Surface Electrical Potentials of Root Cell Plasma Membranes: Implications for Ion Interactions, Rhizotoxicity, and Uptake
Abstract
:1. Introduction
2. Basic Theory of Gouy-Chapman-Stern (GCS) Model
2.1. Computation of the Cell-Surface Potential (ψ0) with a GCS Model
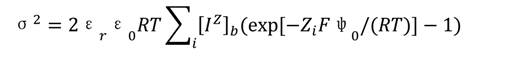
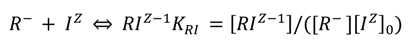
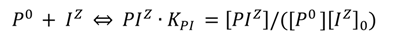
2.2. Data Analysis
3. Application of Cell-Surface Electrical Potential in Studies of Metal Toxicity
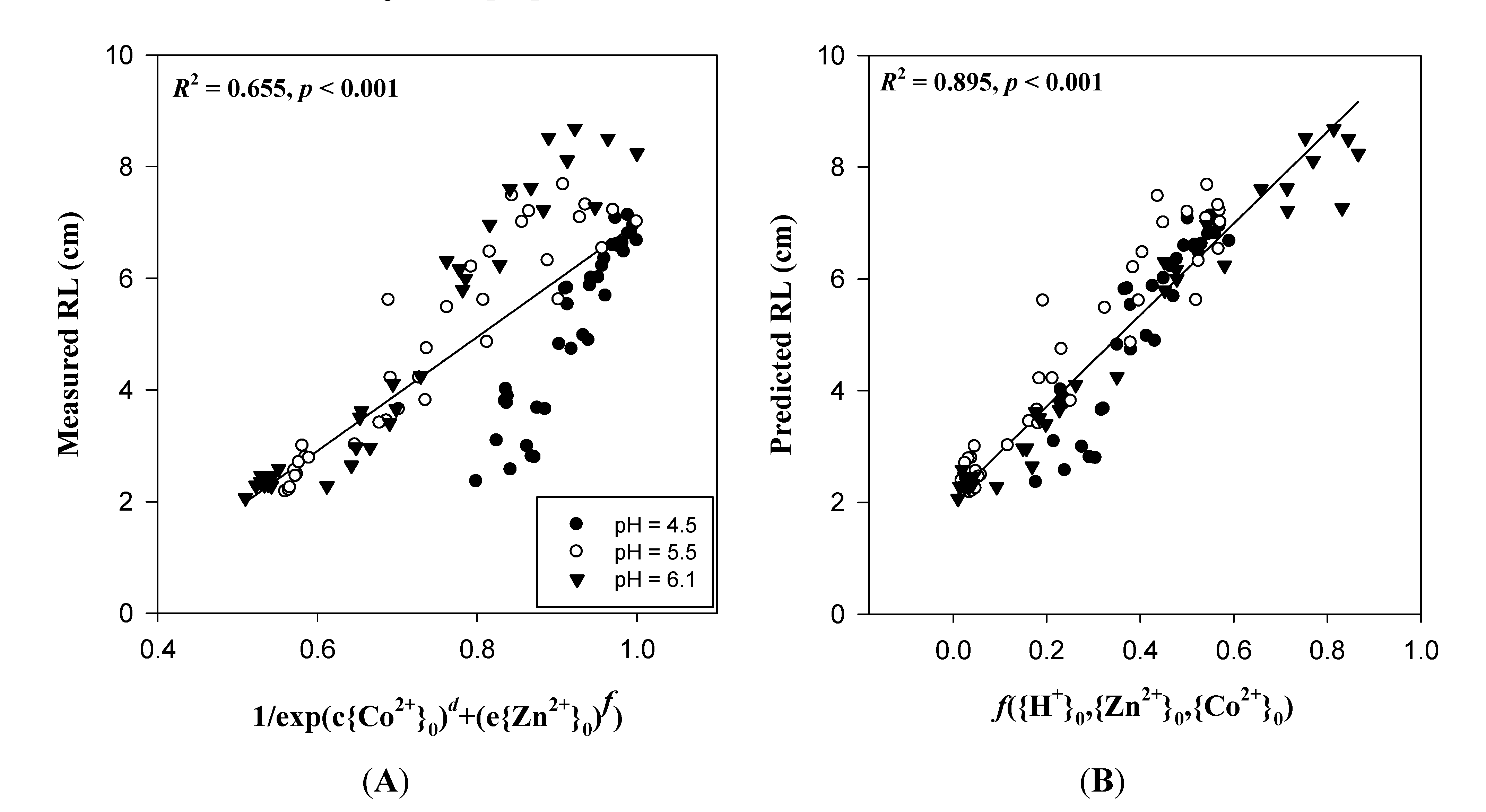
3.1. The Profile of Electrical Potential across the Cell Surface
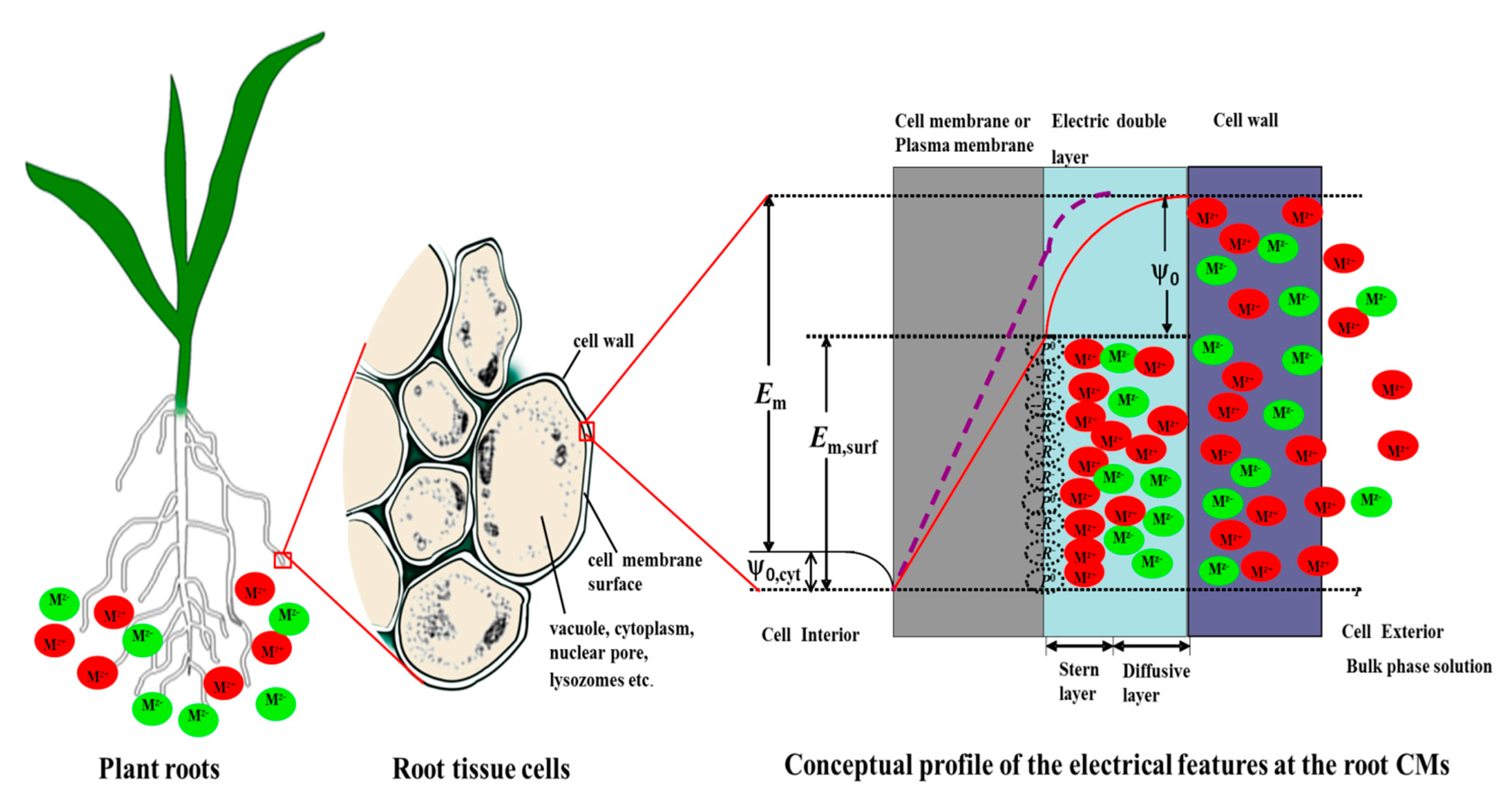
3.2. Verification of the Calculated Values of ψ0, and Some Parameter Values of the GCS Model
3.3. Cationic Toxicants
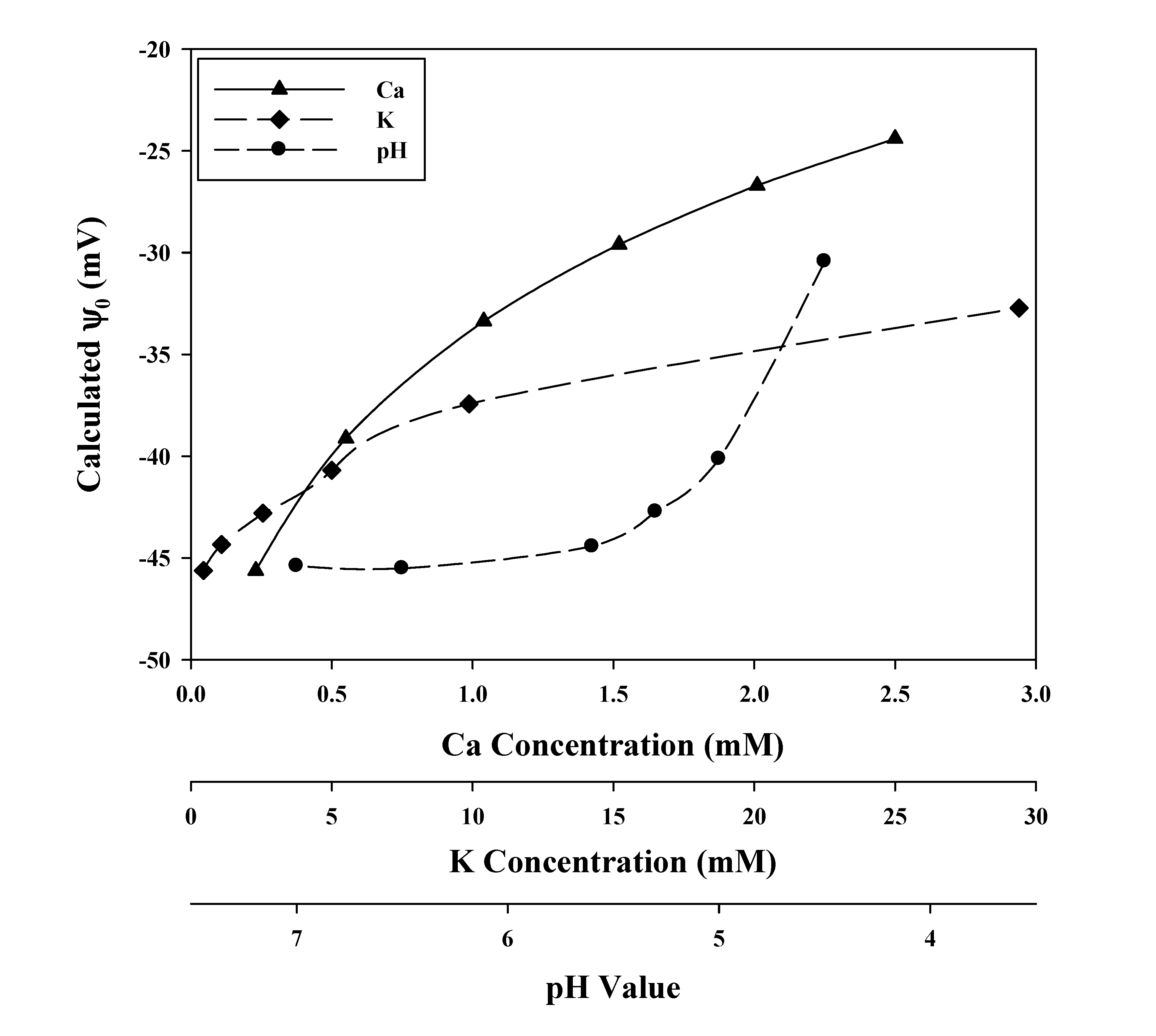
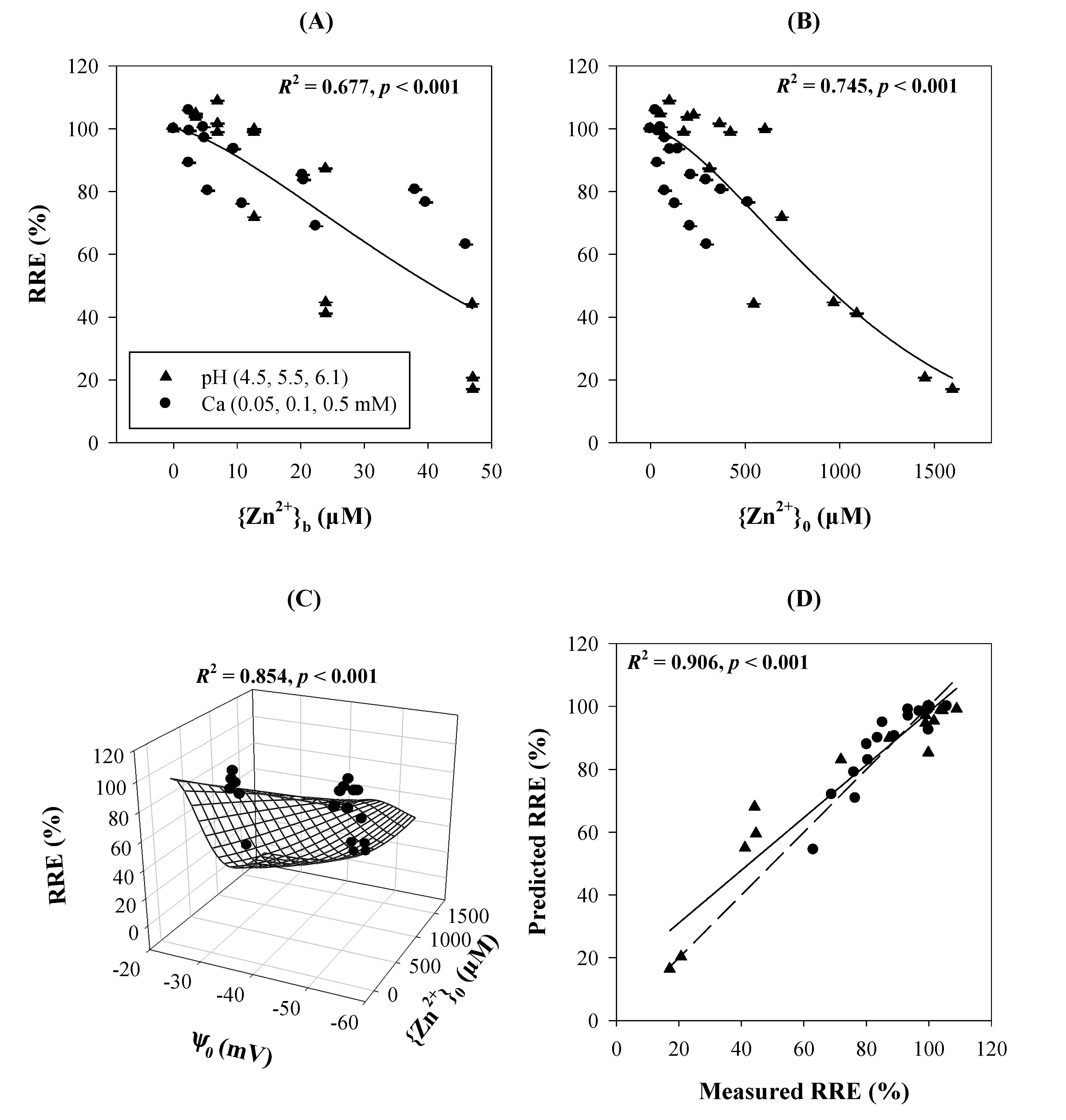

3.4. Anionic Toxicants
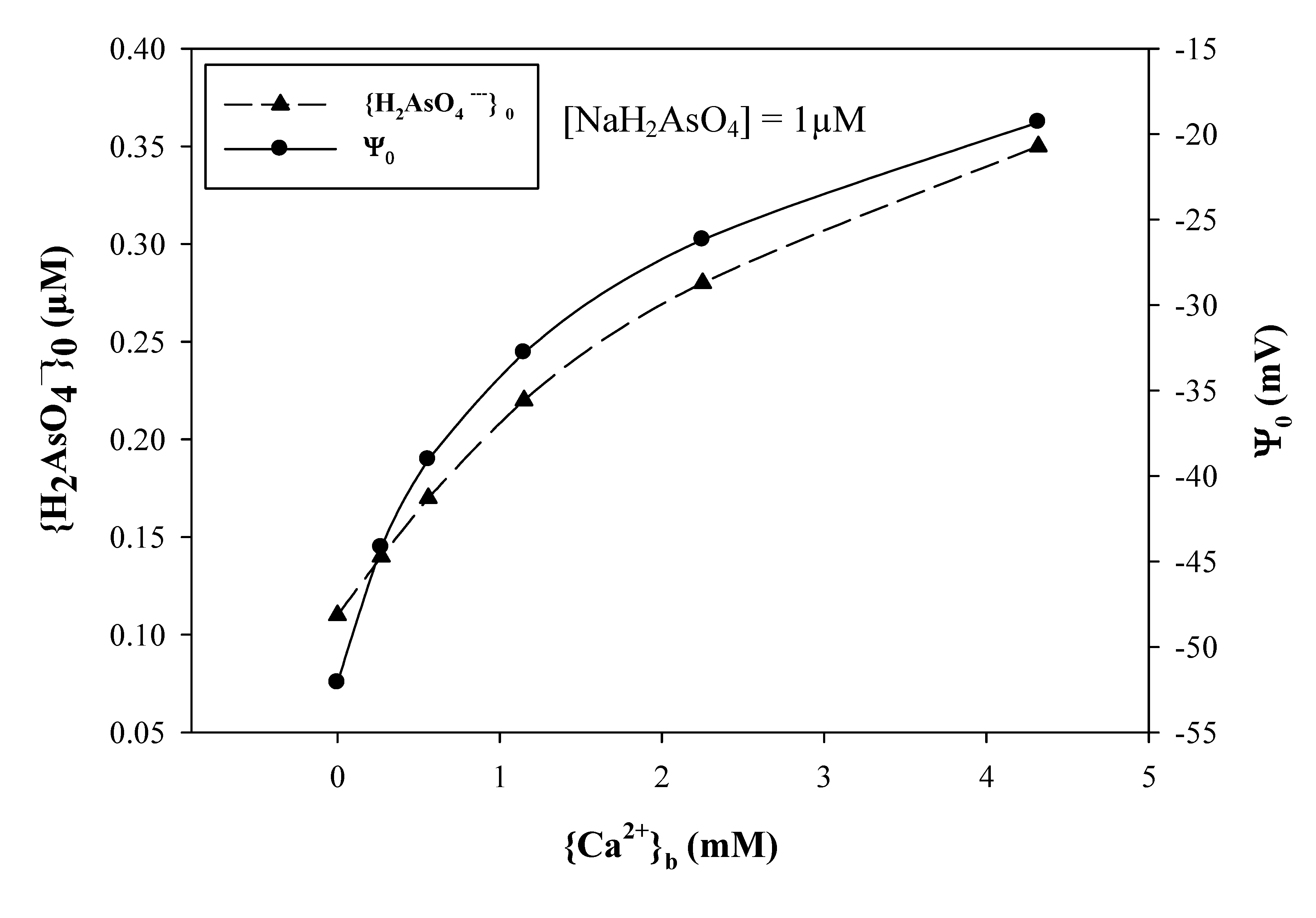
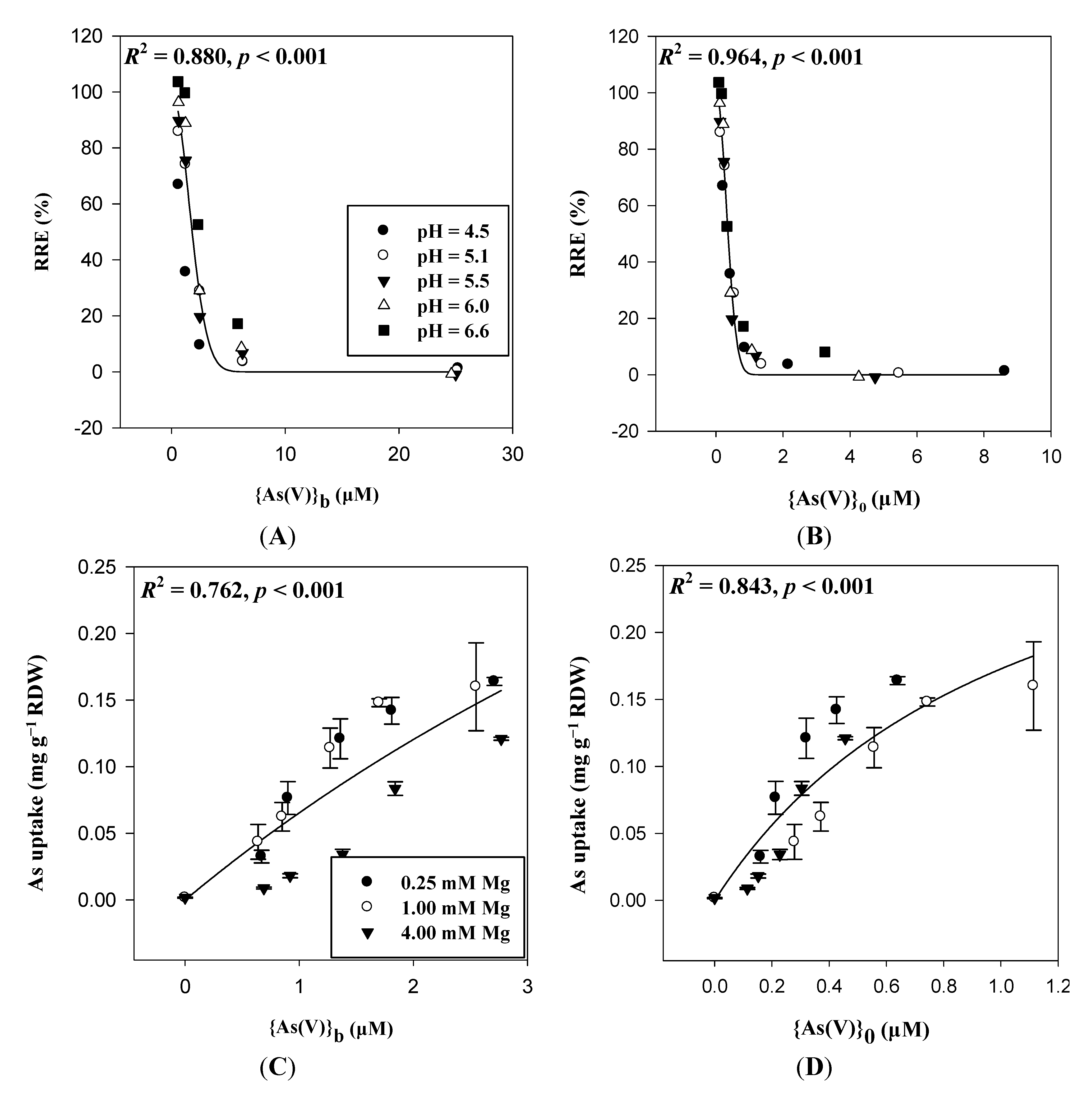
4. Ongoing Use of Electrostatic Principles in Risk Assessment and Modeling
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagajyoti, P.; Lee, K.; Sreekanth, T. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry,Bioavailability,and Risks of Metals; Springer: Berlin, Germany, 2001. [Google Scholar]
- Paquin, P.R.; Gorsuch, J.W.; Apte, S.; Batley, G.E.; Bowles, K.C.; Campbell, P.G.; Delos, C.G.; di Toro, D.M.; Dwyer, R.L.; Galvez, F. The biotic ligand model: A historical overview. Comp. Biochem. Physiol. Part C 2002, 133, 3–35. [Google Scholar]
- Di Toro, D.M.; Allen, H.E.; Bergman, H.L.; Meyer, J.S.; Paquin, P.R.; Santore, R.C. Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ. Toxicol. Chem. 2001, 20, 2383–2396. [Google Scholar]
- Lau, A.; McLaughlin, A.; McLaughlin, S. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. BBA Biomembr. 1981, 645, 279–292. [Google Scholar]
- McLaughlin, S. The electrostatic properties of membranes. Annu. Rev. Biophys. Biophys. Chem. 1989, 18, 113–136. [Google Scholar]
- Kinraide, T.B.; Yermiyahu, U.; Rytwo, G. Computation of surface electrical potentials of plant cell membranes correspondence to published ζ potentials from diverse plant sources. Plant Physiol. 1998, 118, 505–512. [Google Scholar]
- Kinraide, T.B.; Ryan, P.R.; Kochian, L.V. Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol. 1992, 99, 1461–1468. [Google Scholar]
- Kopittke, P.M.; Wang, P.; Menzies, N.W.; Naidu, R.; Kinraide, T.B. A web-accessible computer program for calculating electrical potentials and ion activities at cell-membrane surfaces. Plant Soil 2014, 375, 35–46. [Google Scholar]
- Kobayashi, Y.; Kobayashi, Y.; Watanabe, T.; Shaff, J.E.; Ohta, H.; Kochian, L.V.; Wagatsuma, T.; Kinraide, T.B.; Koyama, H. Molecular and physiological analysis of Al3+ and H+ rhizotoxicities at moderately acidic conditions. Plant Physiol. 2013, 163, 180–192. [Google Scholar]
- Kopittke, P.M.; Kinraide, T.B.; Wang, P.; Blamey, F.P.C.; Reichman, S.M.; Menzies, N.W. Alleviation of Cu and Pb rhizotoxicities in cowpea (Vigna unguiculata) as related to ion activities at root-cell plasma membrane surface. Environ. Sci. Technol. 2011, 45, 4966–4973. [Google Scholar]
- Wang, Y.-M.; Kinraide, T.B.; Wang, P.; Zhou, D.-M.; Hao, X.-Z. Modeling rhizotoxicity and uptake of Zn and Co singly and in binary mixture in wheat in terms of the cell membrane surface electrical potential. Environ. Sci. Technol. 2013, 47, 2831–2838. [Google Scholar]
- Wang, P.; Kinraide, T.B.; Zhou, D.; Kopittke, P.M.; Peijnenburg, W.J. Plasma membrane surface potential: Dual effects upon ion uptake and toxicity. Plant Physiol. 2011, 155, 808–820. [Google Scholar]
- Kinraide, T.B. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol. 1998, 118, 513–520. [Google Scholar]
- Kinraide, T.B. The controlling influence of cell-surface electrical potential on the uptake and toxicity of selenate (SeO42−). Physiol. Plant 2003, 117, 64–71. [Google Scholar]
- Wang, P.; Zhou, D.; Weng, N.; Wang, D.; Peijnenburg, W.J. Calcium and magnesium enhance arsenate rhizotoxicity and uptake in Triticum aestivum. Environ. Toxicol. Chem. 2011, 30, 1642–1648. [Google Scholar]
- Kinraide, T.B. Ion fluxes considered in terms of membrane-surface electrical potentials. Funct. Plant Biol. 2001, 28, 607–618. [Google Scholar]
- Hille, B. Ion Channels of Excitable Membranes; Sinauer Associates, Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Yermiyahu, U.; Kinraide, T.; Huang, P.; Gobran, G. Binding and electrostatic attraction of trace elements to plant root surfaces. In Biogeochemistry of Trace Elements in the Rhizosphere; Elsevier: Amsterdam, The Netherlands, 2005; pp. 365–389. [Google Scholar]
- Kinraide, T.B. Use of a Gouy-Chapman-Stern model for membrane-surface electrical potential to interpret some features of mineral rhizotoxicity. Plant Physiol. 1994, 106, 1583–1592. [Google Scholar]
- Yermiyahu, U.; Rytwo, G.; Brauer, D.; Kinraide, T. Binding and electrostatic attraction of lanthanum (La3+) and aluminum (Al3+) to wheat root plasma membranes. J. Membr. Biol. 1997, 159, 239–252. [Google Scholar]
- Wang, P.; Kinraide, T.B.; Smolders, E.; Zhou, D.-M.; Menzies, N.W.; Thakali, S.; Xia, W.-W.; Hao, X.-Z.; Peijnenburg, W.J.; Kopittke, P.M. An electrostatic model predicting Cu and Ni toxicity to microbial processes in soils. Soil Biol. Biochem. 2013, 57, 720–730. [Google Scholar]
- Shomer, I.; Novacky, A.J.; Pike, S.M.; Yermiyahu, U.; Kinraide, T.B. Electrical potentials of plant cell walls in response to the ionic environment. Plant Physiol. 2003, 133, 411–422. [Google Scholar]
- Kinraide, T.B. Possible influence of cell walls upon ion concentrations at plasma membrane surfaces. Toward a comprehensive view of cell-surface electrical effects upon ion uptake, intoxication, and amelioration. Plant Physiol. 2004, 136, 3804–3813. [Google Scholar]
- Nobel, P. Physicochemical and Environmental Plant Physiology; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Kinraide, T.B.; Wang, P. The surface charge density of plant cell membranes (σ): An attempt to resolve conflicting values for intrinsic σ. J. Exp. Bot. 2010. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Yermiyahu, U. A scale of metal ion binding strengths correlating with ionic charge, Pauling electronegativity, toxicity, and other physiological effects. J. Inorg. Biochem. 2007, 101, 1201–1213. [Google Scholar]
- Kinraide, T.B. Improved scales for metal ion softness and toxicity. Environ. Toxicol. Chem. 2009, 28, 525–533. [Google Scholar]
- Wang, P.; Zhou, D.; Kinraide, T.B.; Luo, X.; Li, L.; Li, D.; Zhang, H. Cell membrane surface potential (ψ0) plays a dominant role in the phytotoxicity of copper and arsenate. Plant Physiol. 2008, 148, 2134–2143. [Google Scholar]
- Wagatsuma, T.; Ezoe, Y. Effect of pH on ionic species of aluminum in medium and on aluminum toxicity under solution culture. Soil Sci. Plant Nutr. 1985, 31, 547–561. [Google Scholar]
- Parker, D.; Zelazny, L.; Kinraide, T. On the phytotoxicity of polynuclear hydroxy-aluminum complexes. Soil Sci. Soc. Am. J. 1989, 53, 789–796. [Google Scholar]
- Kinraide, T.B. Reconsidering the rhizotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminium. J. Exp. Bot. 1997, 48, 1115–1124. [Google Scholar]
- Wang, P.; Menzies, N.W.; Wang, Y.-M.; Zhou, D.-M.; Zhao, F.-J.; Kopittke, P.M. Identifying the species of copper that are toxic to plant roots in alkaline nutrient solutions. Plant Soil 2012, 361, 317–327. [Google Scholar]
- Kobayashi, Y.; Kuroda, K.; Kimura, K.; Southron-Francis, J.L.; Furuzawa, A.; Kimura, K.; Iuchi, S.; Kobayashi, M.; Taylor, G.J.; Koyama, H. Amino acid polymorphisms in strictly conserved domains of a P-type ATPase HMA5 are involved in the mechanism of copper tolerance variation in Arabidopsis. Plant Physiol. 2008, 148, 969–980. [Google Scholar]
- Wang, Y.-M.; Wang, P.; Ni, L.-F.; Hao, X.-Z.; Zhou, D.-M. Assessment of the Zn–Co mixtures rhizotoxicity under Ca deficiency: Using two conventional mixture models based on the cell membrane surface potential. Chemosphere 2014, 112, 232–239. [Google Scholar]
- Jahn, T.P.; Bienert, G.P. MIPS and Their Role in the Exchange of Metalloids; Springer: Berlin, Germany, 2010. [Google Scholar]
- An, Y.-J.; Kim, Y.-M.; Kwon, T.-I.; Jeong, S.-W. Combined effect of copper, cadmium, and lead upon (Cucumis sativus) growth and bioaccumulation. Sci. Total Environ. 2004, 326, 85–93. [Google Scholar]
- Cui, Y.; Zhu, Y.-G.; Zhai, R.; Huang, Y.; Qiu, Y.; Liang, J. Exposure to metal mixtures and human health impacts in a contaminated area in Nanning, China. Environ. Int. 2005, 31, 784–790. [Google Scholar]
- Qiu, H.; Vijver, M.G.; Peijnenburg, W.J. Interactions of cadmium and zinc impact their toxicity to the earthworm Aporrectodea caliginosa. Environ. Toxicol. Chem. 2011, 30, 2084–2093. [Google Scholar]
- Kong, I.C. Joint effects of heavy metal binary mixtures on seed germination, root and shoot growth, bacterial bioluminescence, and gene mutation. J. Environ. Sci. 2013, 25, 889–894. [Google Scholar]
- Vijver, M.G.; Elliott, E.G.; Peijnenburg, W.J.; de Snoo, G.R. Response predictions for organisms water-exposed to metal mixtures: A meta-analysis. Environ. Toxicol. Chem. 2011, 30, 1482–1487. [Google Scholar]
- Vijver, M.G.; Peijnenburg, W.J.; de Snoo, G.R. Toxicological mixture models are based on inadequate assumptions. Environ. Sci. Technol. 2010, 44, 4841–4842. [Google Scholar]
- Yen Le, T.T.; Vijver, M.G.; Jan Hendriks, A.; Peijnenburg, W.J. Modeling toxicity of binary metal mixtures (Cu2+–Ag+, Cu2+–Zn2+) to lettuce, Lactuca sativa, with the biotic ligand model. Environ. Toxicol. Chem. 2013, 32, 137–143. [Google Scholar]
- Kinraide, T.B. Interactions among Ca2+, Na+ and K+ in salinity toxicity: Quantitative resolution of multiple toxic and ameliorative effects. J. Exp. Bot. 1999, 50, 1495–1505. [Google Scholar]
- Wang, P.; Kopittke, P.M.; de Schamphelaere, K.A.; Zhao, F.J.; Zhou, D.M.; Lock, K.; Ma, Y.B.; Peijnenburg, W.J.; McGrath, S.P. Evaluation of an electrostatic toxicity model for predicting Ni2+ toxicity to barley root elongation in hydroponic cultures and in soils. New Phytol. 2011, 192, 414–427. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-M.; Kinraide, T.B.; Wang, P.; Hao, X.-Z.; Zhou, D.-M. Surface Electrical Potentials of Root Cell Plasma Membranes: Implications for Ion Interactions, Rhizotoxicity, and Uptake. Int. J. Mol. Sci. 2014, 15, 22661-22677. https://doi.org/10.3390/ijms151222661
Wang Y-M, Kinraide TB, Wang P, Hao X-Z, Zhou D-M. Surface Electrical Potentials of Root Cell Plasma Membranes: Implications for Ion Interactions, Rhizotoxicity, and Uptake. International Journal of Molecular Sciences. 2014; 15(12):22661-22677. https://doi.org/10.3390/ijms151222661
Chicago/Turabian StyleWang, Yi-Min, Thomas B. Kinraide, Peng Wang, Xiu-Zhen Hao, and Dong-Mei Zhou. 2014. "Surface Electrical Potentials of Root Cell Plasma Membranes: Implications for Ion Interactions, Rhizotoxicity, and Uptake" International Journal of Molecular Sciences 15, no. 12: 22661-22677. https://doi.org/10.3390/ijms151222661
APA StyleWang, Y.-M., Kinraide, T. B., Wang, P., Hao, X.-Z., & Zhou, D.-M. (2014). Surface Electrical Potentials of Root Cell Plasma Membranes: Implications for Ion Interactions, Rhizotoxicity, and Uptake. International Journal of Molecular Sciences, 15(12), 22661-22677. https://doi.org/10.3390/ijms151222661




