Enhanced Levels of Interleukin-8 Are Associated with Hepatitis B Virus Infection and Resistance to Interferon-Alpha Therapy
Abstract
:1. Introduction
2. Results
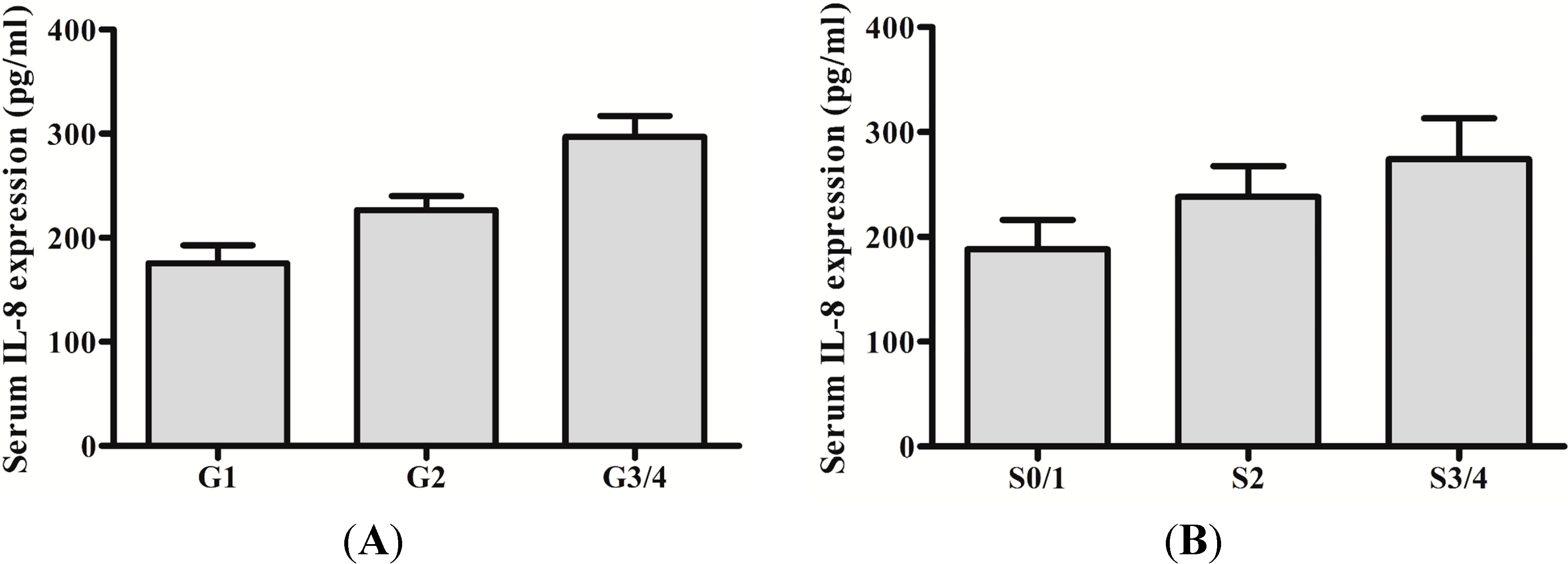
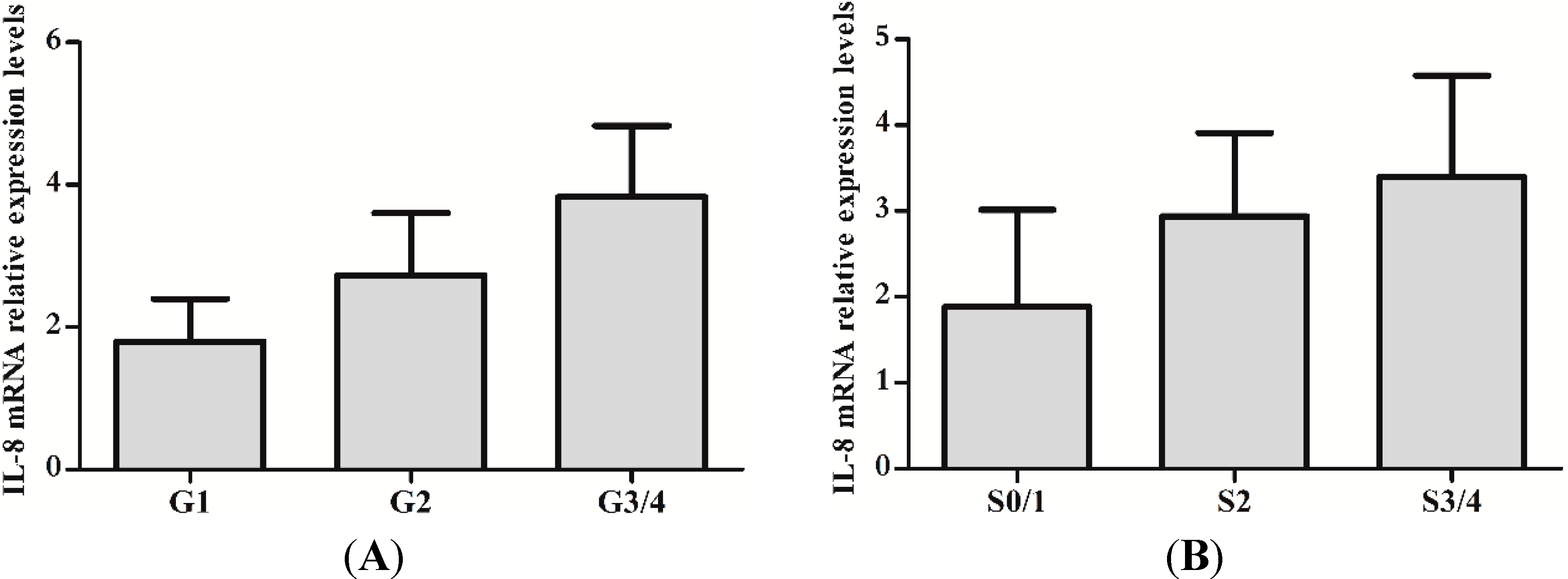
2.1. Serum IL-8 Protein and mRNA Were Augmented in Chronic Hepatitis B (CHB) Patients
| Characteristic | Patients (n = 66) |
|---|---|
| Gender (M/F) | 29/37 |
| Age (Years) | 42.71 ± 13.52 |
| Liver inflammation Grade (1, 2, 3, 4) | 29/24/9/4 |
| Liver fibrosis stage (0, 1, 2, 3, 4) | 39/10/5/12 |
| ALT (Units/L) | 100.28 ± 32.44 |
| PA (mg/L) | 217.97 ± 48.57 |
| HBV DNA (log10 copies per mL) | 5.73 ± 1.95 |
2.2. IL-8 Expression in Liver Tissues of CHB Patients
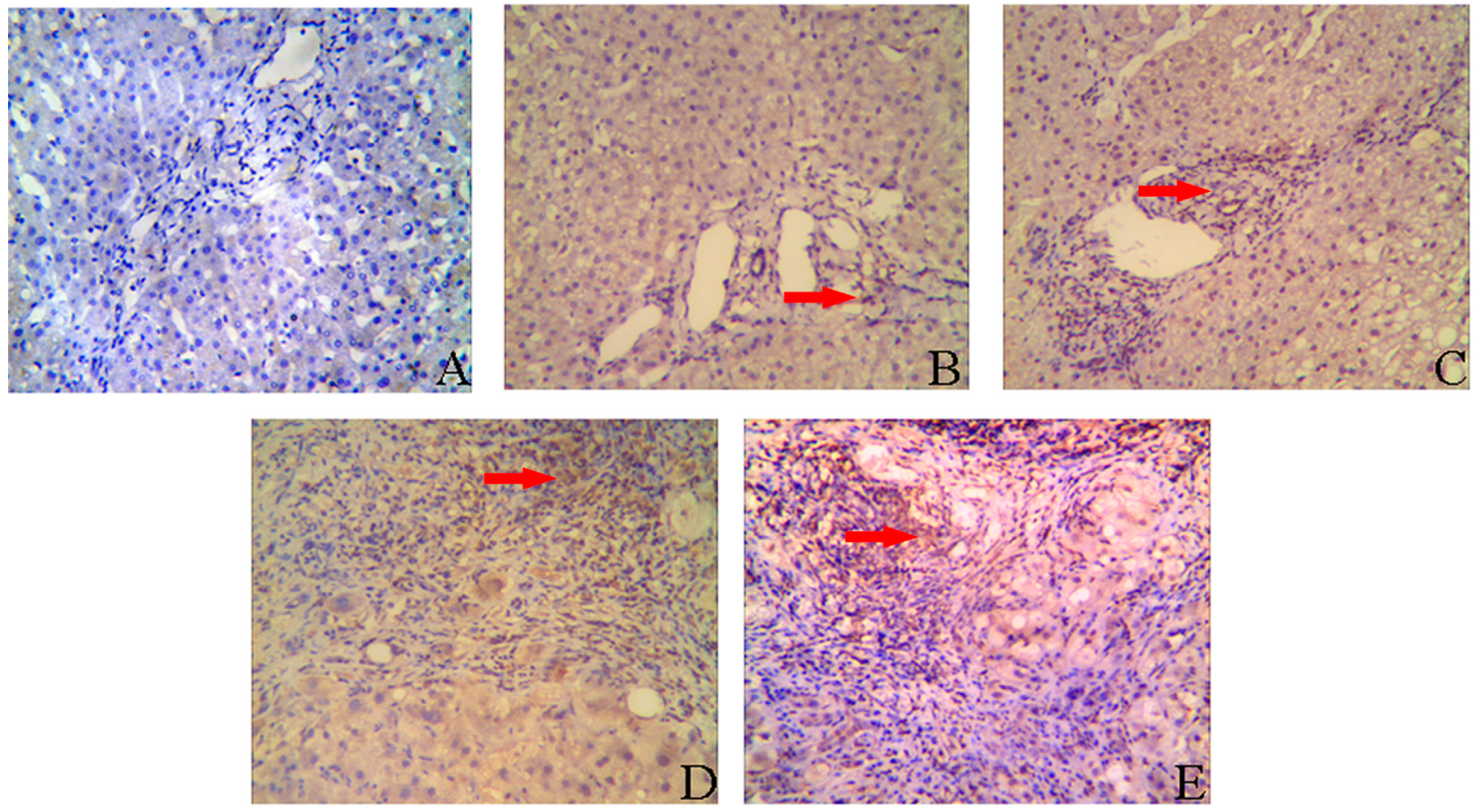
| Inflammation Stages | Cases | Expression of IL-8 | |||
|---|---|---|---|---|---|
| − | + | ++ | +++ | ||
| G1 | 29 | 6 | 19 | 4 | 0 |
| G2 | 24 | 1 | 4 | 15 | 4 |
| G3/4 | 13 | 0 | 0 | 7 | 6 |
| Fibrosis Grades | Cases | Expression of IL-8 | |||
|---|---|---|---|---|---|
| − | + | ++ | +++ | ||
| S0/1 | 39 | 22 | 17 | 0 | 0 |
| S2 | 10 | 2 | 5 | 3 | 0 |
| S3/4 | 17 | 0 | 2 | 6 | 9 |
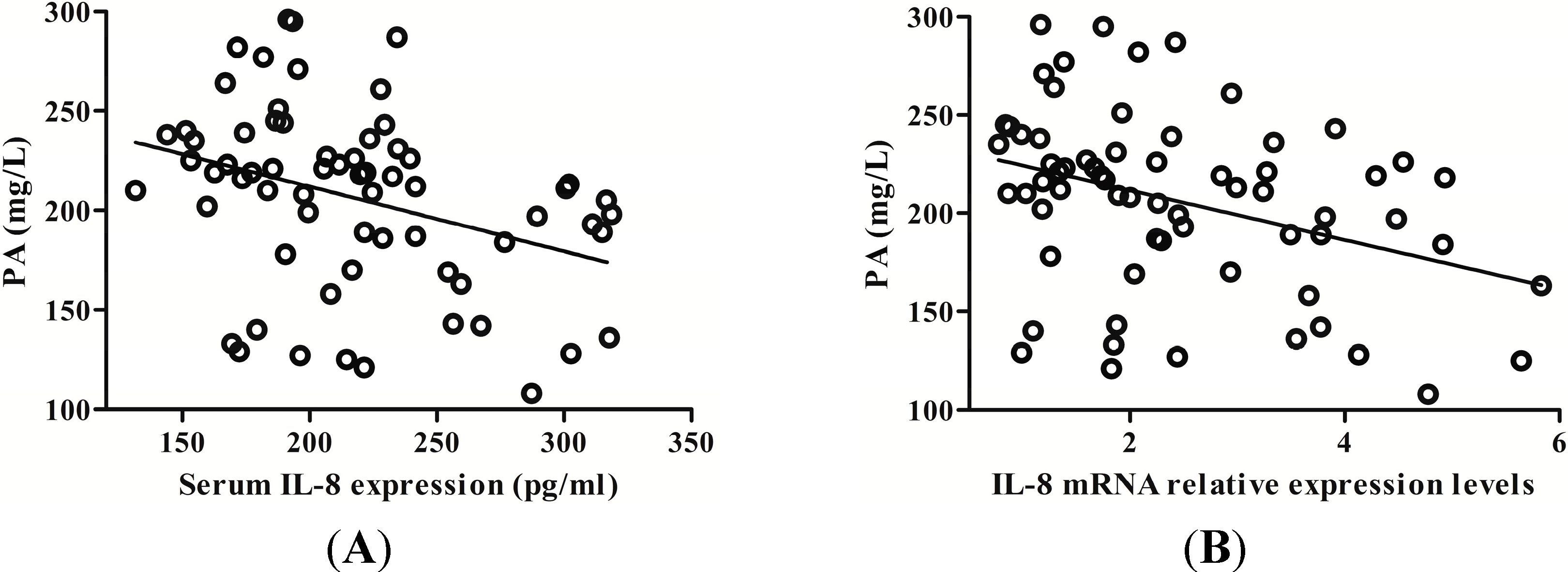
2.3. IL-8 Expression Is Positively Related with Serum Alanine Aminotransferase (ALT) Level in CHB Patients
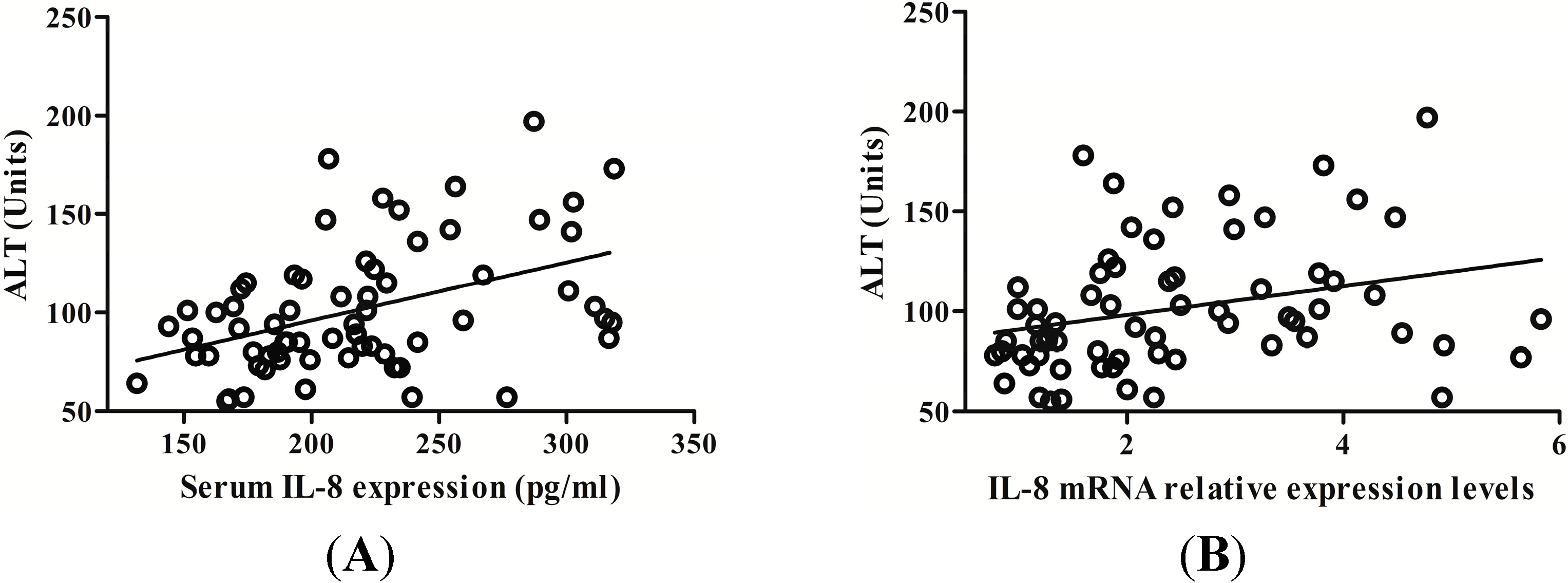
2.5. Levels of IL-8 in Serum and the Peripheral Blood Mononuclear Cells (PBMCs) Responses to Interferon-Alpha (IFN-α) Therapy
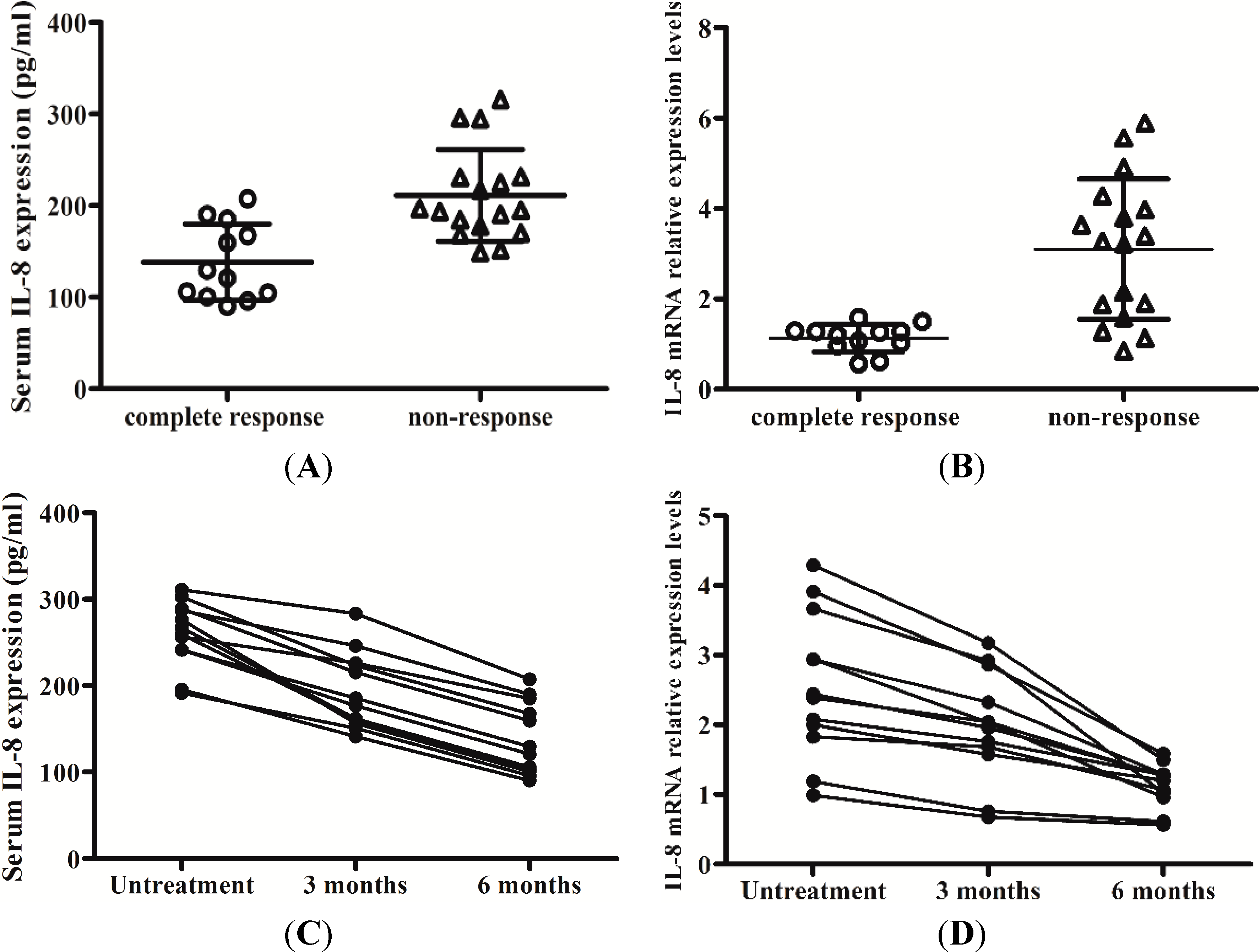
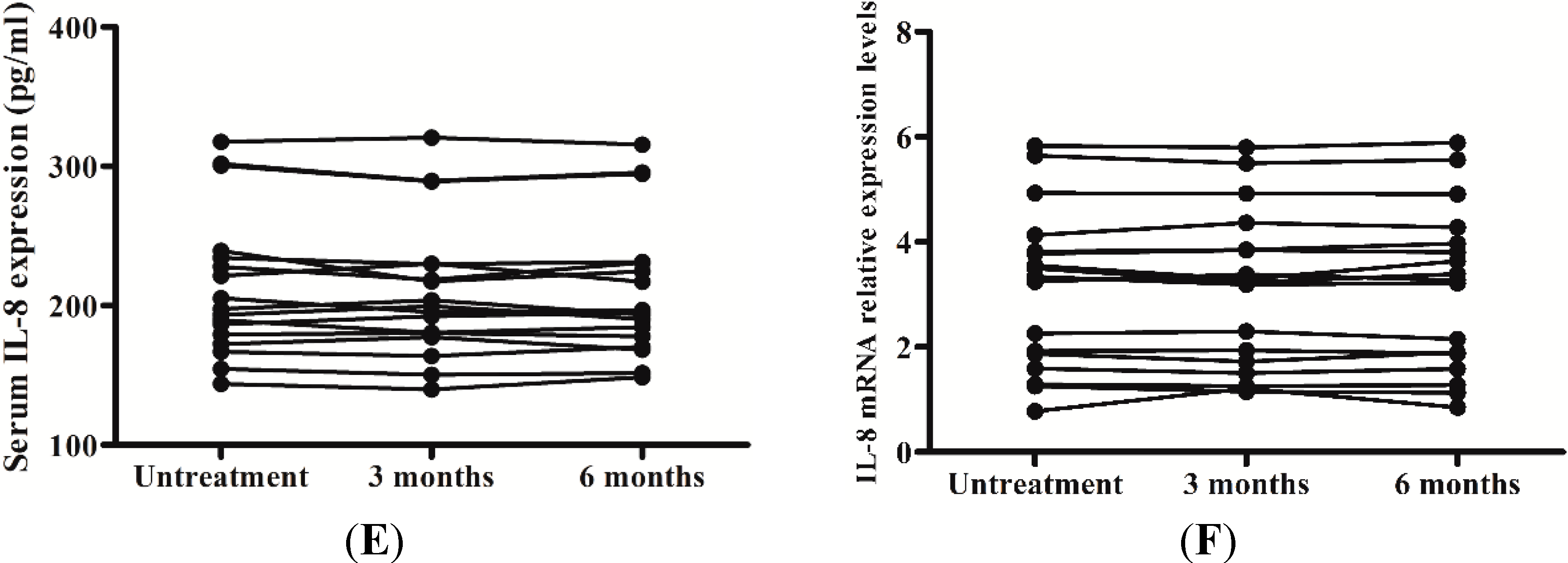
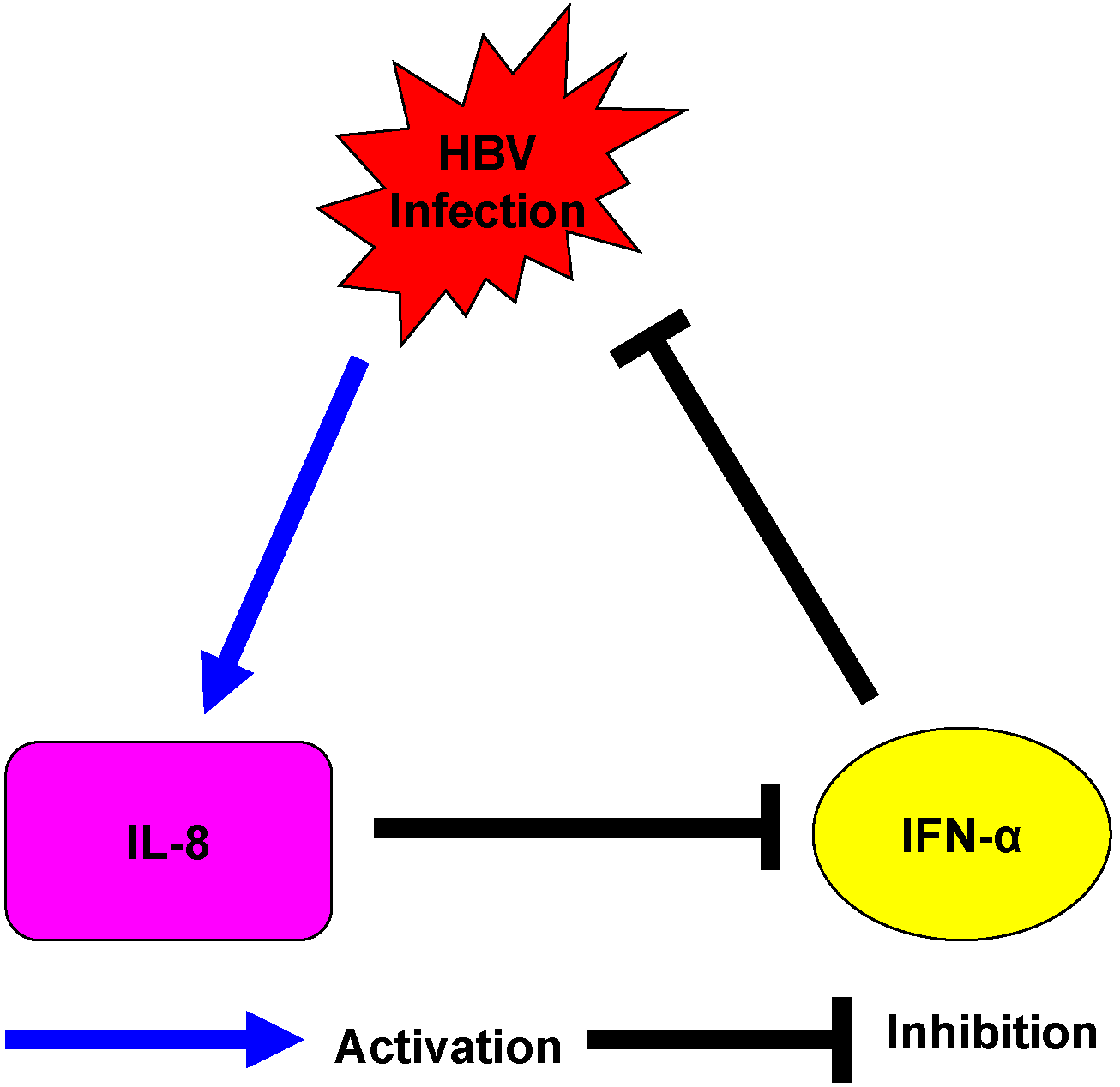
3. Discussion
4. Materials and Methods
4.1. Patients Population
4.2. Peripheral Blood Mononuclear Cells (PBMCs) Isolation and Detection of IL-8 mRNA
| Gene | Forward Primer (5'→3'); Reverse Sequence (5'→3') | Amplicon Size | GenBank Acc. No. |
|---|---|---|---|
| IL-8 | CATACTCCAAACCTTTCCACCCC; TCAGCCCTCTTCAAAAACTTCTCCA | 175 bp | NM_000584 |
| GAPDH | AAGGCTGTGGGCAAGG; TGGAGGAGTGGGTGTCG | 238 bp | NM_001256799 |
| β Tubulin | GCACATAGTAGGCGCTCAAT; ATCTGGAGACCCAGCTTCTT | 175 bp | NM_178014 |
4.3. Detection of IL-8 in Serum
4.4. Immunostaining Procedures
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akbar, S.M.; Horiike, N.; Onji, M. Immune therapy including dendritic cell based therapy in chronic hepatitis B virus infection. World J. Gastroenterol. 2006, 12, 2876–2883. [Google Scholar] [PubMed]
- Chang, T.T.; Liaw, Y.F.; Wu, S.S.; Schiff, E.; Han, K.H.; Lai, C.L.; Safadi, R.; Lee, S.S.; Halota, W.; Goodman, Z.; et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010, 52, 886–893. [Google Scholar] [CrossRef]
- Liaw, Y.F. Reduction of cirrhosis and hepatocellular carcinoma with antiviral therapy in chronic hepatitis B. Hepatology 2013, 58, 1856–1856. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hollinger, F.B. Occult hepatitis B virus infection and hepatocellular carcinoma: A systematic review. J. Viral Hepat. 2014, 21, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.E.; Buster, E.H.; Steyerberg, E.W.; Lesaffre, E.; Janssen, H.L. Prediction of the response to peg-interferon-α in patients with HBeAg positive chronic hepatitis B using decline of HBV DNA during treatment. J. Med. Virol. 2010, 82, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Kittner, J.M.; Sprinzl, M.F.; Grambihler, A.; Weinmann, A.; Schattenberg, J.M.; Galle, P.R.; Schuchmann, M. Adding pegylated interferon to a current nucleos(t)ide therapy leads to HBsAg seroconversion in a subgroup of patients with chronic hepatitis B. J. Clin. Virol. 2012, 54, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, D.; Dev, A.; Nguyen, T.; Sundararajan, V.; Harley, H.; Cheng, W.; Lee, A.; Rusli, F.; Chen, R.; Bell, S.; et al. Efficacy and tolerability of pegylated interferon-α-2a in chronic hepatitis B: A multicenter clinical experience. J. Gastroenterol. Hepatol. 2012, 27, 1447–1453. [Google Scholar] [CrossRef]
- Senturk, H.; Baysal, B.; Tahan, V.; Zerdali, H.; Ozaras, R.; Tabak, F.; Mert, A.; Canbakan, B.; Tabak, O.; Ozbay, G. Long-term effect of interferon therapy in patients with HBeAg positive chronic hepatitis B infection. Dig. Dis. Sci. 2011, 56, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, M.; Zhang, X.; Zhang, W.; Zhang, Z.; Chen, L.; He, J.; Zheng, Y.; Chen, C.; Wang, F.; et al. Hepatitis B virus polymerase impairs interferon-α-induced STA T activation through inhibition of importin-α5 and protein kinase C-δ. Hepatology 2013, 57, 470–482. [Google Scholar]
- Mihm, U.; Herrmann, E.; Sarrazin, U.; von Wagner, M.; Kronenberger, B.; Zeuzem, S.; Sarrazin, C. Association of serum interleukin-8 with virologic response to antiviral therapy in patients with chronic hepatitis C. J. Hepatol. 2004, 40, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signaling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008, 89, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Yen, Y.H.; Hung, C.H.; Lu, S.N.; Wang, J.H.; Wang, J.C.; Chen, C.H.; Kee, K.M.; Hu, T.H.; Changchien, C.S. Liver interleukin-8 messenger RNA expression and interferon sensitivity-determining region mutations relate to treatment response in hepatitis C 1b. Antivir. Ther. 2011, 16, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.W.; Seidler, S.; Gassler, N.; Nattermann, J.; Luedde, T.; Trautwein, C.; Tacke, F. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One 2011, 6, e21381. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Nakamoto, Y.; Mukaida, N.; Kaneko, S. Intrahepatic interleukin-8 production during disease progression of chronic hepatitis C. Cancer Lett. 2007, 251, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.B.; Marsano, L.S.; McClain, C.J. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology 1993, 18, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.; Shalhoub, P.; Lescure, P.; Sabile, A.; Misek, D.E.; Hanash, S.; Bréchot, C.; Beretta, L. An altered cellular response to interferon and up-regulation of interleukin-8 induced by the hepatitis C viral protein NS5A uncovered by microarray analysis. Virology 2002, 295, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Mahé, Y; Mukaida, N.; Kuno, K.; Akiyama, M.; Ikeda, N.; Matsushima, K.; Murakami, S. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor κB and CCAAT/enhancer-binding protein-like cis-elements. J. Biol. Chem. 1991, 266, 13759–13763. [Google Scholar]
- Masumoto, T.; Ohkubo, K.; Yamamoto, K.; Ninomiya, T.; Abe, M.; Akbar, S.M.; Michitaka, K.; Horiike, N.; Onji, M. Serum IL-8 levels and localization of IL-8 in liver from patients with chronic viral hepatitis. Hepatogastroenterology 1998, 45, 1630–1634. [Google Scholar] [PubMed]
- Mahmood, S.; Sho, M.; Yasuhara, Y.; Kawanaka, M.; Niiyama, G.; Togawa, K.; Ito, T.; Takahashi, N.; Kinoshita, M.; Yamada, G. Clinical significance of intrahepatic interleukin-8 in chronic hepatitis C patients. Hepatol. Res. 2002, 24, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wang, X.L.; Liu, P. Detection of serum TNF-α, IFN-β, IL-6 and IL-8 in patients with hepatitis B. World J. Gastroenterol. 1999, 5, 38–40. [Google Scholar] [PubMed]
- Yu, Y.; Gong, R.; Mu, Y.; Chen, Y.; Zhu, C.; Sun, Z.; Chen, M.; Liu, Y.; Zhu, Y.; Wu, J. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8 and cyclooxygenase-2. J. Immunol. 2011, 187, 4844–4860. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.C.; Poage, G.M.; den Hollander, P.; Tsimelzon, A.; Hill, J.; Panupinthu, N.; Zhang, Y.; Mazumdar, A.; Hilsenbeck, S.G.; Mills, G.B.; et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013, 73, 3470–3480. [Google Scholar] [CrossRef]
- Bauer, M.; Gräbsch, C.; Gminski, R.; Ollmann, A.I.; Borm, P.; Dietz, A.; Herbarth, O.; Wichmann, G. Cement-related particles interact with proinflammatory IL-8 chemokine from human primary oropharyngeal mucosa cells and human epithelial lung cancer cell line A549. Environ. Toxicol. 2012, 27, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Unger, B.L.; Comstock, A.T.; Angel, K.A.; Mancuso, P.; Martinez, F.J.; Sajjan, U.S. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax 2013, 68, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Brunetto, M.; Reynolds, G.; Christophides, T.; Kennedy, P.T.; Lampertico, P.; Das, A.; Lopes, A.R.; Borrow, P.; Williams, K.; et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J. Exp. Med. 2007, 204, 667–680. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.W.; Huang, Y.Q.; Gong, Z.J. Interferon-alpha induces high expression of APOBEC3G and STAT-1 in Vitro and in Vivo. Int. J. Mol. Sci. 2010, 11, 3501–3512. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Khabar, K.S.; Paschal, D.M.; Ezelle, H.J.; Duverlie, G.; Barber, G.N.; Levy, D.E.; Mukaida, N.; Gretch, D.R. Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. J. Virol. 2001, 75, 6095–6106. [Google Scholar] [CrossRef] [PubMed]
- Pollicino, T.; Bellinghieri, L.; Restuccia, A.; Raffa, G.; Musolino, C.; Alibrandi, A.; Teti, D.; Raimondo, G. Hepatitis B virus (HBV) induces the expression of interleukin-8 that in turn reduces HBV sensitivity to interferon-alpha. Virology 2013, 444, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, JA.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Guan, S.-H.; Zhang, H.; Pan, Y.; Wu, Y.-Y.; Wang, A.-H.; Sun, B.-B. Enhanced Levels of Interleukin-8 Are Associated with Hepatitis B Virus Infection and Resistance to Interferon-Alpha Therapy. Int. J. Mol. Sci. 2014, 15, 21286-21298. https://doi.org/10.3390/ijms151121286
Yang K, Guan S-H, Zhang H, Pan Y, Wu Y-Y, Wang A-H, Sun B-B. Enhanced Levels of Interleukin-8 Are Associated with Hepatitis B Virus Infection and Resistance to Interferon-Alpha Therapy. International Journal of Molecular Sciences. 2014; 15(11):21286-21298. https://doi.org/10.3390/ijms151121286
Chicago/Turabian StyleYang, Kai, Shi-He Guan, Hao Zhang, Ying Pan, Yuan-Yuan Wu, Ai-Hua Wang, and Bei-Bei Sun. 2014. " Enhanced Levels of Interleukin-8 Are Associated with Hepatitis B Virus Infection and Resistance to Interferon-Alpha Therapy" International Journal of Molecular Sciences 15, no. 11: 21286-21298. https://doi.org/10.3390/ijms151121286
APA StyleYang, K., Guan, S.-H., Zhang, H., Pan, Y., Wu, Y.-Y., Wang, A.-H., & Sun, B.-B. (2014). Enhanced Levels of Interleukin-8 Are Associated with Hepatitis B Virus Infection and Resistance to Interferon-Alpha Therapy. International Journal of Molecular Sciences, 15(11), 21286-21298. https://doi.org/10.3390/ijms151121286