Lunasin Sensitivity in Non-Small Cell Lung Cancer Cells Is Linked to Suppression of Integrin Signaling and Changes in Histone Acetylation
Abstract
:1. Introduction
2. Results
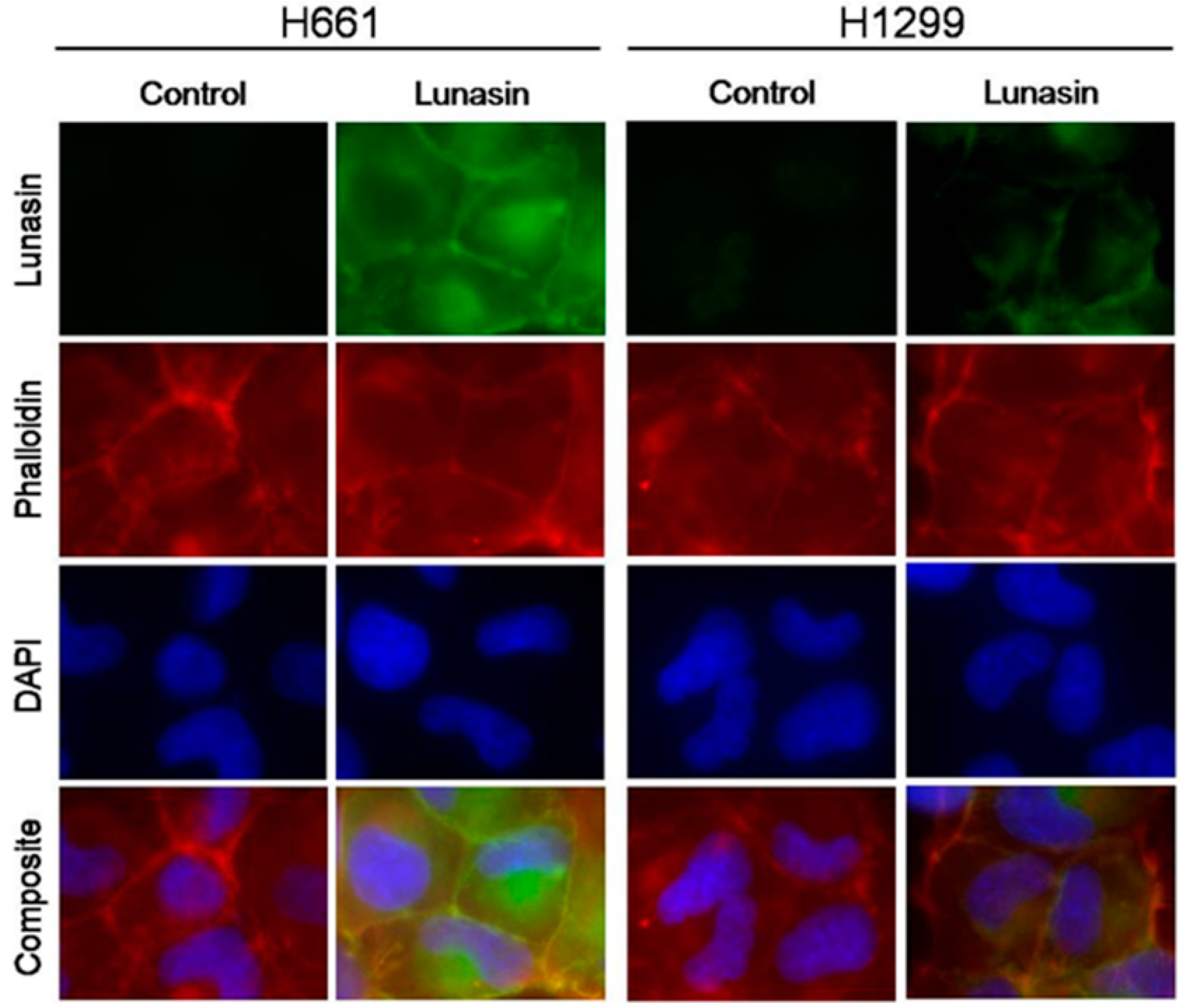
2.1. Lunasin Sensitivity Is Associated with Increased Lunasin Uptake
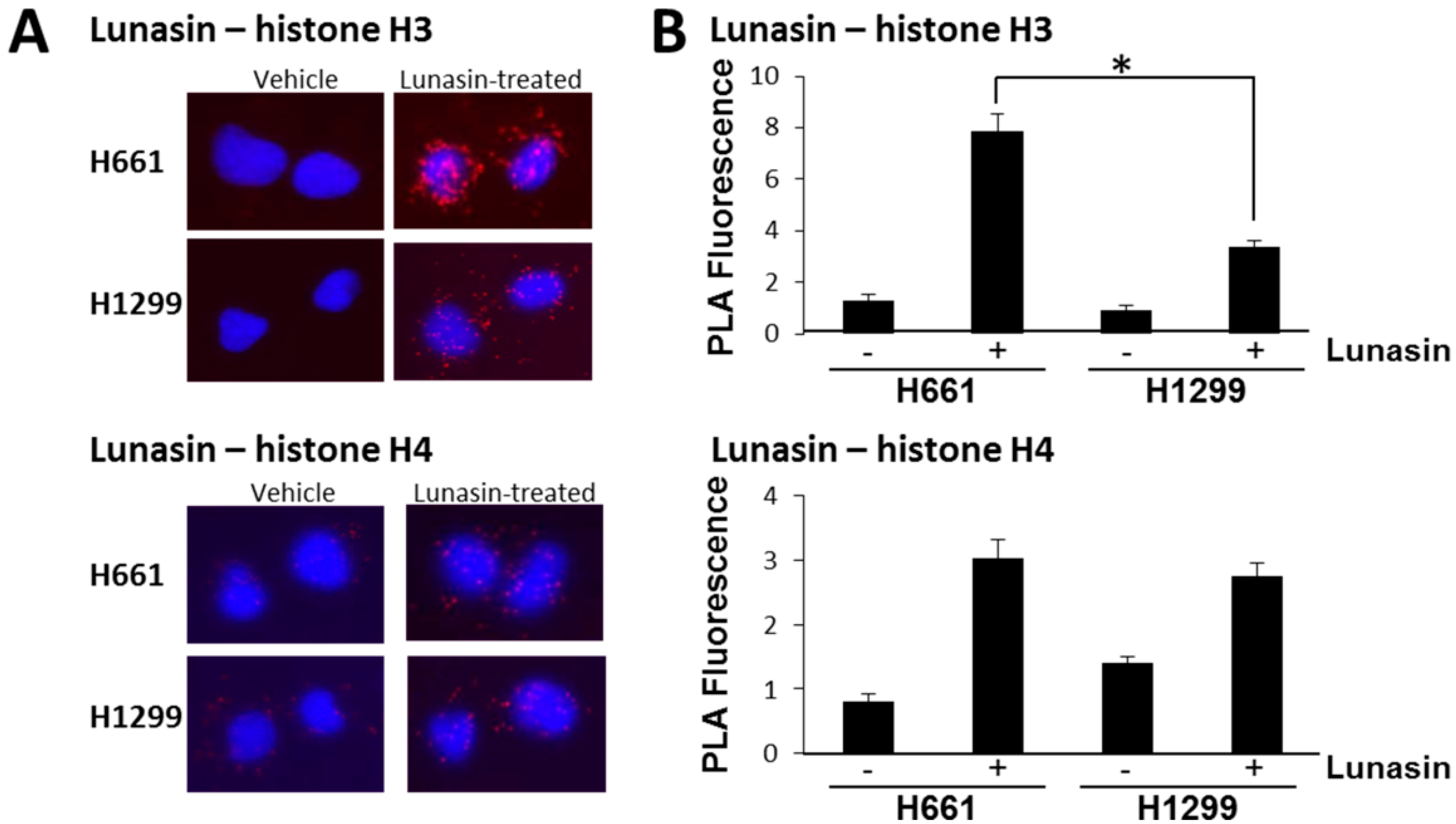
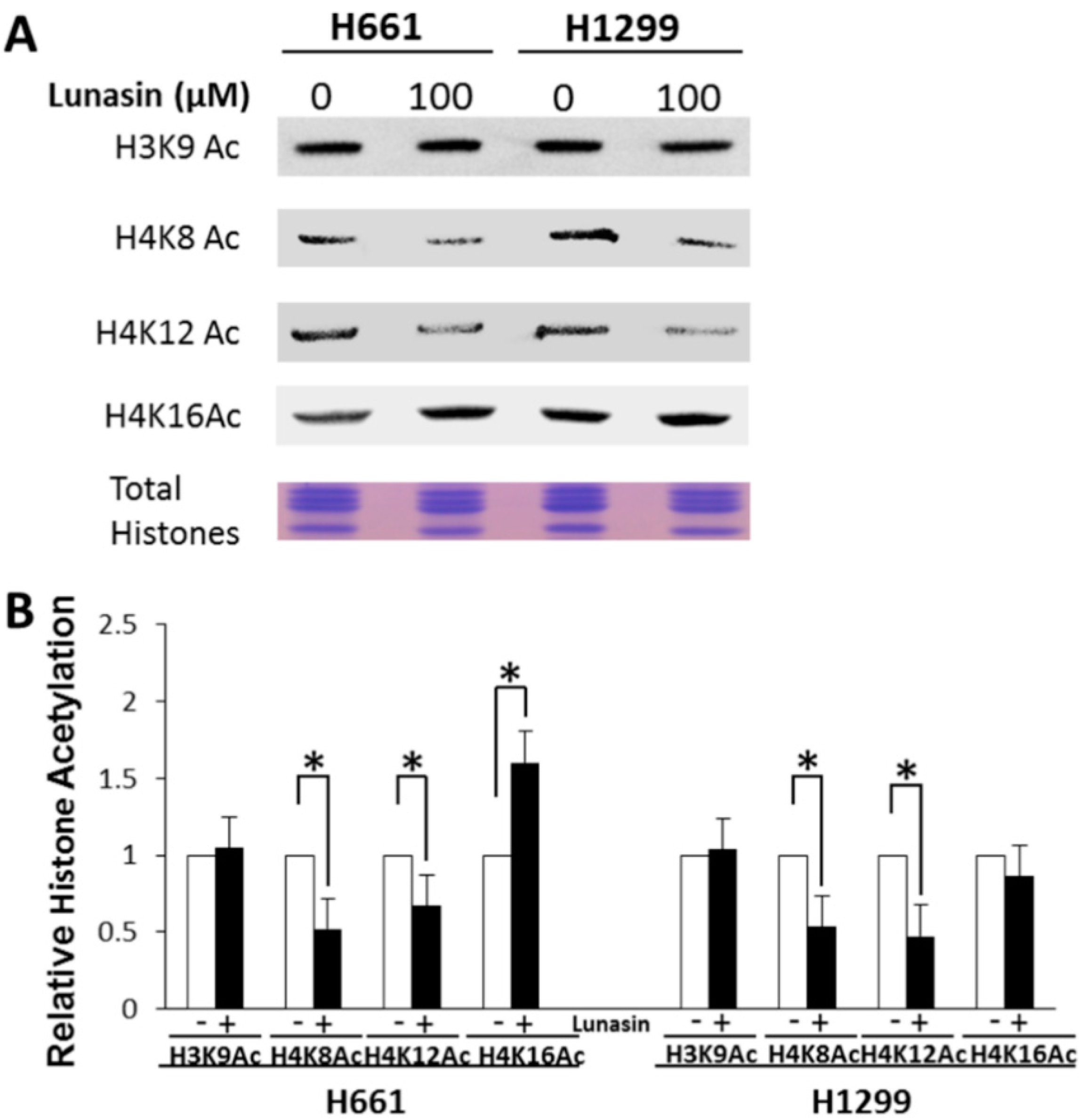
2.2. Lunasin Binds Histones in Situ and Affects Histone Acetylation
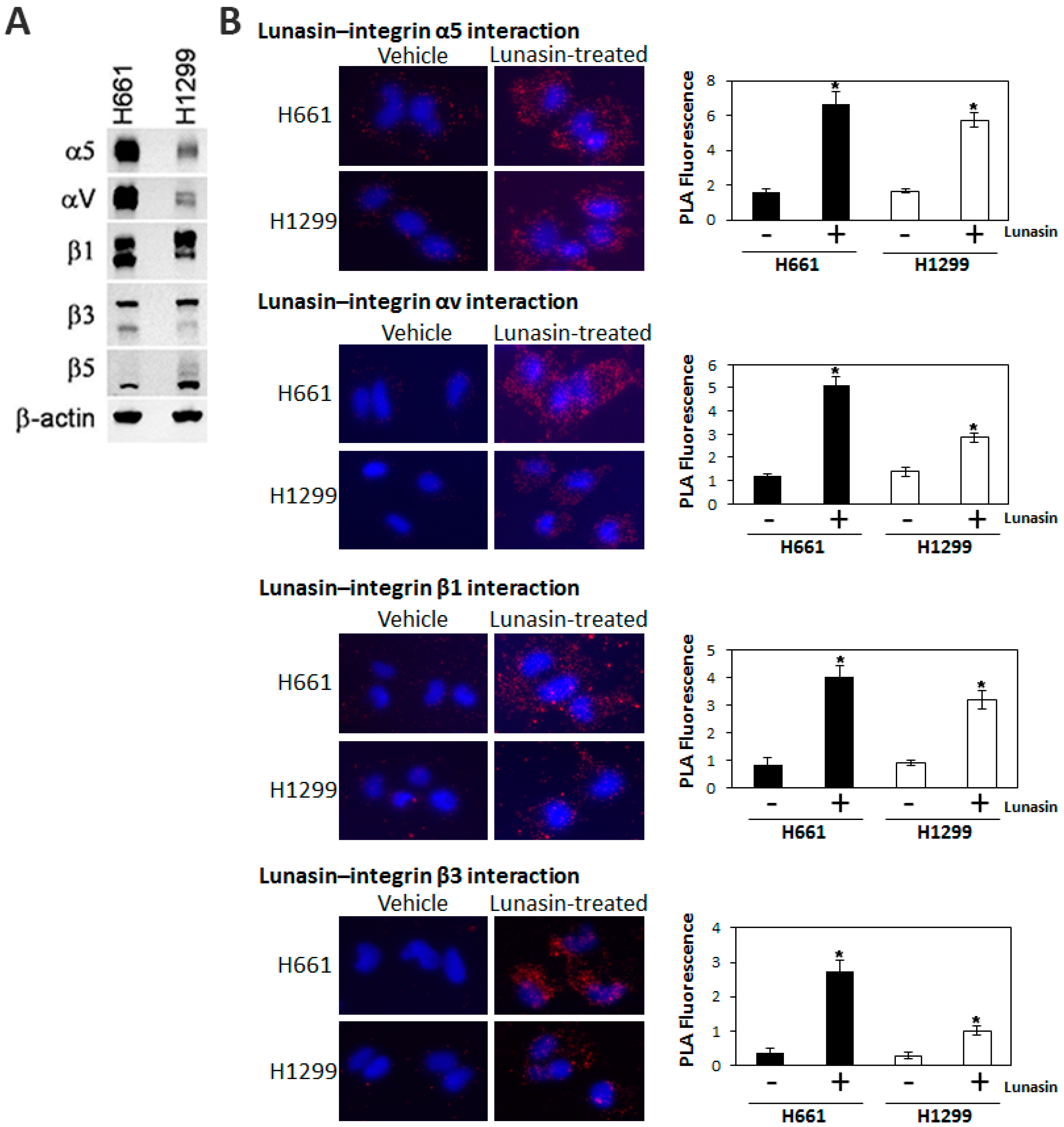
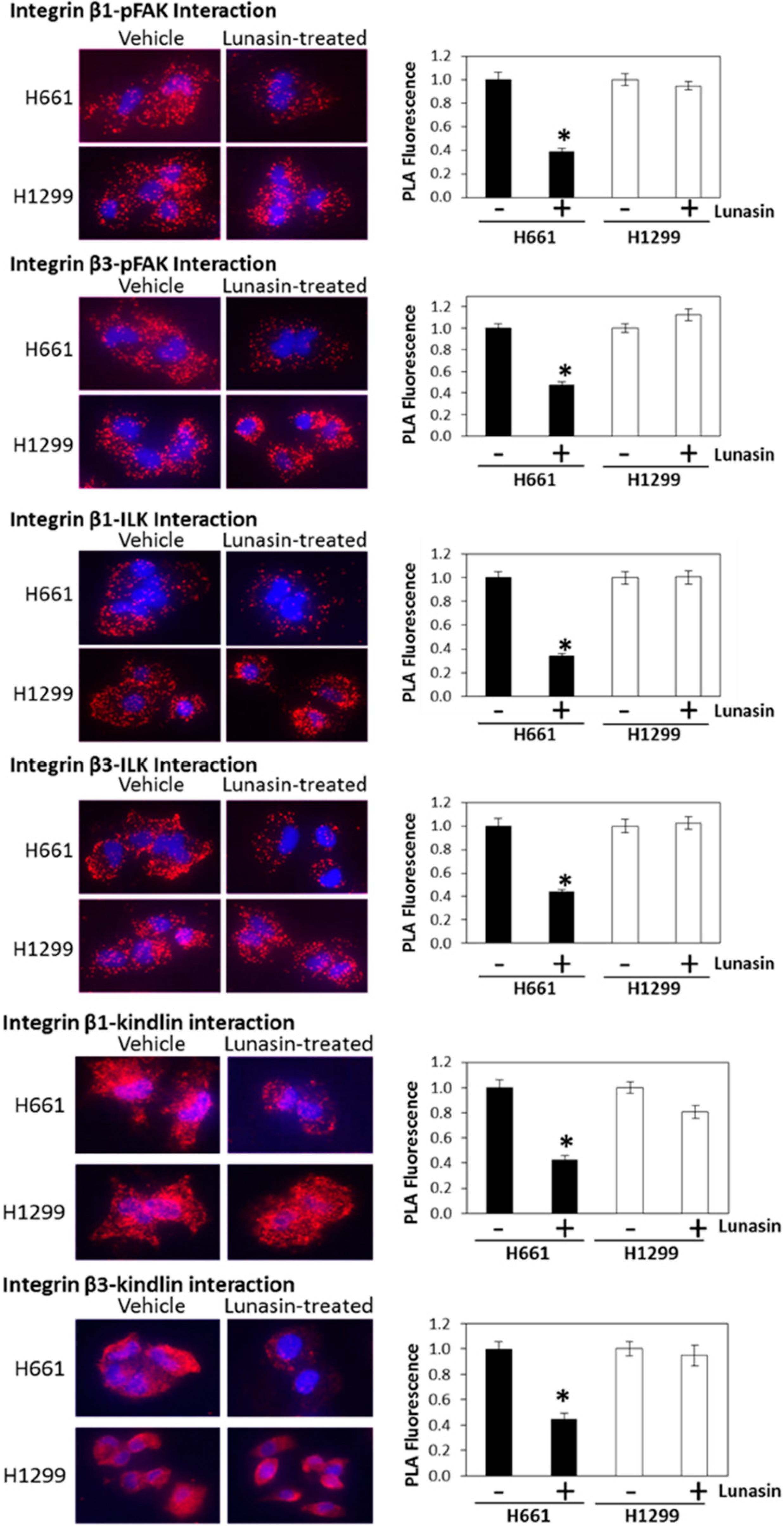
2.3. Interaction of Lunasin with Specific Integrin Subunits
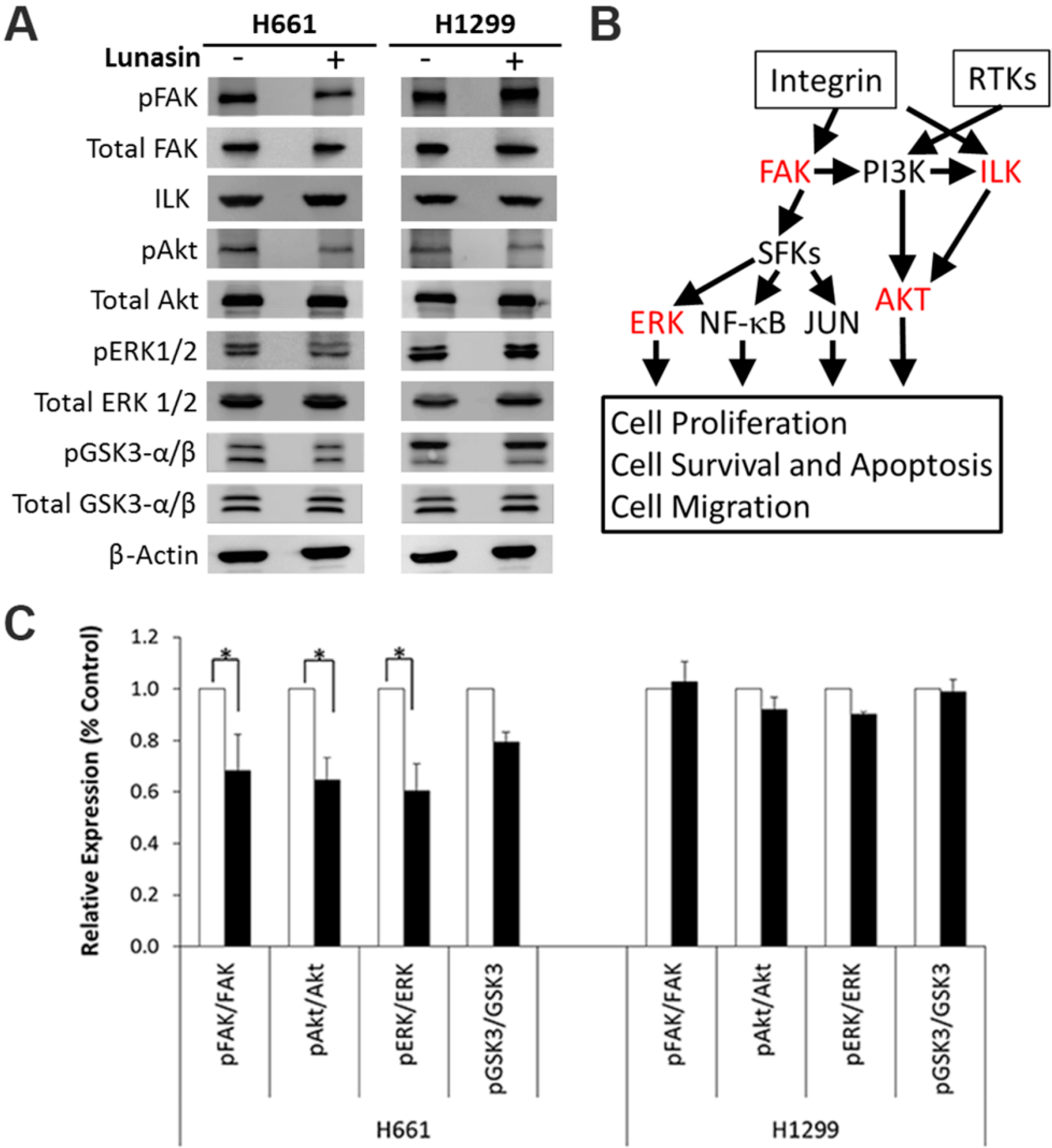
2.4. Lunasin Disrupts Integrin Signaling
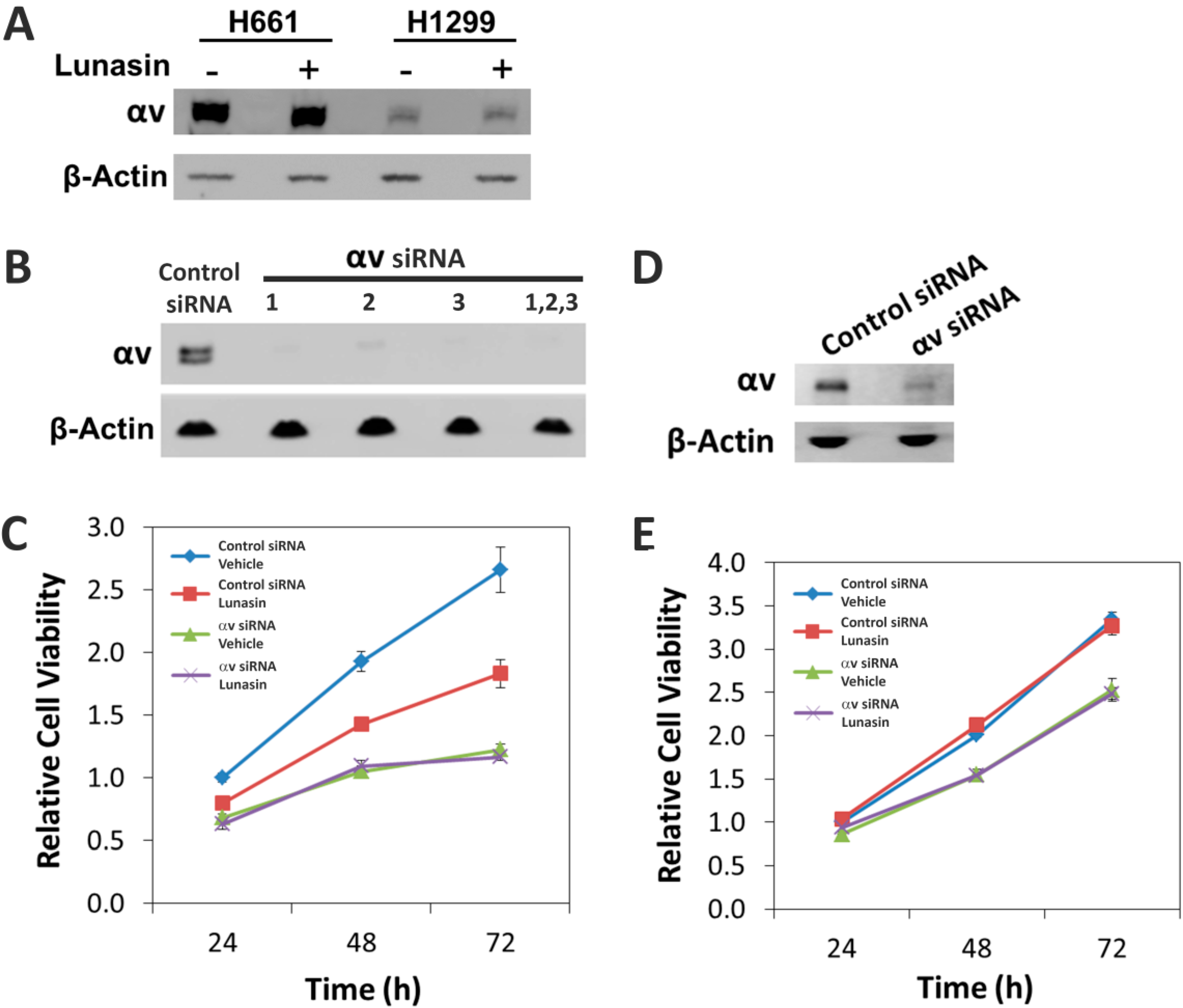
2.5. The Alpha-v Integrin Subunit Is Required for NSCLC H661 Cell Proliferation
3. Discussion
4. Experimental Section
4.1. Cell Lines
4.2. Reagents
4.3. Lunasin Uptake Immunocytochemistry
4.4. Histone Acetylation Analyses
4.5. In Situ Proximity Ligation Assays (PLA)
4.6. Integrin Subunit Analysis by Immunoblotting
4.7. Integrin Signaling Immunoblot Analyses
4.8. SiRNA-Mediated Knockdown of Integrin αv
4.9. Proliferation Assays
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Lumen, B.O. Lunasin: A novel cancer preventive seed peptide that modifies chromatin. J. AOAC Int. 2008, 91, 932–935. [Google Scholar] [PubMed]
- De Mejia, E.G.; Dia, V.P. The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metastasis Rev. 2010, 29, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Chemopreventive properties of Peptide Lunasin: A review. Protein Pept. Lett. 2013, 20, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Seber, L.E.; Barnett, B.W.; McConnell, E.J.; Hume, S.D.; Cai, J.; Boles, K.; Davis, K.R. Scalable purification and characterization of the anticancer lunasin peptide from soybean. PLoS One 2012, 7, e35409. [Google Scholar] [CrossRef] [PubMed]
- Odani, S.; Koide, T.; Ono, T. Amino acid sequence of a soybean (Glycine max) seed polypeptide having a poly(l-aspartic acid) structure. J. Biol. Chem. 1987, 262, 10502–10505. [Google Scholar] [PubMed]
- Hernandez-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Lunasin, a novel seed peptide for cancer prevention. Peptides 2009, 30, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Galvez, A.F.; Chen, N.; Macasieb, J.; de Lumen, B.O. Chemopreventive property of a soybean peptide (lunasin) that binds to deacetylated histones and inhibits acetylation. Cancer Res. 2001, 61, 7473–7478. [Google Scholar] [PubMed]
- Jeong, H.J.; Lam, Y.; de Lumen, B.O. Barley lunasin suppresses ras-induced colony formation and inhibits core histone acetylation in mammalian cells. J. Agric. Food Chem. 2002, 50, 5903–5908. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.; Galvez, A.; de Lumen, B.O. Lunasin suppresses E1A-mediated transformation of mammalian cells but does not inhibit growth of immortalized and established cancer cell lines. Nutr. Cancer 2003, 47, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hernandez-Ledesma, B.; de Lumen, B.O. Lunasin, a novel seed peptide, sensitizes human breast cancer MDA-MB-231 cells to aspirin-arrested cell cycle and induced apoptosis. Chem. Biol. Interact. 2010, 186, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hernandez-Ledesma, B.; Jeong, H.J.; Park, J.H.; de Lumen, B.O. Complementary roles in cancer prevention: Protease inhibitor makes the cancer preventive peptide lunasin bioavailable. PLoS One 2010, 5, e8890. [Google Scholar] [CrossRef] [PubMed]
- De Mejia, E.G.; Wang, W.; Dia, V.P. Lunasin, with an arginine-glycine-aspartic acid motif, causes apoptosis to L1210 leukemia cells by activation of caspase-3. Mol. Nutr. Food Res. 2010, 54, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Mejia, E.G. Lunasin promotes apoptosis in human colon cancer cells by mitochondrial pathway activation and induction of nuclear clusterin expression. Cancer Lett. 2010, 295, 44–53. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.J.; Devapatia, B.; Yaddanapudi, K.; Davis, K.R. The soybean-derived peptide lunasin inhibits non-small cell lung cancer cell proliferation by suppressing phosphorylation of the retinoblastoma protein. 2014; in review. [Google Scholar]
- Cam, A.; Sivaguru, M.; Gonzalez de Mejia, E. Endocytic mechanism of internalization of dietary peptide lunasin into macrophages in inflammatory condition associated with cardiovascular disease. PLoS One 2013, 8, e72115. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Jeong, J.B.; Kim, D.S.; de Lumen, B.O. Inhibition of core histone acetylation by the cancer preventive peptide lunasin. J. Agric. Food Chem. 2007, 55, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Jeong, J.B.; Kim, D.S.; Park, J.H.; Lee, J.B.; Kweon, D.H.; Chung, G.Y.; Seo, E.W.; de Lumen, B.O. The cancer preventive peptide lunasin from wheat inhibits core histone acetylation. Cancer Lett. 2007, 255, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Jeong, H.J.; Park, J.H.; Lee, S.H.; Lee, J.R.; Lee, H.K.; Chung, G.Y.; Choi, J.D.; de Lumen, B.O. Cancer-preventive peptide lunasin from Solanum nigrum L. inhibits acetylation of core histones H3 and H4 and phosphorylation of retinoblastoma protein (Rb). J. Agric. Food Chem. 2007, 55, 10707–10713. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Relationship between lunasin’s sequence and its inhibitory activity of histones H3 and H4 acetylation. Mol. Nutr. Food Res. 2011, 55, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ledesmaa, B.; Hsiehb, C.C.; de Lumenb, B.O. Lunasin, a seed peptide with promising cancer preventive properties. Protein Pept. Lett. 2012, 4, 424–432. [Google Scholar]
- Galvez, A.F.; Huang, L.; Magbanua, M.M.; Dawson, K.; Rodriguez, R.L. Differential expression of thrombospondin (THBS1) in tumorigenic and nontumorigenic prostate epithelial cells in response to a chromatin-binding soy peptide. Nutr. Cancer 2011, 63, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Pabona, J.M.; Dave, B.; Su, Y.; Montales, M.T.; de Lumen, B.O.; de Mejia, E.G.; Rahal, O.M.; Simmen, R.C. The soybean peptide lunasin promotes apoptosis of mammary epithelial cells via induction of tumor suppressor PTEN: Similarities and distinct actions from soy isoflavone genistein. Genes Nutr. 2013, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- De Mejia, E.G.; Dia, V.P. Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-kappaB pathway in the macrophage. Peptides 2009, 30, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Cam, A.; de Mejia, E.G. RGD-peptide lunasin inhibits Akt-mediated NF-κB activation in human macrophages through interaction with the αVβ3 integrin. Mol. Nutr. Food Res. 2012, 56, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; de Mejia, E.G. Differential gene expression of RAW 264.7 macrophages in response to the RGD peptide lunasin with and without lipopolysaccharide stimulation. Peptides 2011, 32, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- De Mejia, E.; de Lumen, B. Soybean bioactive peptides: A new horizon in preventing chronic diseases. Sex. Reprod. Menop. 2006, 4, 91–95. [Google Scholar] [CrossRef]
- Dia, V.P.; Gonzalez de Mejia, E. Lunasin potentiates the effect of oxaliplatin preventing outgrowth of colon cancer metastasis, binds to alpha5beta1 integrin and suppresses FAK/ERK/NF-kappaB signaling. Cancer Lett. 2011, 313, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Weibrecht, I.; Leuchowius, K.J.; Clausson, C.M.; Conze, T.; Jarvius, M.; Howell, W.M.; Kamali-Moghaddam, M.; Soderberg, O. Proximity ligation assays: A recent addition to the proteomics toolbox. Expert Rev. Proteomics 2010, 7, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S. Lunasin: Attenuating effects on tumour growth in systemic malignancies. Food Chem. 2014, 150. [Google Scholar] [CrossRef] [PubMed]
- Elsasser, S.J.; D’Arcy, S. Towards a mechanism for histone chaperones. Biochim. Biophys. Acta 2012, 1819, 211–221. [Google Scholar] [CrossRef]
- Parthun, M.R. Histone acetyltransferase 1: More than just an enzyme? Biochim. Biophys. Acta 2012, 1819, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.A.; Kuo, Y.M.; Andrews, A.J. Differences in specificity and selectivity between CBP and p300 acetylation of histone H3 and H3/H4. Biochemistry 2013, 52, 5746–5759. [Google Scholar] [CrossRef] [PubMed]
- Rothbart, S.B.; Strahl, B.D. Interpreting the language of histone and DNA modifications. Biochim. Biophys. Acta 2014, 1839, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.J.; Shah, J.S.; Cole, A.J. Histones and their modifications in ovarian cancer-drivers of disease and therapeutic targets. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef]
- Chang, C.S.; Pillus, L. Collaboration between the essential Esa1 acetyltransferase and the Rpd3 deacetylase is mediated by H4K12 histone acetylation in Saccharomyces cerevisiae. Genetics 2009, 183, 149–160. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, G.; Rickards, B.; Flint, S.J. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell 2008, 30, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, S.E.; Green, A.R.; Rakha, E.A.; Powe, D.G.; Ahmed, R.A.; Collins, H.M.; Soria, D.; Garibaldi, J.M.; Paish, C.E.; Ammar, A.A.; et al. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res. 2009, 69, 3802–3809. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.V.; Carrer, A.; Shah, S.; Snyder, N.W.; Wei, S.; Venneti, S.; Worth, A.J.; Yuan, Z.F.; Lim, H.W.; Liu, S.; et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell MeTable 2014, 20, 306–319. [Google Scholar] [CrossRef]
- Morse, E.M.; Brahme, N.N.; Calderwood, D.A. Integrin cytoplasmic tail interactions. Biochemistry 2014, 53, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, E. Signalling complexes at the cell-matrix interface. Curr. Opin. Struct. Biol. 2014, 29, 10–16. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.L.; Picard, M. Integrins as therapeutic targets. Trends Pharmacol. Sci. 2012, 33, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Paolillo, M.; Sanchez-Hernandez, Y.; Curti, D.; Ciusani, E.; Serra, M.; Colombo, L.; Schinelli, S. A small-molecule RGD-integrin antagonist inhibits cell adhesion, cell migration and induces anoikis in glioblastoma cells. Int. J. Oncol. 2013, 42, 83–92. [Google Scholar] [PubMed]
- Zeng, Z.Z.; Jia, Y.; Hahn, N.J.; Markwart, S.M.; Rockwood, K.F.; Livant, D.L. Role of focal adhesion kinase and phosphatidylinositol 3'-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res. 2006, 66, 8091–8099. [Google Scholar] [CrossRef] [PubMed]
- Hersey, P.; Sosman, J.; O’Day, S.; Richards, J.; Bedikian, A.; Gonzalez, R.; Sharfman, W.; Weber, R.; Logan, T.; Buzoianu, M.; et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin αvβ3, ±dacarbazine in patients with stage IV metastatic melanoma. Cancer 2010, 116, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Bell-McGuinn, K.M.; Matthews, C.M.; Ho, S.N.; Barve, M.; Gilbert, L.; Penson, R.T.; Lengyel, E.; Palaparthy, R.; Gilder, K.; Vassos, A.; et al. A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol. Oncol. 2011, 121, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Park, J.; Manning, C.; Goehlmann, H.W.; Marshall, D.J. Metastatic signature in lung cancer is associated with sensitivity to anti-integrin αV monoclonal antibody intetumumab. Genes Chromosom. Cancer 2014, 53, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Morello, V.; Cabodi, S.; Sigismund, S.; Camacho-Leal, M.P.; Repetto, D.; Volante, M.; Papotti, M.; Turco, E.; Defilippi, P. Beta1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells. Oncogene 2011, 30, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Stampolidis, P.; Ullrich, A.; Iacobelli, S. LGALS3BP, lectin galactoside-binding soluble 3 binding protein, promotes oncogenic cellular events impeded by antibody intervention. Oncogene 2013. [Google Scholar] [CrossRef]
- Knowles, L.M.; Gurski, L.A.; Engel, C.; Gnarra, J.R.; Maranchie, J.K.; Pilch, J. Integrin αvβ3 and fibronectin upregulate Slug in cancer cells to promote clot invasion and metastasis. Cancer Res. 2013, 73, 6175–6184. [Google Scholar] [CrossRef] [PubMed]
- Katoh, D.; Nagaharu, K.; Shimojo, N.; Hanamura, N.; Yamashita, M.; Kozuka, Y.; Imanaka-Yoshida, K.; Yoshida, T. Binding of αvβ1 and αvβ6 integrins to tenascin-C induces epithelial-mesenchymal transition-like change of breast cancer cells. Oncogenesis 2013, 2. [Google Scholar] [CrossRef]
- Shechter, D.; Dormann, H.L.; Allis, C.D.; Hake, S.B. Extraction, purification and analysis of histones. Nat. Protoc. 2007, 2, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inaba, J.; McConnell, E.J.; Davis, K.R. Lunasin Sensitivity in Non-Small Cell Lung Cancer Cells Is Linked to Suppression of Integrin Signaling and Changes in Histone Acetylation. Int. J. Mol. Sci. 2014, 15, 23705-23724. https://doi.org/10.3390/ijms151223705
Inaba J, McConnell EJ, Davis KR. Lunasin Sensitivity in Non-Small Cell Lung Cancer Cells Is Linked to Suppression of Integrin Signaling and Changes in Histone Acetylation. International Journal of Molecular Sciences. 2014; 15(12):23705-23724. https://doi.org/10.3390/ijms151223705
Chicago/Turabian StyleInaba, Junichi, Elizabeth J. McConnell, and Keith R. Davis. 2014. "Lunasin Sensitivity in Non-Small Cell Lung Cancer Cells Is Linked to Suppression of Integrin Signaling and Changes in Histone Acetylation" International Journal of Molecular Sciences 15, no. 12: 23705-23724. https://doi.org/10.3390/ijms151223705
APA StyleInaba, J., McConnell, E. J., & Davis, K. R. (2014). Lunasin Sensitivity in Non-Small Cell Lung Cancer Cells Is Linked to Suppression of Integrin Signaling and Changes in Histone Acetylation. International Journal of Molecular Sciences, 15(12), 23705-23724. https://doi.org/10.3390/ijms151223705



