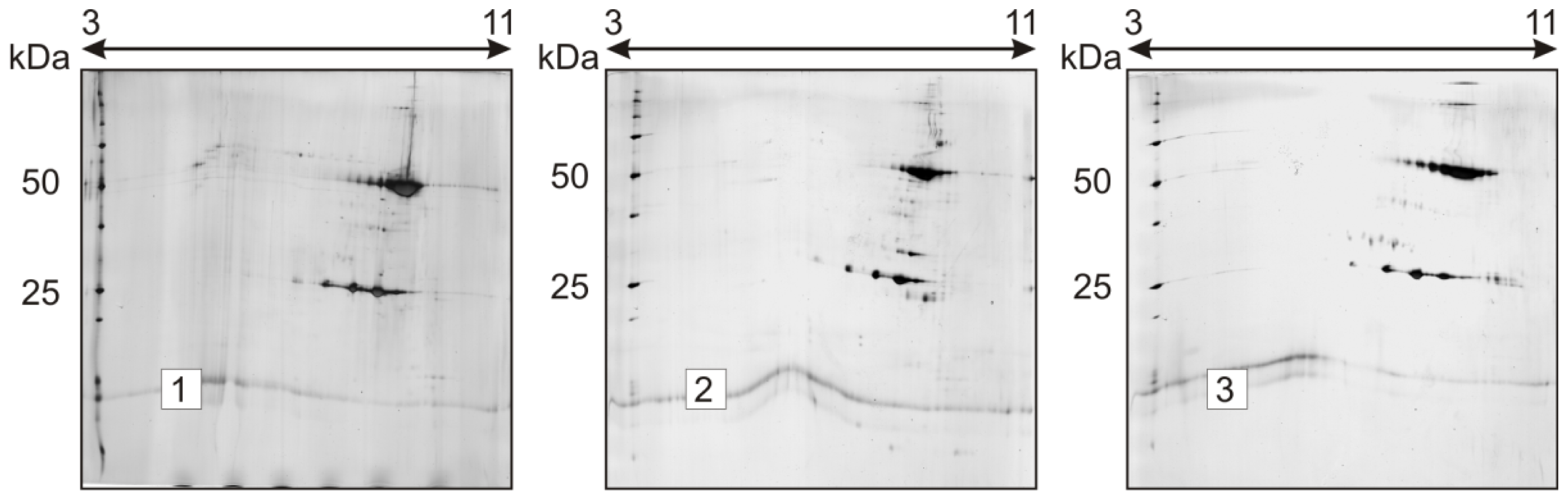
2.1. Charge Heterogeneity Study
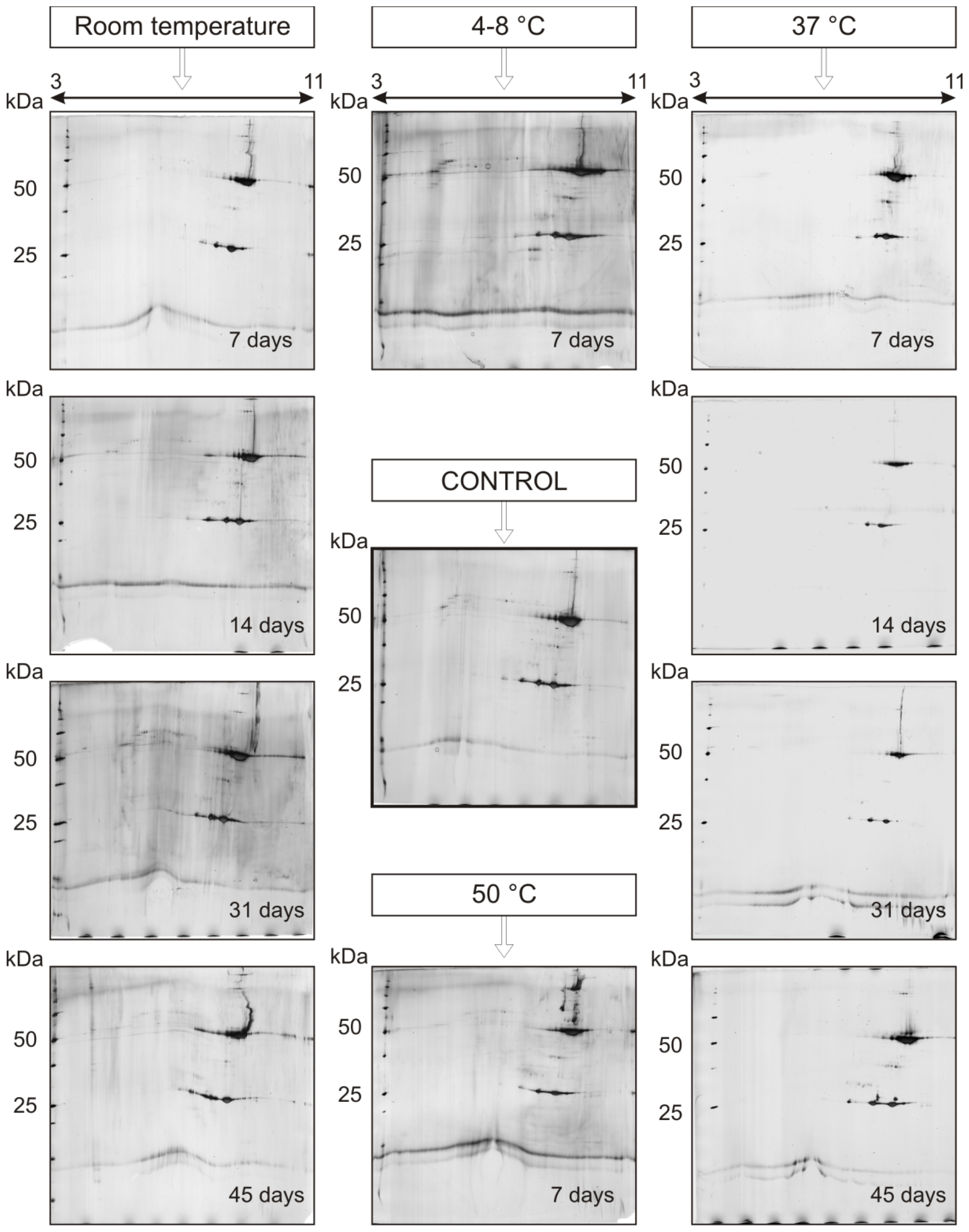
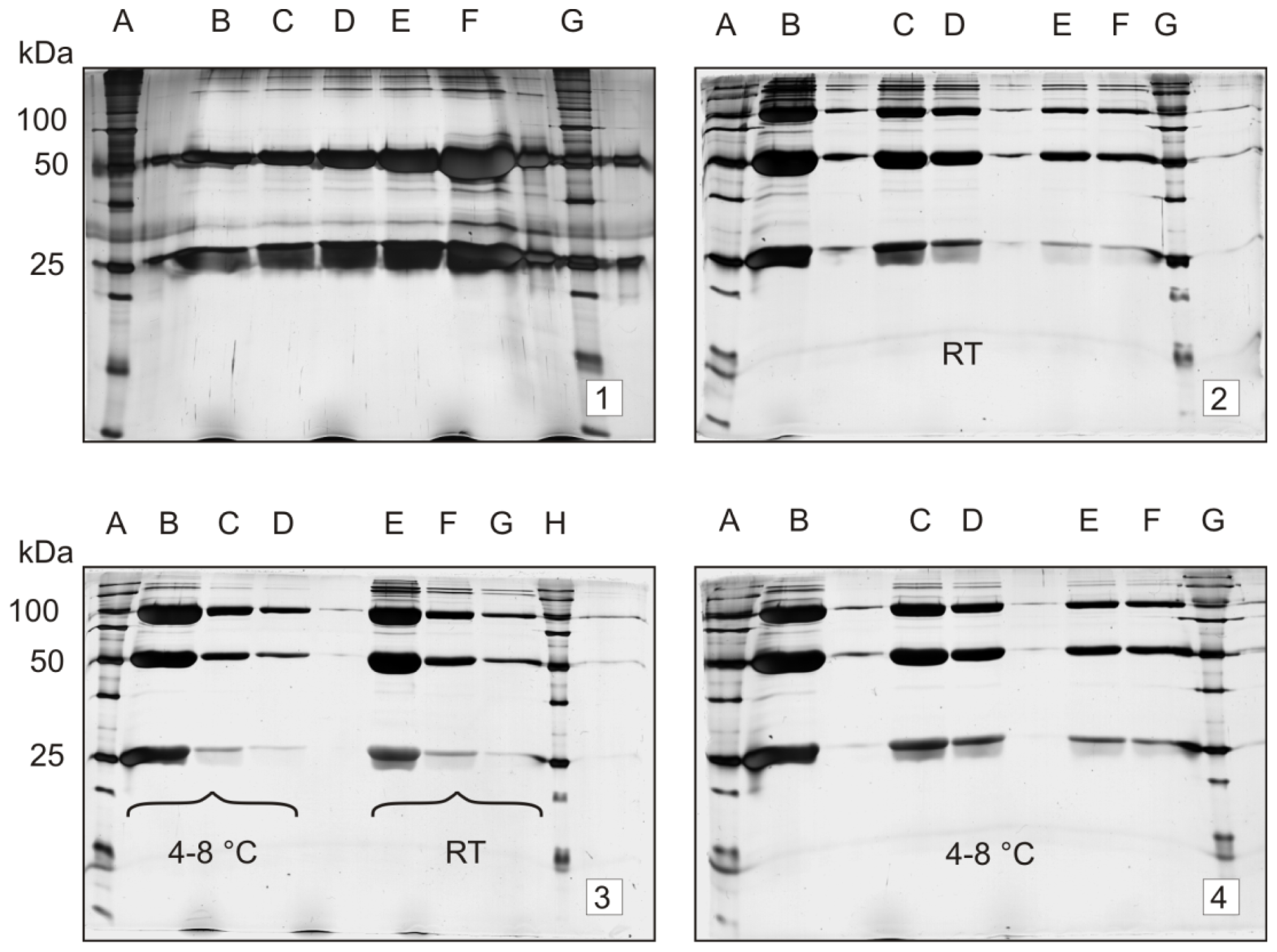
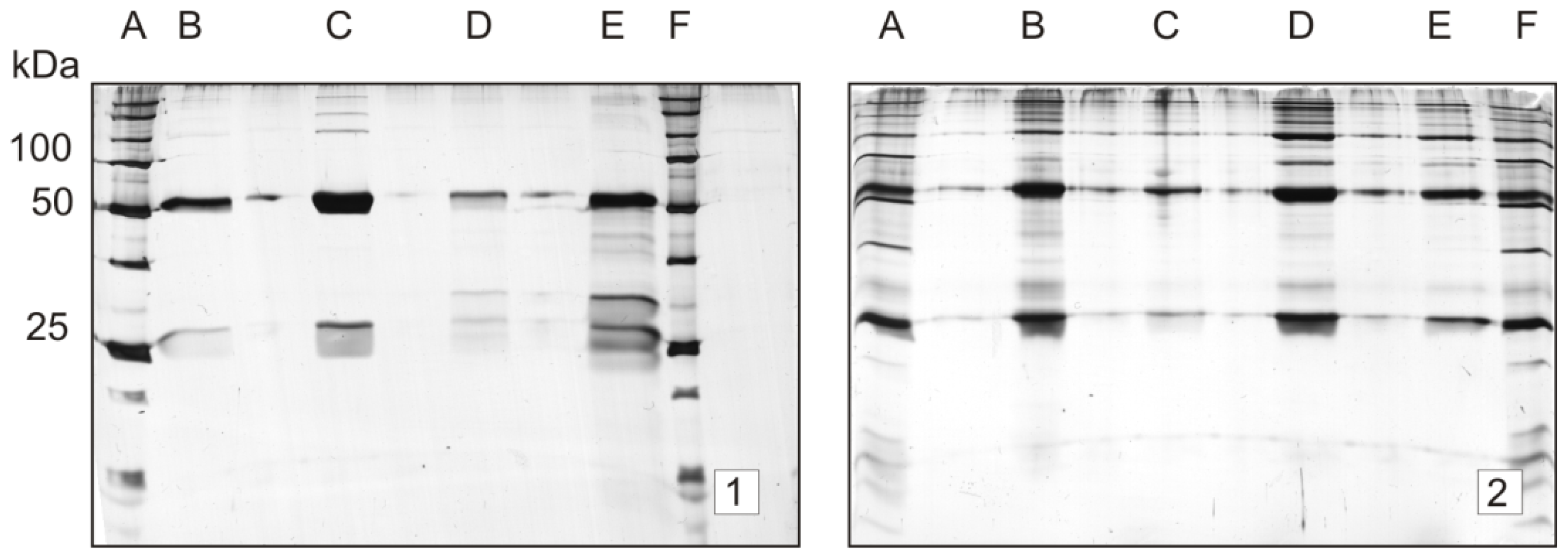
2.2. Stability Study
3. Experimental Section
3.1. Isoelectric Focusing (IEF)
3.2. Second Dimension SDS-PAGE
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsD.N. developed the methods and performed the experiments, analyzed data, and co-wrote the manuscript. E.U. and C.N. co-wrote the manuscript, B.L. supervised the project, analyzed data and co-wrote the manuscript.
References
- Schlags, W.; Lachmann, B.; Walther, M.; Kratzel, M.; Noe, C.R. Two-dimensional electrophoresis of recombinant human erythropoietin: A future method for the European Pharmacopoeia? Proteomics 2002, 2, 679–682. [Google Scholar]
- Nebija, D.; Kopelent-Frank, H.; Urban, E.; Noe, C.R.; Lachmann, B. Comparison of two-dimensional gel electrophoresis patterns and MALDI-TOF MS analysis of therapeutic recombinant monoclonal antibodies trastuzumab and rituximab. J. Pharm. Biomed. Anal 2011, 56, 684–691. [Google Scholar]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu protooncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar]
- Herceptin Prescribing. Available online: http://www.gene.com/download/pdf/herceptin_prescribing.pdf (accessed on 10 September 2013).
- Harris, R.J.; Kabakoff, B.; Macchi, F.D.; Shen, F.J.; Kwong, M.; Andya, J.D.; Shire, S.J.; Bjork, N.; Totpal, K.; Chen, A.B. Identification of multiple sources of charge heterogeneity in a recombinant antibody. J. Chromatogr. B 2001, 752, 233–245. [Google Scholar]
- Protein Entry P01857, IGHG1. Available online: http://ca.expasy.org/uniprot/P01857 (accessed on 30 July 2008).
- Wilkins, M.R.; Lindskog, I.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Hochstrasser, D.F.; Appel, R.D. Detailed peptide characterization using peptide mass—A world-wide web accessible tool. Electrophoresis 1997, 18, 403–408. [Google Scholar]
- Kamoda, S.; Nomura, C.; Kinoshita, M.; Nishiura, S.; Ishikawa, R.; Kakehi, K.; Kawasaki, N.; Hayakawa, T. Profiling analysis of oligosaccharides in antibody pharmaceuticals by capillary electrophoresis. J. Chromatogr. A 2004, 1050, 211–216. [Google Scholar]
- European Medicines Agency. Available online: http://www.ema.europa.eu/ema/ (accessed on 10 September 2013).
- U.S. Food and Drug Administration. Available online: http://www.fda.gov/ (accessed on 10 September 2013).
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.; Henner, D.; Wong, W.L.; Rowland, A.M.; Kotts, C.; Carver, M.E.; Shepard, H.M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar]
- U.S. Food and Drug Administration. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails (accessed on 18 November 2008).
- Hunt, G.; Moorhouse, K.G.; Chen, A.B. Capillary isoelectric focusing and sodium dodecyle sulfate capillary gel electrophoresis of recombinant humanized monoclonal; antibody HER2. J. Chromatogr. A 1996, 744, 295–301. [Google Scholar]
- U.S. Pharmacopeial Convention. 10 September 2013. Available online: https://mc.usp.org/monographs/trastuzumab-0-2.
- United States Pharmacopoeial Convention, USP36-NF31, 2013: U.S. Pharmacopoeia National Formulary Biotechnology-Derived Articles—Capillary Electrophoresis <1053>; United States Pharmacopoeial Convention: Rockville, MD, USA, 2012.
- Damen, C.W.N.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H. Quantitative aspects of the analysis of the monoclonal antibody using high-performance liquid chromatography coupled with electrospray mass spectrometry. J. Pharm. Biomed. Anal 2008, 46, 449–455. [Google Scholar]
- Maple, L.; Lathrop, R.; Bozich, S.; Harman, W.; Tacey, R.; Kelley, M.; Danilkovitch-Miagkova, A. Development and validation of ELISA for Herceptin detection in human serum. J. Immunol. Methods 2004, 295, 169–182. [Google Scholar]
- Jamieson, D.; Cresti, N.; Verril, M.W.; Boddy, A.V. Development and validation of cell-based ELISA for the quantification of trastuzumab in human plasma. J. Immunol. Methods 2009, 345, 106–111. [Google Scholar]
- Jenkins, N. Modifications of therapeutic proteins: Challenges and prospects. Cytotechnology 2007, 53, 121–125. [Google Scholar]
- Wan, H.Z.; Kaneshiro, S.; Frenz, J.; Cacia, J. Rapid method for monitoring galactosylation levels during recombinant antibody production by electrospray mass spectrometry with selective-ion monitoring. J. Chromatogr. A 2001, 913, 437–446. [Google Scholar]
- Gennaro, L.A.; Salas-Solano, O. On-line CE-LIF-MS technology for the direct characterization of N-linked glycans from therapeutic antibodies. Anal. Chem 2008, 80, 3838–3845. [Google Scholar]
- Ma, S.; Nashabeh, W. Carbohydrate analysis of a chimeric recombinant monoclonal antibody by capillary electrophoresis with laser-induced fluorescence detection. Anal. Chem 1999, 71, 5185–5192. [Google Scholar]
- Nakano, M.; Higo, D.; Arai, E.; Nakagawa, T.; Kakehi, K.; Taniguchi, N.; Kondo, A. Capillary electrophoresis-electrospray ionization mass spectrometry for rapid and sensitive N-glycan analysis of glycoproteins as 9-fluorenylmethyl derivatives. Glycobiology 2009, 19, 135–143. [Google Scholar]
- Stadlmann, J.; Pabst, M.; Kolarich, D.; Kunert, R.; Altmann, F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics 2008, 8, 2858–2871. [Google Scholar]
- Yu, Y.Q.; Ahn, J.; Gilar, M. Application Note: Trastuzumab Glycan Batch-to-Batch Profiling Using a UPLC/FLR/Mass Spectrometry Platform; Waters Corporation: Milford, MA, USA. 10 September 2013.
- Harris, R.J. Processing of C-terminal lysine and arginine residues of proteins isolated from mammalian cell culture. J. Chromatogr. A 1995, 705, 129–134. [Google Scholar]
- Cleland, J.L.; Lam, X.; Kendrick, B.; Yang, J.; Yang, T.H.; Overcashier, D.; Brooks, D.; Hsu, C.; Carpenter, J.F. A specific molar ratio of stabilizer to protein is required for storage stability of a lyophylised monoclonal antibody. J. Pharm. Sci 2001, 90, 310–321. [Google Scholar]
- Kaiser, J.; Kramer, I. Physiochemical stability of diluted trastuzumab infusion solutions in polypropylene infusion bags. Int. J. Pharm. Compd 2011, 15, 515–520. [Google Scholar]
- Pabari, R.M.; Ryan, B.; McCarthy, C.; Ramtoola, Z. Effect of microencapsulation shear stress on the structural integrity and biological activity of a model monoclonal antibody, trastuzumab. Pharmaceutics 2011, 3, 510–524. [Google Scholar]
- Demeule, B.; Palais, C.; Machaidze, G.; Gurny, R.; Arvinte, T. New methods allowing the detection of protein aggregates: A case study on trastuzumab. MAbs 2009, 1, 142–150. [Google Scholar]
- Peters, B.J.; Capelle, M.A.; Arvinte, T.; van de Garde, E.M. Validation of an automated method for compounding monoclonal antibody patient doses: Case studies of Avastin (bevacizumab), Remicade (infliximab) and Herceptin (trastuzumab). MAbs 2013, 5, 162–170. [Google Scholar]
- Arvinte, T.; Palais, C.; Green-Trexler, E.; Gregory, S.; Mach, H.; Narasimhan, C.; Shameem, M. Aggregation of biopharmaceuticals in human plasma and human serum: Implications for drug research and development. MAbs 2013, 5, 491–500. [Google Scholar]
- Demeule, B.; Lawrence, M.J.; Drake, A.F.; Gurny, R.; Arvinte, T. Characterization of protein aggregation: The case of a therapeutic immunoglobulin. Biochim. Biophys. Acta 2007, 1774, 146–153. [Google Scholar]
- Rabilloud, T. Two-dimensional gel electrophoresis in proteomics: Old, old fashioned, but it still climbs up the mountains. Proteomics 2002, 2, 3–10. [Google Scholar]
- Rabilloud, T.; Chevallet, M.; Luche, S.; Lelong, C. Two-dimensional gel electrophoresis in proteomics: Past, present and future. J. Proteomics 2010, 73, 2064–2077. [Google Scholar] [Green Version]
- Thiede, B.; Höhenwarter, W.; Krah, A.; Mattow, J.; Schmi, M.; Schmidt, F.; Jungblut, F.R. Peptide mass fingerprinting. Methods 2005, 35, 237–247. [Google Scholar]
- Q5C: Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products, 1995. Available online: http://www.ich.org (accessed on 20 September 2013).
- Wang, W.; Singh, S.; Zeng, D.L.; King, K.; Nema, S. Antibody structure, instability, and formulation. J. Pharm. Sci 2007, 96, 1–26. [Google Scholar]
- Falconar, R. Therapeutic protein stability and formulation. In Biopharmaceutical Production Technology; Subramanian, G., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Berkshire, UK, 2012; pp. 165–183. [Google Scholar]
- Kamoda, S.; Ishikawa, R.; Kakehi, K. Capillary electrophoresis with laser-induced fluorescence detection for detailed studies on N-linked oligosaccharide profile of therapeutic recombinant monoclonal antibodies. J. Chromatogr. A 2006, 1133, 332–339. [Google Scholar]
- Rabilloud, T.; Valette, C.; Lawrence, J.J. Sample application by in-gel rehydration improves the resolution of two-dimensional electrophoresis with immobilized pH gradients in the first dimension. Electrophoresis 1994, 15, 1552–1558. [Google Scholar]
- Görg, A.; Postel, W.; Günther, S. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 1988, 9, 531–546. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature 1970, 227, 680–685. [Google Scholar]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant-proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nebija, D.; Noe, C.R.; Urban, E.; Lachmann, B. Quality Control and Stability Studies with the Monoclonal Antibody, Trastuzumab: Application of 1D- vs. 2D-Gel Electrophoresis. Int. J. Mol. Sci. 2014, 15, 6399-6411. https://doi.org/10.3390/ijms15046399
Nebija D, Noe CR, Urban E, Lachmann B. Quality Control and Stability Studies with the Monoclonal Antibody, Trastuzumab: Application of 1D- vs. 2D-Gel Electrophoresis. International Journal of Molecular Sciences. 2014; 15(4):6399-6411. https://doi.org/10.3390/ijms15046399
Chicago/Turabian StyleNebija, Dashnor, Christian R. Noe, Ernst Urban, and Bodo Lachmann. 2014. "Quality Control and Stability Studies with the Monoclonal Antibody, Trastuzumab: Application of 1D- vs. 2D-Gel Electrophoresis" International Journal of Molecular Sciences 15, no. 4: 6399-6411. https://doi.org/10.3390/ijms15046399
APA StyleNebija, D., Noe, C. R., Urban, E., & Lachmann, B. (2014). Quality Control and Stability Studies with the Monoclonal Antibody, Trastuzumab: Application of 1D- vs. 2D-Gel Electrophoresis. International Journal of Molecular Sciences, 15(4), 6399-6411. https://doi.org/10.3390/ijms15046399



