MicroRNAs Associated with the Efficacy of Photodynamic Therapy in Biliary Tract Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Phototoxicity Experiments
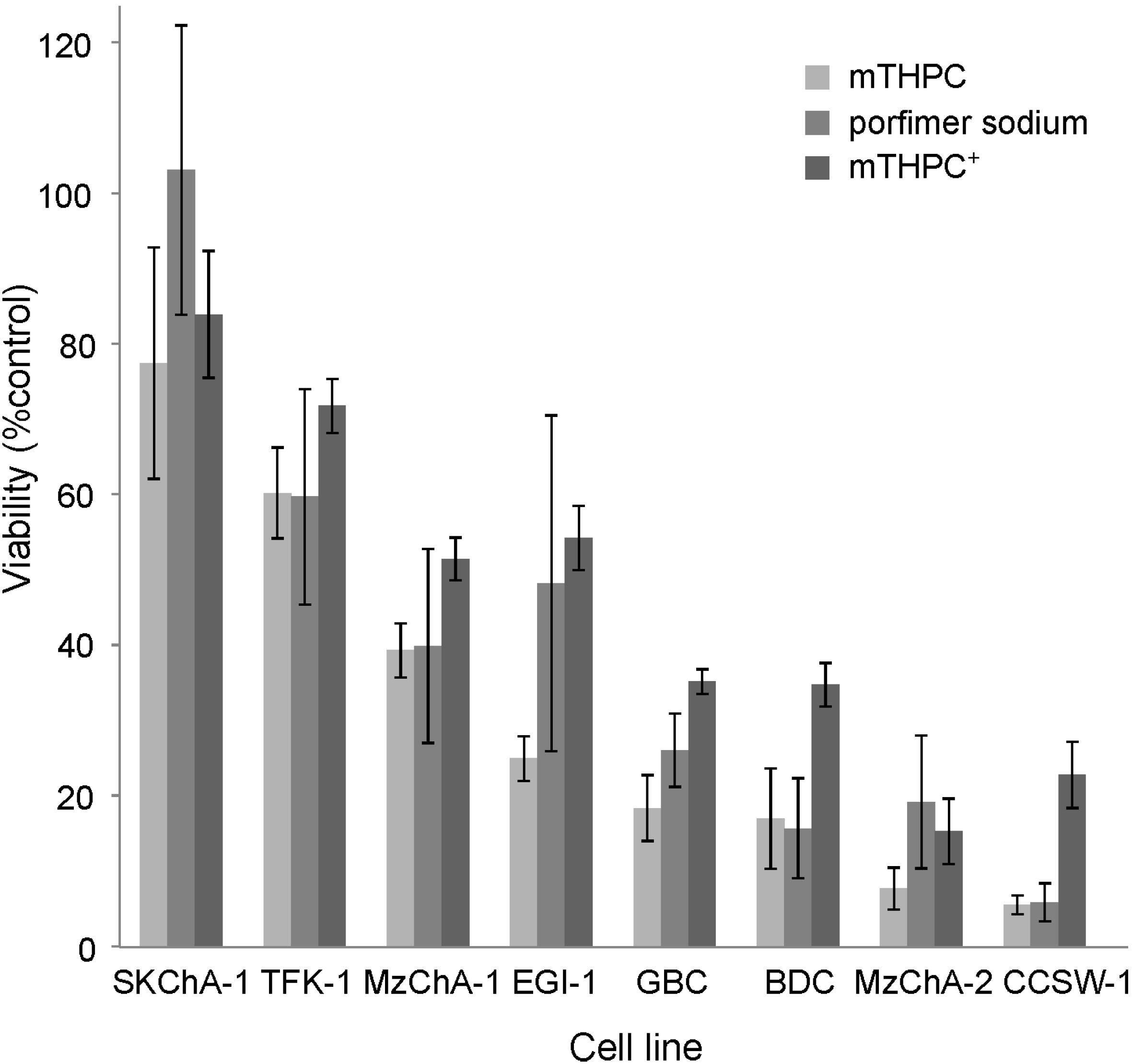
2.1.2. MicroRNA Expression Profiling
| Cell Line | n miRs | n Cell Lines | n miRs |
|---|---|---|---|
| BDC | 332 | 8 | 200 |
| CCSW | 379 | 7 | 62 |
| EGI | 352 | 6 | 48 |
| GBC | 349 | 5 | 37 |
| MzChA1 | 416 | 4 | 48 |
| MzChA2 | 445 | 3 | 37 |
| SkChA1 | 412 | 2 | 56 |
| TFK | 359 | 1 | 96 |
| - | 0 | 170 | |
| total | 754 | ||
2.1.3. PDT Efficiency and MicroRNA Expression
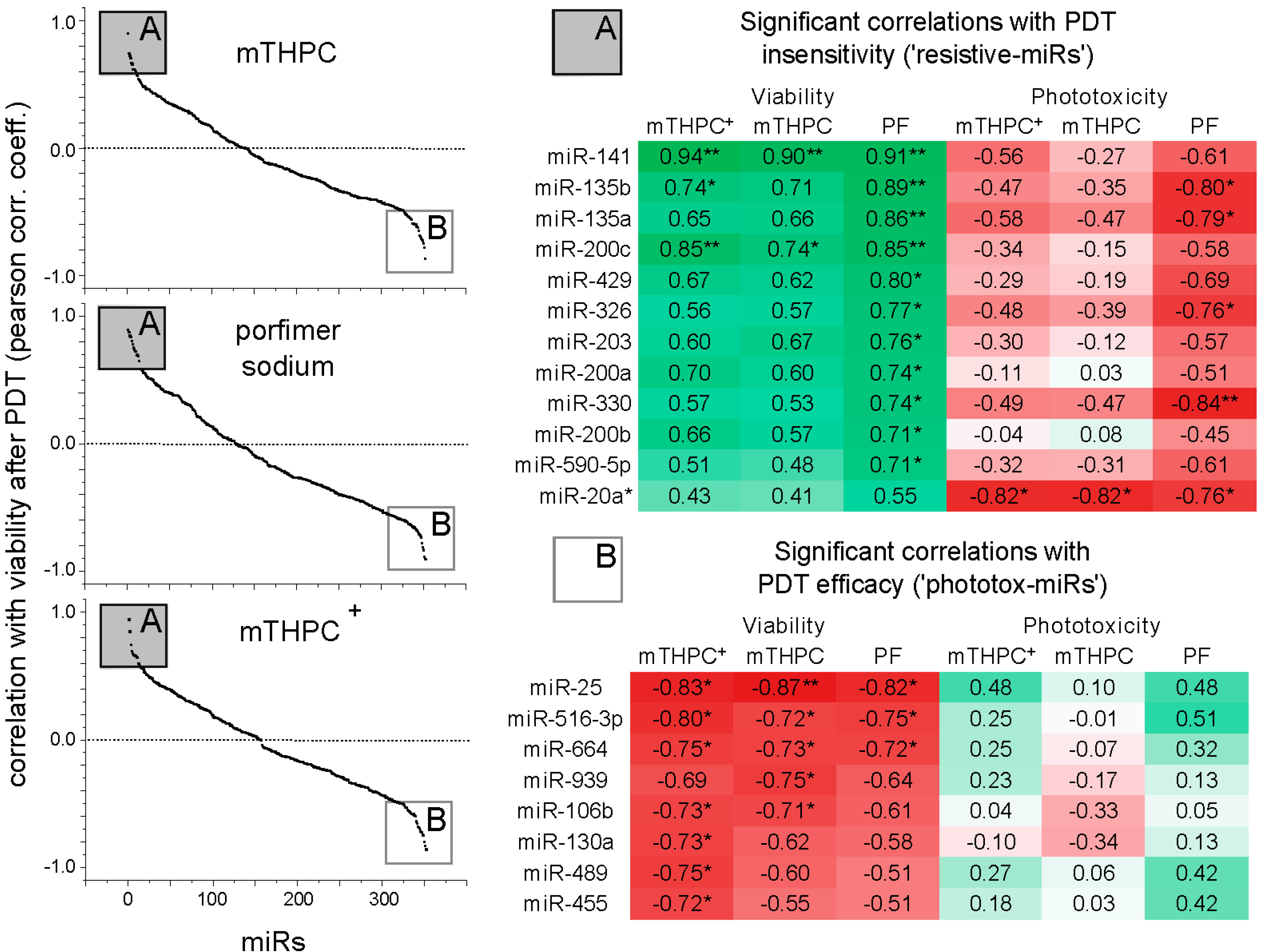
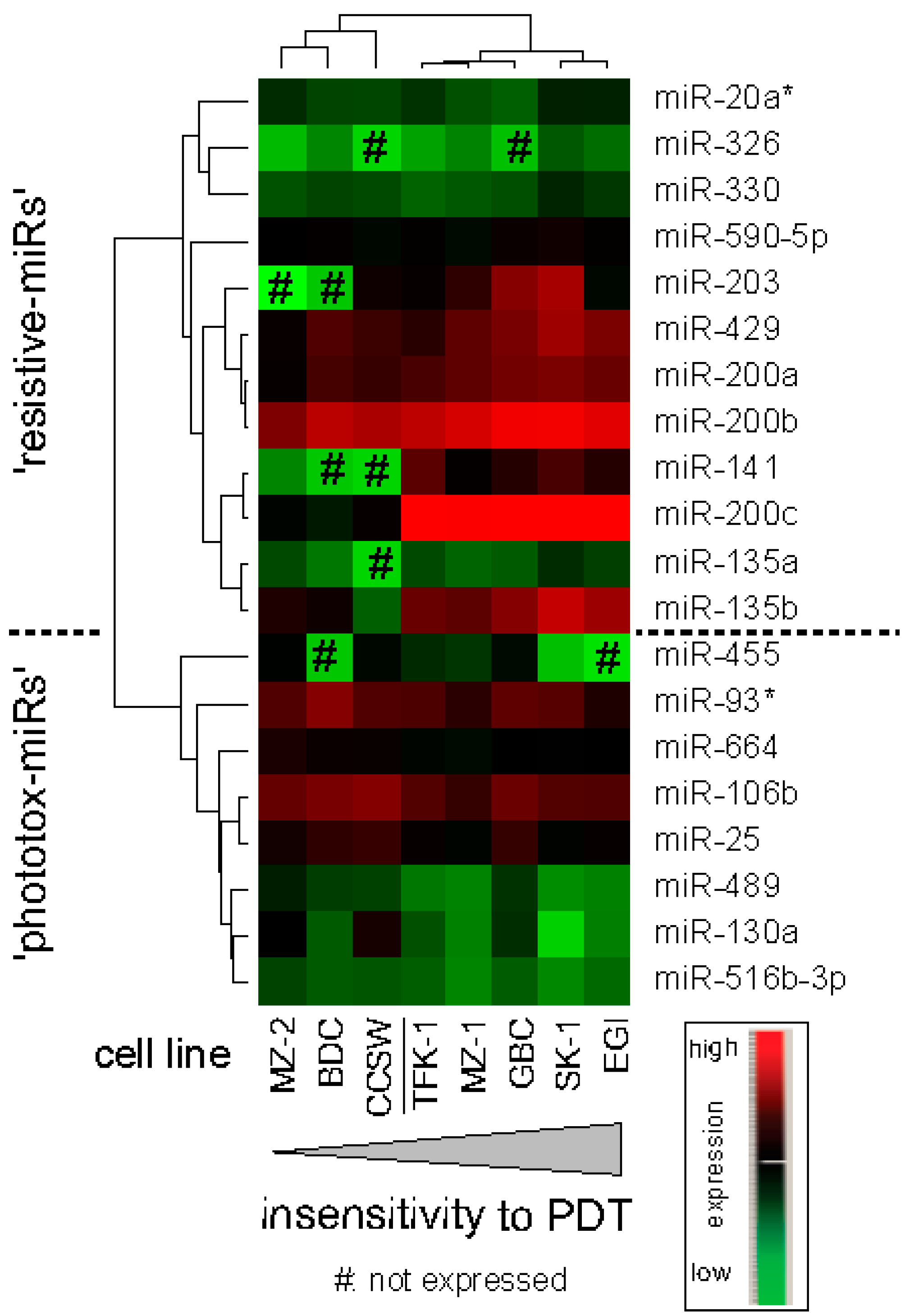
2.1.4. Correlation Analysis: miR Expression and PDT Efficiency, PS Uptake, Glutathione (GSH) Levels and Markers of Differentiation and Proliferation
2.1.5. In Silico Target Prediction and Bioinformatic Approach
| (Phenotypic) Markers | Resistive-miRs | Phototox-miRs | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 141 | 135b | 135a | 200c | 429 | 326 | 203 | 200a | 330 | 200b | 590-5p | 20a* | 25 | 516b-3p | 664 | 93* | 106b | 130a | 489 | 455 | |
| Uptake | −0.70 | −0.60 | −0.47 | −0.74 † | −0.63 | −0.43 | −0.55 | −0.74 † | −0.39 | −0.74 † | −0.52 | −0.03 | 0.65 | 0.72 † | 0.67 | 0.64 | 0.79 † | 0.94 †† | 0.54 | 0.65 |
| Ck19 | 0.24 | 0.50 | −0.01 | 0.23 | 0.72 † | 0.11 | 0.50 | 0.66 | 0.14 | 0.75 † | 0.17 | −0.19 | −0.30 | −0.63 | −0.33 | −0.05 | −0.36 | −0.70 | −0.29 | −0.54 |
| Ck8/18 | 0.39 | 0.64 | 0.37 | 0.35 | 0.72 † | 0.39 | 0.11 | 0.59 | 0.48 | 0.77 † | 0.73 † | 0.35 | −0.18 | −0.40 | −0.04 | 0.09 | −0.09 | −0.55 | −0.03 | −0.68 |
| E-Cadherin | 0.51 | 0.52 | 0.27 | 0.47 | 0.40 | 0.06 | −0.05 | 0.49 | −0.10 | 0.53 | 0.34 | 0.25 | −0.16 | −0.39 | −0.21 | −0.19 | −0.37 | −0.34 | −0.16 | −0.64 |
| Vimentin | −0.58 | −0.66 | −0.39 | −0.64 | −0.44 | −0.18 | −0.38 | −0.67 | 0.24 | −0.51 | −0.07 | 0.05 | 0.47 | 0.67 | 0.78 † | 0.53 | 0.78 † | 0.45 | 0.42 | 0.18 |
| Cyclin D1 | −0.42 | −0.42 | −0.17 | −0.23 | −0.09 | 0.08 | −0.45 | −0.29 | 0.57 | −0.21 | 0.00 | 0.35 | 0.40 | 0.38 | 0.63 | 0.64 | 0.70 | 0.26 | 0.11 | −0.21 |
| Ki67 | −0.58 | −0.38 | −0.15 | −0.53 | −0.23 | 0.06 | −0.78 † | −0.48 | 0.40 | −0.30 | 0.20 | 0.08 | 0.58 | 0.65 | 0.78 † | 0.65 | 0.73 † | 0.40 | 0.73 † | −0.05 |
| GSH | 0.24 | 0.21 | 0.11 | 0.20 | 0.27 | 0.12 | 0.40 | 0.29 | 0.12 | 0.33 | 0.36 | −0.19 | −0.15 | −0.18 | −0.06 | −0.21 | −0.33 | −0.72 † | −0.16 | −0.44 |
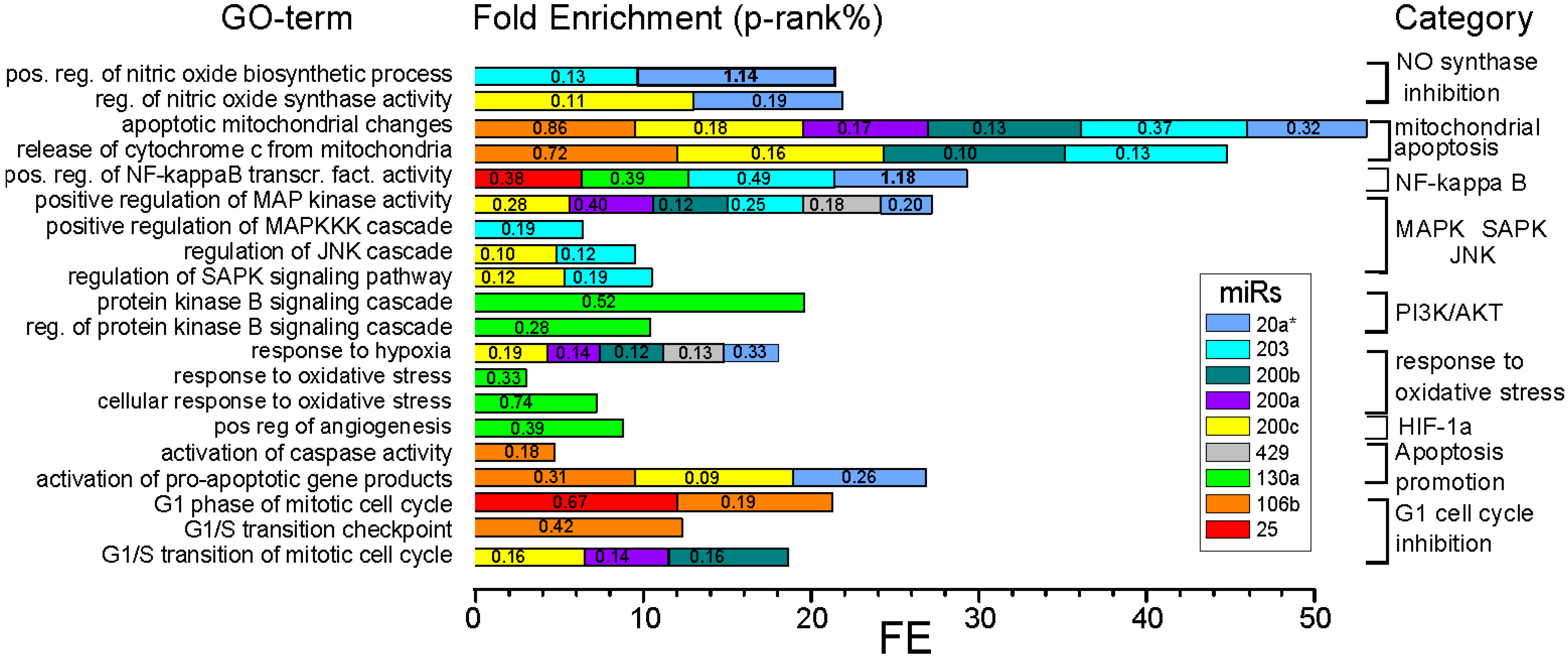
| Function | MiR | Possible Impact on PDT Efficiency via: | Association with Cancer Phenotype |
|---|---|---|---|
| “Phototox-miRs” | -130a | neg. corr. with GSH (*), targets: PRDX3, SESN2, HIF-1α, SIRT6/7, NOS3, AKT1, GO: pos. reg. angiogenesis, protein B kinase signaling, pos. reg. NF-κB transcription factor activity | - |
| -25 | targets: AKT2, SESN3, BCL2, NOX4, HMOX2, GO: pos. reg. NF-κB transcription factor activity, G1/S cycle inhibition | induction of G1/S arrest via Wnt-inhibition (β-cat) [25] Associated with aggressive phenotype (e.g., gastric cancer) [26,27] | |
| -93* | targets: AKT1, SESN2, NOX4, NFE2, MGST1, Influence on Sp1 and Nrf2 TF [28,29] and PTEN/Akt signaling pathway [30] | Associated with aggressive phenotype (e.g., gastric and breast cancer) [26,27] | |
| “Resistive-miRs” | -141 | targets: BACH1, KEAP1, TGF-β1, Nrf2, NOX1 | GO: EMT |
| -200a | GO: apoptotic mitochondrial changes, pos. regulation of MAP kinase activity, targets: KEAP1, activation of Nrf2 and NAD(P)H-quinone oxidoreductase 1 [31] | suppression of Wnt (β-cat) and anti-proliferative function [32], miR family 200 is negatively associated with EMT (ZEB1/ZEB2) [33,34,35] | |
| -200c/-203 | target: BAX, GO: apoptotic mitochondrial changes, release of cytochrome c from mitochondria, activation of proapoptotic gene products, pos. regulation of MAPKKK cascade, regulation of c-Jun N-terminal kinases (JNK) cascade, regulation of stress-activated protein kinase signaling pathway | neg. corr. with Ki67 (*), GO: EMT, 200c: targets CD44 [36,37], which is associated with high ROS levels and EMT [38], 203: “anti-stemness-miR”, which is down-regulated by CD44 [39,40] | |
| 20a* | GO: activation of pro-apoptotic gene products, apoptotic mitochondrial changes, positive regulation of MAP kinase activity | - |
2.2. Discussion
2.2.1. MiR Expression and PDT Insensitivity
2.2.2. Cancer Phenotype and Susceptibility to PDT
3. Experimental Section
3.1. Substances and Cell Culture
3.2. Photodynamic Therapy and Phototoxicity Experiments
3.3. MicroRNA Expression Profiling
3.4. Bioinformatical Approach and Literature Search
3.5. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jarnagin, W.R.; Shoup, M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004, 24, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.N.; Gores, G.J. Cholangiocarcinoma. Gastroenterology 2005, 128, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, P.; Jonas, S.; Bechstein, W.O.; Lohmann, R.; Radke, C.; Kling, N.; Wex, C.; Lobeck, H.; Hintze, R. Extended resections for hilar cholangiocarcinoma. Ann. Surg. 1999, 230, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Berr, F. Photodynamic therapy for cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ortner, M.E.; Caca, K.; Berr, F.; Liebetruth, J.; Mansmann, U.; Huster, D.; Voderholzer, W.; Schachschal, G.; Mossner, J.; Lochs, H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology 2003, 125, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Witzigmann, H.; Berr, F.; Ringel, U.; Caca, K.; Uhlmann, D.; Schoppmeyer, K.; Tannapfel, A.; Wittekind, C.; Mossner, J.; Hauss, J.; et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: Palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann. Surg. 2006, 244, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Bai, Y.; Ma, S.R.; Liu, F.; Li, Z.S. Systematic review: Photodynamic therapy for unresectable cholangiocarcinoma. J. Hepatobiliary Pancreat Sci. 2010, 17, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, T.; Wolkersdorfer, G.; Neureiter, D.; Salmhofer, H.; Berr, F. Photodynamic therapy for non-resectable perihilar cholangiocarcinoma. Photochem. Photobiol. Sci. 2009, 8, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Kiesslich, T.; Oberdanner, C.B.; Krammer, B. Apoptosis following photodynamic tumor therapy: Induction, mechanisms and detection. Curr. Pharm. Des. 2005, 11, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, T.; Berlanda, J.; Plaetzer, K.; Krammer, B.; Berr, F. Comparative characterization of the efficiency and cellular pharmacokinetics of foscan- and foslip-based photodynamic treatment in human biliary tract cancer cell lines. Photochem. Photobiol. Sci. 2007, 6, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, T.; Neureiter, D.; Alinger, B.; Jansky, G.L.; Berlanda, J.; Mkrtchyan, V.; Ocker, M.; Plaetzer, K.; Berr, F. Uptake and phototoxicity of meso-tetrahydroxyphenyl chlorine are highly variable in human biliary tract cancer cell lines and correlate with markers of differentiation and proliferation. Photochem. Photobiol. Sci. 2010, 9, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Voorhoeve, P.M. Micrornas: Oncogenes, tumor suppressors or master regulators of cancer heterogeneity? Biochim. Biophys. Acta 2010, 1805, 72–86. [Google Scholar]
- Al-Ali, B.M.; Ress, A.L.; Gerger, A.; Pichler, M. Micrornas in renal cell carcinoma: Implications for pathogenesis, diagnosis, prognosis and therapy. Anticancer Res. 2012, 32, 3727–3732. [Google Scholar] [PubMed]
- Schwarzenbacher, D.; Balic, M.; Pichler, M. The role of micrornas in breast cancer stem cells. Int. J. Mol. Sci. 2013, 14, 14712–14723. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. Microrna expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- De Groen, P.C.; Gores, G.J.; LaRusso, N.F.; Gunderson, L.L.; Nagorney, D.M. Biliary tract cancers. N. Engl. J. Med. 1999, 341, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Dweep, H.; Sticht, C.; Pandey, P.; Gretz, N. Mirwalk—Database: Prediction of possible mirna binding sites by “walking” the genes of three genomes. J. Biomed. Inform. 2011, 44, 839–847. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Pieslinger, A.; Plaetzer, K.; Oberdanner, C.B.; Berlanda, J.; Mair, H.; Krammer, B.; Kiesslich, T. Characterization of a simple and homogeneous irradiation device based on light-emitting diodes: A possible low-cost supplement to conventional light sources for photodynamic treatment. Med. Laser Appl. 2006, 21, 277–283. [Google Scholar] [CrossRef]
- Wang, K.K.; Mitra, S.; Foster, T.H. Photodynamic dose does not correlate with long-term tumor response to mTHPC-PDT performed at several drug-light intervals. Med. Phys. 2008, 35, 3518–3526. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; el Naqa, I.M. Prediction of both conserved and nonconserved microrna targets in animals. Bioinformatics 2008, 24, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, T.; Alinger, B.; Wolkersdorfer, G.W.; Ocker, M.; Neureiter, D.; Berr, F. Active Wnt signalling is associated with low differentiation and high proliferation in human biliary tract cancer in vitro and in vivo and is sensitive to pharmacological inhibition. Int. J. Oncol. 2010, 36, 49–58. [Google Scholar] [PubMed]
- Anton, R.; Chatterjee, S.S.; Simundza, J.; Cowin, P.; Dasgupta, R. A systematic screen for micro-rnas regulating the canonical wnt pathway. PLoS One 2011, 6, e26257. [Google Scholar]
- Zhang, R.; Wang, W.; Li, F.; Zhang, H.; Liu, J. Microrna-106b~25 expressions in tumor tissues and plasma of patients with gastric cancers. Med. Oncol. 2014, 31. [Google Scholar] [CrossRef]
- Espinosa-Parrilla, Y.; Munoz, X.; Bonet, C.; Garcia, N.; Vencesla, A.; Yiannakouris, N.; Naccarati, A.; Sieri, S.; Panico, S.; Huerta, J.M.; et al. Genetic association of gastric cancer with mirna clusters including the cancer-related genes mir29, mir25, mir93 and mir106: Results from the epic-eurgast study. Int. J. Cancer 2014, 135, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Ronghe, A.M.; Chatterjee, A.; Bhat, N.K.; Bhat, H.K. MicroRNA-93 regulates Nrf2 expression and is associated with breast carcinogenesis. Carcinogenesis 2013, 34, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Muthusamy, S.; Liang, R.; Sarojini, H.; Wang, E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech. Ageing Dev. 2011, 132, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tian, J.; Zhang, L.; Chen, Y.; Hao, Q. Involvement of microrna-93, a new regulator of pten/akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012, 586, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Eades, G.; Yang, M.; Yao, Y.; Zhang, Y.; Zhou, Q. miR-200a regulates Nrf2 activation by targeting keap1 mRNA in breast cancer cells. J. Biol. Chem. 2011, 286, 40725–40733. [Google Scholar] [CrossRef] [PubMed]
- Saydam, O.; Shen, Y.; Wurdinger, T.; Senol, O.; Boke, E.; James, M.F.; Tannous, B.A.; Stemmer-Rachamimov, A.O.; Yi, M.; Stephens, R.M.; et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing e-cadherin and activating the wnt/beta-catenin signaling pathway. Mol. Cell Biol. 2009, 29, 5923–5940. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The mir-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of e-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef] [PubMed]
- Mongroo, P.S.; Rustgi, A.K. The role of the mir-200 family in epithelial-mesenchymal transition. Cancer Biol. Ther. 2010, 10, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the e-cadherin repressors zeb1 and zeb2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, R.T.; Qian, H.Q.; Wei, J.; Xie, L.; Shen, J.; Yang, M.; Qian, X.P.; Yu, L.X.; Jiang, X.Q.; et al. Targeted delivery of miR-200c/doc to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles. Biomaterials 2013, 34, 7191–7203. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.; Yu, C.C.; Chiou, G.Y.; Chen, Y.W.; Huang, P.I.; Chien, C.S.; Tseng, L.M.; Chu, P.Y.; Lu, K.H.; Chang, K.W.; et al. MicroRNA-200c attenuates tumour growth and metastasis of presumptive head and neck squamous cell carcinoma stem cells. J. Pathol. 2011, 223, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hung, P.H.; Chen, Y.J. Cd44 is associated with the aggressive phenotype of nasopharyngeal carcinoma through redox regulation. Int. J. Mol. Sci. 2013, 14, 13266–13281. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, Y.; Wang, J.; Chen, J.; Yang, C.; Cai, K.; Wang, X.; Shi, F.; Dou, J. MicroRNA-200c overexpression inhibits tumorigenicity and metastasis of CD117+CD44+ ovarian cancer stem cells by regulating epithelial-mesenchymal transition. J. Ovarian Res. 2013, 6. [Google Scholar] [CrossRef]
- Ju, S.Y.; Chiou, S.H.; Su, Y. Maintenance of the stemness in CD44 HCT-15 and hct-116 human colon cancer cells requires miR-203 suppression. Stem Cell Res. 2013, 12, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.; Fuereder, J.; Karbiener, M.; Scheideler, M.; Ress, A.L.; Neureiter, D.; Kemmerling, R.; Dietze, O.; Wiederstein, M.; Berr, F.; et al. Comprehensive analysis of alterations in the mirnome in response to photodynamic treatment. J. Photochem. Photobiol. B 2013, 120, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T. Photodynamic therapy induces microRNA-210 and -296 expression in hela cells. J. Biophotonics 2010, 3, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Beissbarth, T.; Speed, T.P. Gostat: Find statistically overrepresented gene ontologies within a group of genes. Bioinformatics 2004, 20, 1464–1465. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, X. Computational methods for the identification of microrna targets. Open Access Bioinform. 2010, 2, 29–39. [Google Scholar]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Responses of cancer cells induced by photodynamic therapy. J. Healthc. Eng. 2013, 4, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Sablina, A.A.; Feinstein, E.; Koonin, E.V.; Chumakov, P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial ahpd. Science 2004, 304, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Cho, C.S.; Park, S.; Yu, S.; Kang, S.W.; Rhee, S.G. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J. Biol. Chem. 2004, 279, 41975–41984. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; von Tiehl, K.F.; Rucker, N.; Schwarz, M.A.; Gill, P.S.; Gomer, C.J. Antiangiogenic treatment enhances photodynamic therapy responsiveness in a mouse mammary carcinoma. Cancer Res. 2000, 60, 4066–4069. [Google Scholar] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Skarlatos, J.; Corti, L.; Blandamura, S.; Piazza, M.; Gatter, K.C.; Harris, A.L. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001, 61, 1830–1832. [Google Scholar] [PubMed]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Cassar, S.E.; Niles, D.J.; Puskas, J.A.; Frelinger, J.G.; Foster, T.H. Photodynamic therapy mediates the oxygen-independent activation of hypoxia-inducible factor 1α. Mol. Cancer Ther. 2006, 5, 3268–3274. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, J.E. Sirt7 an emerging sirtuin: Deciphering newer roles. J. Physiol. Pharmacol. 2013, 64, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Gomer, C.J. Avastin enhances photodynamic therapy treatment of kaposiʼs sarcoma in a mouse tumor model. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.T.; Zhang, B.; Zhou, Q.; Shen, C.X.; Wang, C.Q. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene spred-1. J. Mol. Cell. Cardiol. 2012, 53, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Fontana, J.T.; Ou, Z.; Jones, D.W.; Ackerman, A.W.; Oldham, K.T.; Yu, J.; Sessa, W.C.; Pritchard, K.A., Jr. Heat shock protein 90 and tyrosine kinase regulate eNOS NO* generation but not NO* bioactivity. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H561–H569. [Google Scholar] [CrossRef]
- Roviezzo, F.; Cuzzocrea, S.; di Lorenzo, A.; Brancaleone, V.; Mazzon, E.; di Paola, R.; Bucci, M.; Cirino, G. Protective role of PI3-kinase-Akt-eNOS signalling pathway in intestinal injury associated with splanchnic artery occlusion shock. Br. J. Pharmacol. 2007, 151, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Sessa, W.C. eNOS at a glance. J. Cell Sci. 2004, 117, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Granville, D.J.; Carthy, C.M.; Jiang, H.; Levy, J.G.; McManus, B.M.; Matroule, J.Y.; Piette, J.; Hunt, D.W. Nuclear factor-κB activation by the photochemotherapeutic agent verteporfin. Blood 2000, 95, 256–262. [Google Scholar] [PubMed]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [PubMed]
- Yuan, Z.Q.; Feldman, R.I.; Sun, M.; Olashaw, N.E.; Coppola, D.; Sussman, G.E.; Shelley, S.A.; Nicosia, S.V.; Cheng, J.Q. Inhibition of jnk by cellular stress- and tumor necrosis factor α-induced Akt2 through activation of the Nfκb pathway in human epithelial cells. J. Biol. Chem. 2002, 277, 29973–29982. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Kochevar, I.E. Singlet oxygen-induced activation of Akt/protein kinase b is independent of growth factor receptors. Photochem. Photobiol. 2003, 78, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Promotion of PDT efficacy by a bcl-2 antagonist. Photochem. Photobiol. 2008, 84, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Corti, L.; Skarlatos, J.; Giatromanolaki, A.; Krammer, B.; Blandamura, S.; Piazza, M.; Verwanger, T.; Schnitzhofer, G.; Kostandelos, J.; et al. Clinical and experimental evidence of bcl-2 involvement in the response to photodynamic therapy. Anticancer Res. 2001, 21, 663–668. [Google Scholar] [PubMed]
- Srivastava, M.; Ahmad, N.; Gupta, S.; Mukhtar, H. Involvement of bcl-2 and bax in photodynamic therapy-mediated apoptosis. Antisense bcl-2 oligonucleotide sensitizes rif 1 cells to photodynamic therapy apoptosis. J. Biol. Chem. 2001, 276, 15481–15488. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Y.; Wang, Z.; Wang, L.; Wei, X.; Zhang, B.; Wen, Z.; Fang, H.; Pang, Q.; Yi, F. Regulation of nadph oxidase activity is associated with mirna-25-mediated nox4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010, 32, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Yung, L.H.; Wong, W.T.; Liu, J.; Leung, F.P.; Liu, L.; Chen, Y.; Kong, S.K.; Kwan, K.M.; Ng, S.M.; et al. Bone morphogenic protein-4 induces endothelial cell apoptosis through oxidative stress-dependent p38mapk and jnk pathway. J. Mol. Cell. Cardiol. 2012, 52, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Tong, B.M.; Wang, R.; Chen, J.P.; Foo, S.; Chong, H.C.; Wang, X.L.; Ang, G.Y.; Chiba, S.; Tan, N.S. Nox4-dependent ROS modulation by amino endoperoxides to induce apoptosis in cancer cells. Cell Death Dis. 2013, 4. [Google Scholar] [CrossRef]
- Weyergang, A.; Berg, K.; Kaalhus, O.; Peng, Q.; Selbo, P.K. Photodynamic therapy targets the mtor signaling network in vitro and in vivo. Mol. Pharm. 2009, 6, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Curnow, A.; McIlroy, B.W.; Postle-Hacon, M.J.; Porter, J.B.; MacRobert, A.J.; Bown, S.G. Enhancement of 5-aminolaevulinic acid-induced photodynamic therapy in normal rat colon using hydroxypyridinone iron-chelating agents. Br. J. Cancer 1998, 78, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Xiang, Y.; Zhang, Y.; Zhao, X.; Zhou, L.; Gao, X. The balance mediated by mirnas and the heme oxygenase 1 feedback loop contributes to biological effects. J. Cell. Biochem. 2013, 114, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Ishii, M.; Kawashima, K.; Kodama, T.; Sugano, K.; Fujimoto, K.; Hirao, Y. Sirna-mediated knockdown of the heme synthesis and degradation pathways: Modulation of treatment effect of 5-aminolevulinic acid-based photodynamic therapy in urothelial cancer cell lines. Photochem. Photobiol. 2009, 85, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Nowis, D.; Legat, M.; Grzela, T.; Niderla, J.; Wilczek, E.; Wilczynski, G.M.; Glodkowska, E.; Mrowka, P.; Issat, T.; Dulak, J.; et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene 2006, 25, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lambrecht, R.W.; Ghaziani, T.; Donohue, S.E.; Bonkovsky, H.L. Role of bach-1 in regulation of heme oxygenase-1 in human liver cells: Insights from studies with small interfering rnas. J. Biol. Chem. 2004, 279, 51769–51774. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Weissmann, N.; Grimminger, F.; Hegel, C.; Bader, L.; Rose, F.; Fink, L.; Ghofrani, H.A.; Schermuly, R.T.; Schmidt, H.H.; et al. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Q.; Xue, P.; Lu, H.W.; Zheng, Q.; Wen, Z.L.; Shao, Z.J. Hematoporphyrin derivative-mediated photodynamic therapy inhibits tumor growth in human cholangiocarcinoma in vitro and in vivo. Hepatol. Res. 2009, 39, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Regulation of miRNA Expression by Low-Level Laser Therapy (LLLT) and Photodynamic Therapy (PDT). Int. J. Mol. Sci. 2013, 14, 13542–13558. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Liu, L.X.; Pan, S.H.; Wang, C.Y.; Fu, Q.F. Therapeutic effect of photodynamic therapy using hematoporphyrin monomethyl ether (hmme) on human cholangiocarcinoma cell line qbc939. Neoplasma 2010, 57, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Luo, Y.; Wang, Z.; Bi, S.; Song, D.; Dai, Y.; Wang, T.; Qiu, L.; Wen, L.; et al. Aldose reductase regulates miR-200a-3p/141–3p to coordinate keap1-nrf2, TGFβ1/2, and zeb1/2 signaling in renal mesangial cells and the renal cortex of diabetic mice. Free Radic. Biol. Med. 2013, 67C, 91–102. [Google Scholar]
- Bhowmick, R.; Girotti, A.W. Pro-survival and pro-growth effects of stress-induced nitric oxide in a prostate cancer photodynamic therapy model. Cancer Lett. 2014, 343, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Sitnik-Busch, T.M.; Vaughan, L.A. Potentiation of photodynamic therapy antitumor activity in mice by nitric oxide synthase inhibition is fluence rate dependent. Photochem. Photobiol. 1999, 70, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Rucker, N.; Wong, S.; Luna, M.; Gomer, C.J. Survivin, a member of the inhibitor of apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer Res. 2007, 67, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Sun, J.; Cecic, I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: Relevance for tumor response. Cancer Res. 2005, 65, 1018–1026. [Google Scholar] [PubMed]
- Nonaka, M.; Ikeda, H.; Inokuchi, T. Inhibitory effect of heat shock protein 70 on apoptosis induced by photodynamic therapy in vitro. Photochem. Photobiol. 2004, 79, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kleban, J.; Mikes, J.; Horvath, V.; Sackova, V.; Hofmanova, J.; Kozubik, A.; Fedorocko, P. Mechanisms involved in the cell cycle and apoptosis of HT-29 cells pre-treated with mk-886 prior to photodynamic therapy with hypericin. J. Photochem. Photobiol. B 2008, 93, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and mir-205 regulate epithelial to mesenchymal transition by targeting zeb1 and sip1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.; Ress, A.L.; Winter, E.; Stiegelbauer, V.; Karbiener, M.; Schwarzenbacher, D.; Scheideler, M.; Ivan, C.; Jahn, S.W.; Kiesslich, T.; et al. miR-200a regulates epithelial to mesenchymal transition-related gene expression and determines prognosis in colorectal cancer patients. Br. J. Cancer 2014, 110, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Ekshyyan, O.; Aw, T.Y. Decreased susceptibility of differentiated pc12 cells to oxidative challenge: Relationship to cellular redox and expression of apoptotic protease activator factor-1. Cell Death Differ. 2005, 12, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Sugihara, H.; Watanabe, M.; Sawayama, H.; Iwatsuki, M.; Baba, Y.; Okabe, H.; Hidaka, K.; Yokoyama, N.; Miyake, K.; et al. Macrophage-derived reactive oxygen species suppress miR-328 targeting CD44 in cancer cells and promote redox adaptation. Carcinogenesis 2013, 35, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Gammon, L.; Biddle, A.; Heywood, H.K.; Johannessen, A.C.; Mackenzie, I.C. Sub-sets of cancer stem cells differ intrinsically in their patterns of oxygen metabolism. PLoS One 2013, 8, e62493. [Google Scholar]
- Giannoni, E.; Buricchi, F.; Grimaldi, G.; Parri, M.; Cialdai, F.; Taddei, M.L.; Raugei, G.; Ramponi, G.; Chiarugi, P. Redox regulation of anoikis: Reactive oxygen species as essential mediators of cell survival. Cell Death Differ. 2008, 15, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Park, S.H.; Cieply, B.; Schupp, J.; Killiam, E.; Zhang, F.; Rimm, D.L.; Frisch, S.M. A pathway for the control of anoikis sensitivity by e-cadherin and epithelial-to-mesenchymal transition. Mol. Cell. Biol. 2011, 31, 4036–4051. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Tan, M.J.; Huang, R.L.; Tan, C.K.; Chong, H.C.; Pal, M.; Lam, C.R.; Boukamp, P.; Pan, J.Y.; Tan, S.H.; et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2−:H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell 2011, 19, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.; Clement, M.V. Superoxide anion: Oncogenic reactive oxygen species? Int. J. Biochem. Cell Biol. 2007, 39, 1297–1304. [Google Scholar] [CrossRef]
- Kemmerling, R.; Alinger, B.; Dietze, O.; Bosmuller, H.C.; Ocker, M.; Wolkersdorfer, G.W.; Berr, F.; Neureiter, D.; Kiesslich, T. Association of stem cell marker expression pattern and survival in human biliary tract cancer. Int. J. Oncol. 2012, 41, 511–522. [Google Scholar] [PubMed]
- Berlanda, J.; Kiesslich, T.; Engelhardt, V.; Krammer, B.; Plaetzer, K. Comparative in vitro study on the characteristics of different photosensitizers employed in PDT. J. Photochem. Photobiol. B 2010, 100, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Dysart, J.S.; Patterson, M.S. Characterization of photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys. Med. Biol. 2005, 50, 2597–2616. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Rimington, C.; Malik, Z. Photoinduced degradation and modification of photofrin II in cells in vitro. Photochem. Photobiol. 1988, 47, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.G.; Foster, T.H. Oxygen diffusion and reaction kinetics in the photodynamic therapy of multicell tumour spheroids. Phys. Med. Biol. 1994, 39, 2161–2181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Mirdb: A microRNA target prediction and functional annotation database with a wiki interface. RNA 2008, 14, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Robin, A.M.; Katakowski, M.; Tong, L.; Espiritu, M.; Singh, G.; Chopp, M. Photodynamic therapy with photofrin in combination with buthionine sulfoximine (BSO) of human glioma in the nude rat. Lasers Med. Sci. 2003, 18, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.P.; Mollenauer, M.N.; Xu, Z.; Turck, C.W.; Weiss, A. Akt-dependent phosphorylation specifically regulates cot induction of NF-κB-dependent transcription. Mol. Cell. Biol. 2002, 22, 5962–5974. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.C.; Wong, S.; Gomer, C.J. Photodynamic therapy mediated induction of early response genes. Cancer Res. 1994, 54, 1374–1380. [Google Scholar] [PubMed]
- Xia, Y.; Wang, J.; Liu, T.J.; Yung, W.K.; Hunter, T.; Lu, Z. c-Jun downregulation by HDAC3-dependent transcriptional repression promotes osmotic stress-induced cell apoptosis. Mol. Cell 2007, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.; Danesh, F.R. Identification of microrna-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J. Biol. Chem. 2010, 285, 23457–23465. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Wu, R.F.; Gu, Y.; Yang, Y.S.; Yang, M.C.; Nwariaku, F.E.; Terada, L.S. Involvement of traf4 in oxidative activation of c-Jun N-terminal kinase. J. Biol. Chem. 2002, 277, 28051–28057. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, A.; Mayr, C.; Bach, D.; Illig, R.; Plaetzer, K.; Berr, F.; Pichler, M.; Neureiter, D.; Kiesslich, T. MicroRNAs Associated with the Efficacy of Photodynamic Therapy in Biliary Tract Cancer Cell Lines. Int. J. Mol. Sci. 2014, 15, 20134-20157. https://doi.org/10.3390/ijms151120134
Wagner A, Mayr C, Bach D, Illig R, Plaetzer K, Berr F, Pichler M, Neureiter D, Kiesslich T. MicroRNAs Associated with the Efficacy of Photodynamic Therapy in Biliary Tract Cancer Cell Lines. International Journal of Molecular Sciences. 2014; 15(11):20134-20157. https://doi.org/10.3390/ijms151120134
Chicago/Turabian StyleWagner, Andrej, Christian Mayr, Doris Bach, Romana Illig, Kristjan Plaetzer, Frieder Berr, Martin Pichler, Daniel Neureiter, and Tobias Kiesslich. 2014. "MicroRNAs Associated with the Efficacy of Photodynamic Therapy in Biliary Tract Cancer Cell Lines" International Journal of Molecular Sciences 15, no. 11: 20134-20157. https://doi.org/10.3390/ijms151120134
APA StyleWagner, A., Mayr, C., Bach, D., Illig, R., Plaetzer, K., Berr, F., Pichler, M., Neureiter, D., & Kiesslich, T. (2014). MicroRNAs Associated with the Efficacy of Photodynamic Therapy in Biliary Tract Cancer Cell Lines. International Journal of Molecular Sciences, 15(11), 20134-20157. https://doi.org/10.3390/ijms151120134







