Endothelial Semaphorin 7A Promotes Inflammation in Seawater Aspiration-Induced Acute Lung Injury
Abstract
:1. Introduction
2. Results
2.1. Changes of Lung Histopathology and Pro-Inflammation Cytokines after Seawater Instillation
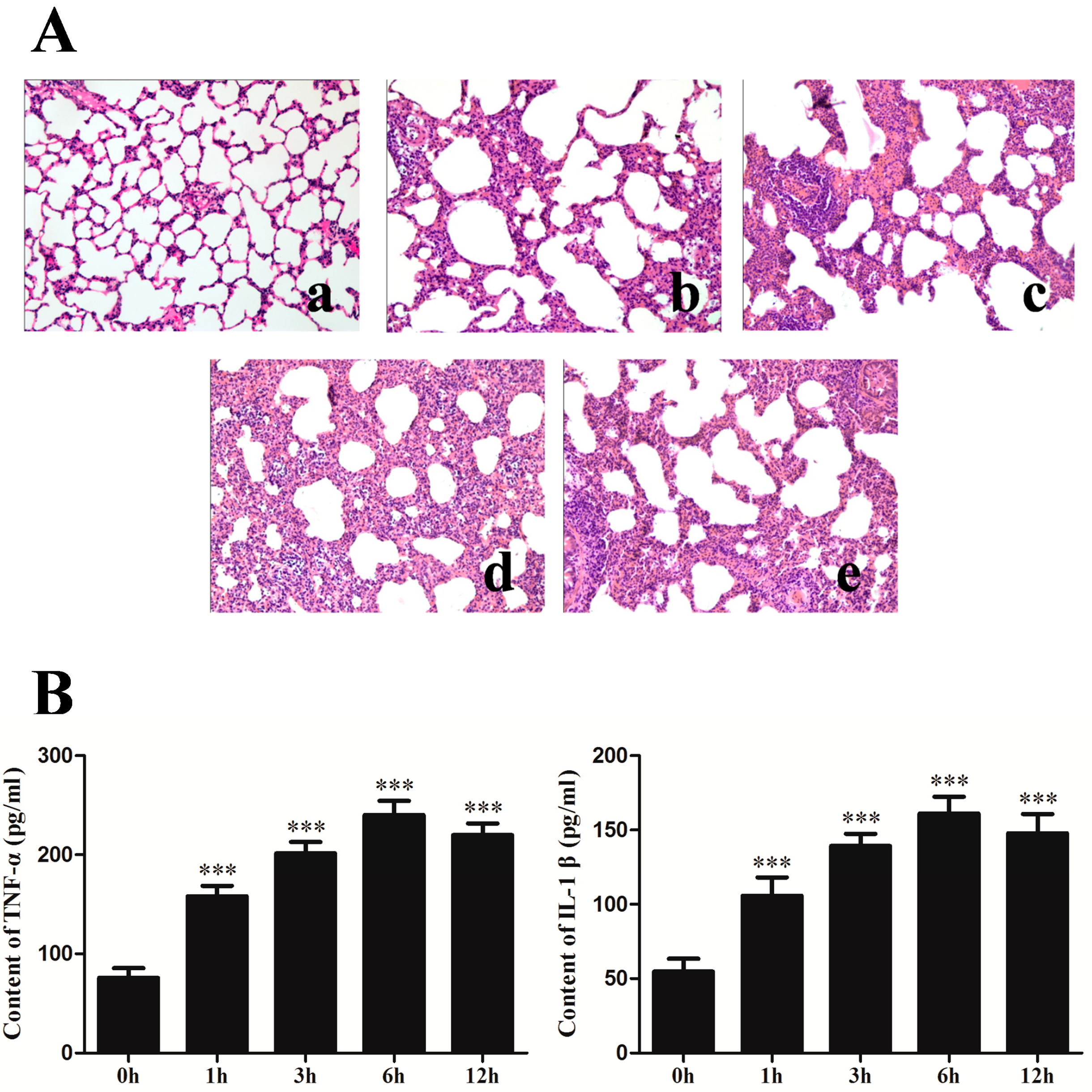
2.2. Rat Pulmonary Microvascular Endothelial Cell (RPMVEC) Characteristics
2.3. Changes of Semaphorin 7A (SEMA7A) Expression in Seawater Aspiration-Induced Acute Lung Injury (ALI)
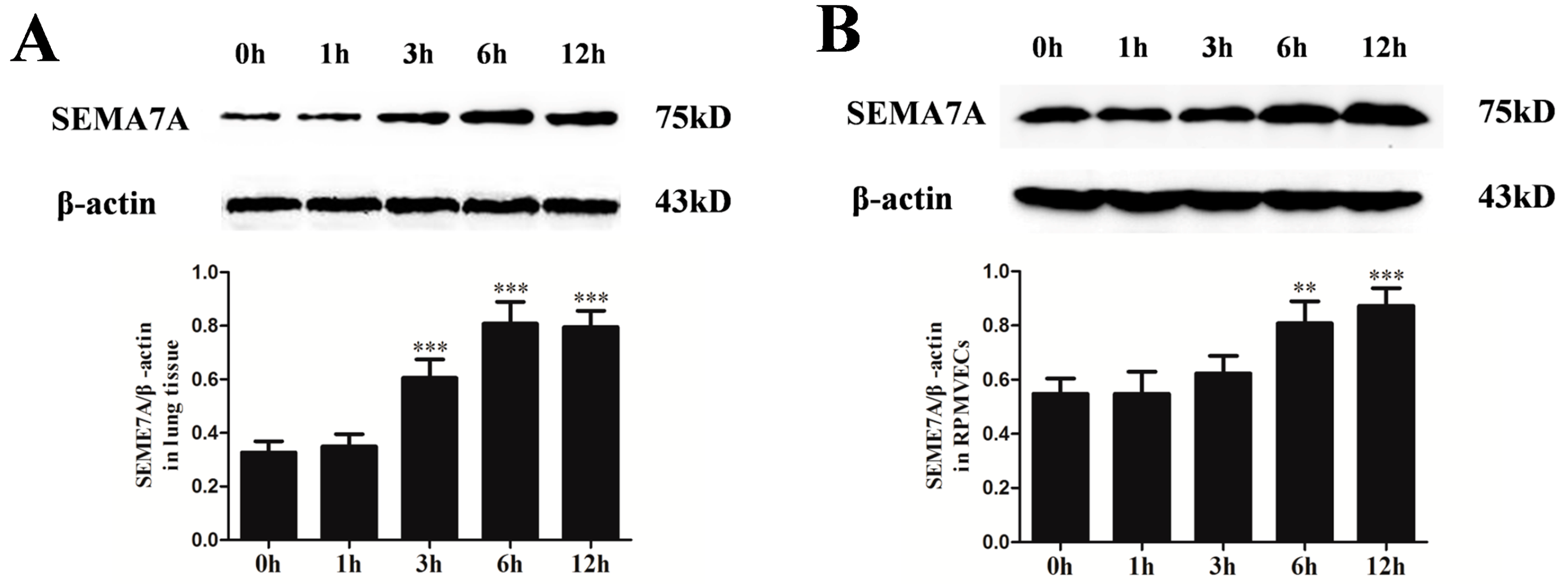
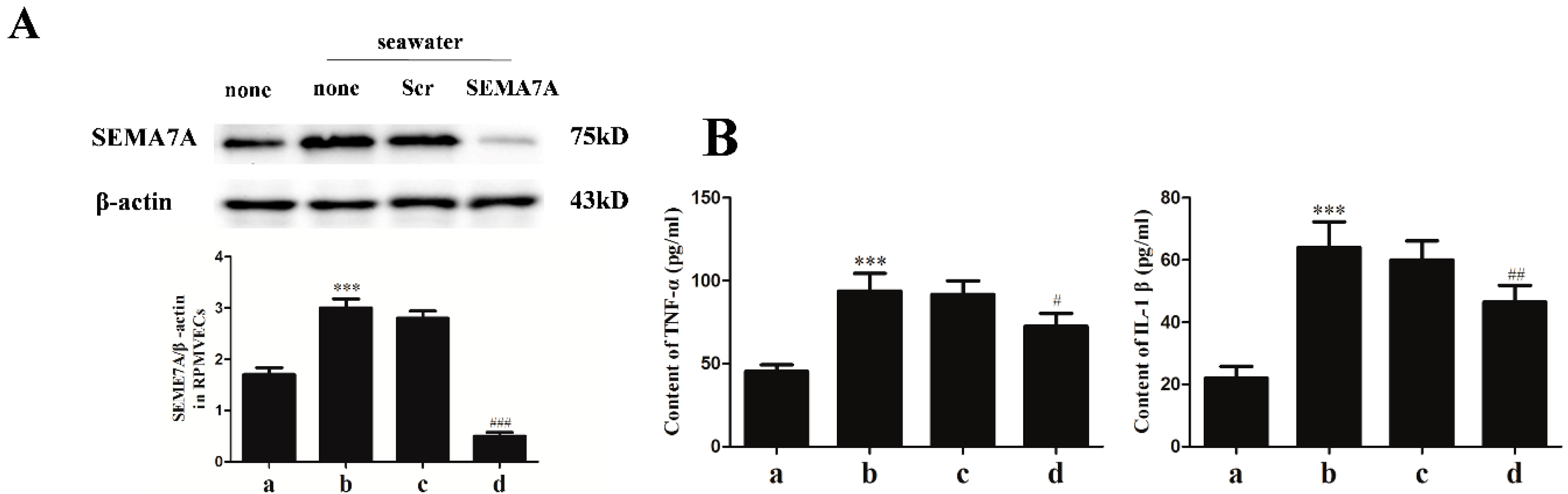
2.4. Specific siRNA against SEMA7A
2.5. Endothelial SEMA7A Promotes Pro-Inflammation Cytokines Production in Seawater Aspiration-Induced ALI
2.6. Endothelial SEMA7A Promotes Cytoskeletal Remodeling and Monolayer Permeability in RPMVECs Stimulated by Seawater
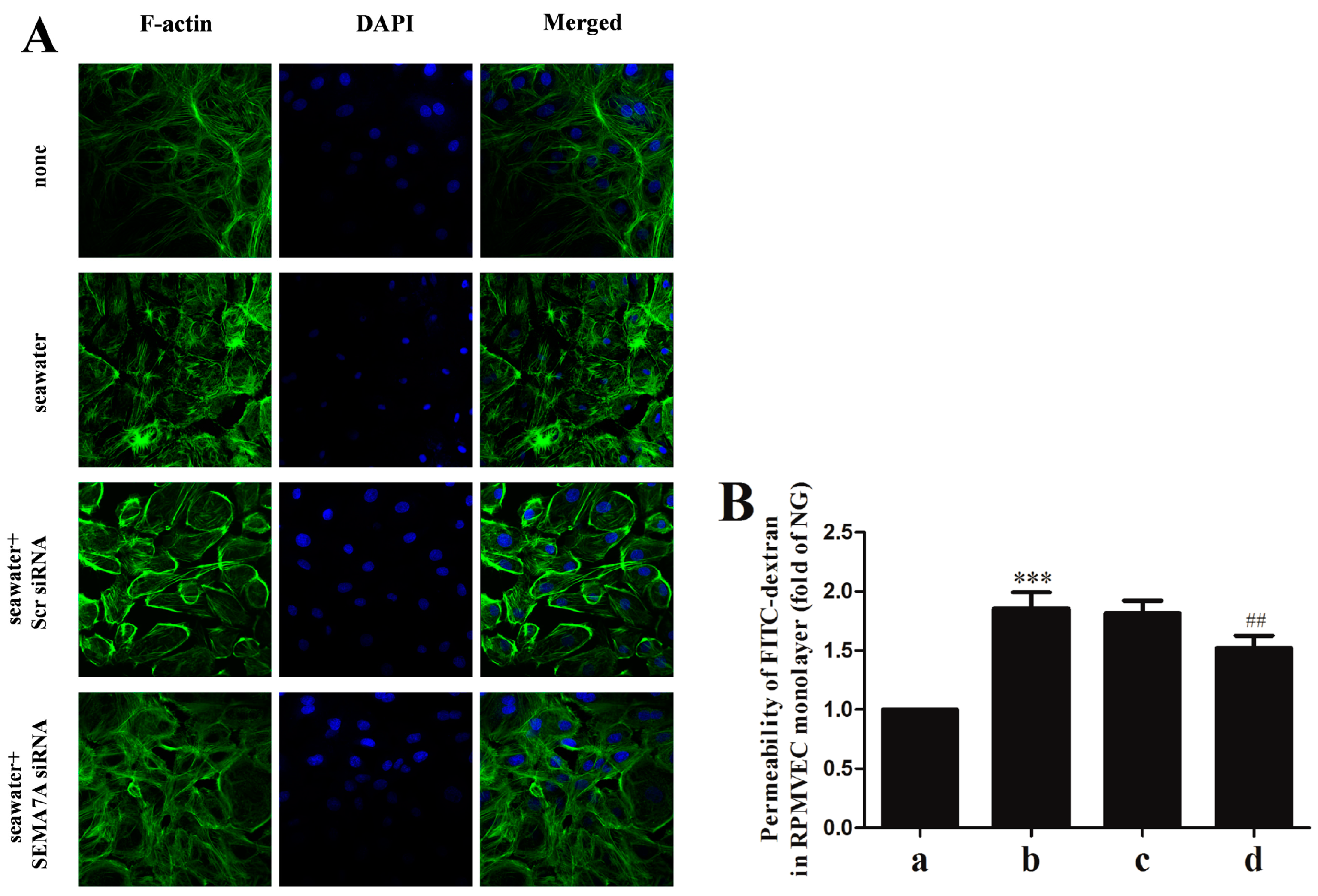
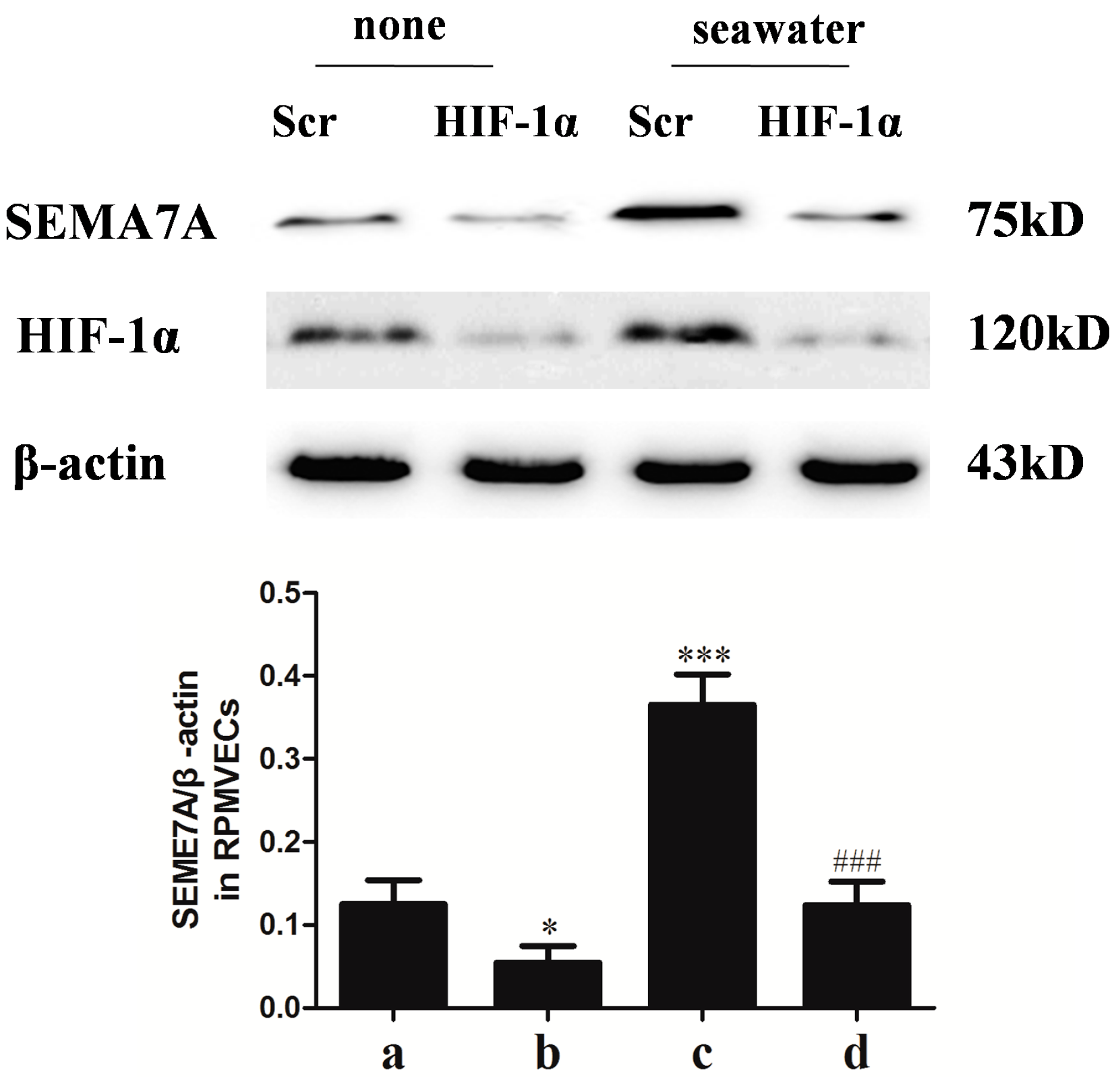
2.7. Hypoxia-Inducible Factor (HIF)-1α Mediated the Seawater-Stimulated Increase of SEMA7A in RPMVECs
3. Discussion
4. Experimental Section
4.1. Animals Preparation
4.2. Drug and Reagents
4.3. Lung Morphology
4.4. Measurement of Pro-Inflammation Cytokines
4.5. RPMVECs Culture and siRNA Transfection
4.6. Determination of SEMA7A mRNA
4.7. Western Blot Analysis
4.8. Immunofluorescent Staining
4.9. Measurement of RPMVECs Monolayer Permeability
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frutos-Vivar, F.; Ferguson, N.D.; Esteban, A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2006, 27, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, L.M.; Koch, T. Submersion and asphyxial injury. Crit. Care Med. 2002, 30, S402–S408. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-Y.; Zhang, B.; Li, J.-H.; Fan, Q.-X.; Zhang, Y.; Mu, D.-G.; Li, W.-P.; Xu, M.; Zhao, P.-T.; Jin, F.-G.; et al. Sodium tanshinone iia sulfonate attenuates seawater aspiration-induced acute pulmonary edema by up-regulating Na+,K+-ATPase activity. Exp. Lung Res. 2011, 37, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Duan, Y.; Peng, C.; Feng, H.; Zhou, P.; Meng, J.; Xue, Z.; Xu, Q. Dexamethasone treatment attenuates early seawater instillation-induced acute lung injury in rabbits. Pharmacol. Res. 2006, 53, 372–379. [Google Scholar]
- Konrad, F.M.; Witte, E.; Vollmer, I.; Stark, S.; Reutershan, J. Adenosine receptor A2b on hematopoietic cells mediates LPS-induced migration of PMNs into the lung interstitium. Am. J. Physiol. 2012, 303, L425–L438. [Google Scholar]
- Walmsley, S.R.; Print, C.; Farahi, N.; Peyssonnaux, C.; Johnson, R.S.; Cramer, T.; Sobolewski, A.; Condliffe, A.M.; Cowburn, A.S.; Johnson, N.; et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J. Exp. Med. 2005, 201, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Puschel, A.W.; Adams, R.H.; Betz, H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron 1995, 14, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin, A.L.; Matthes, D.J.; Goodman, C.S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 1993, 75, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shepherd, I.; Li, J.; Renzi, M.J.; Chang, S.; Raper, J. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron 1995, 14, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; Downs, A.-M.; Fosberry, A.; Hayes, P.D.; Michalovich, D.; Murdoch, P.; Moores, K.; Fox, J.; Deen, K.; Pettman, G.; et al. Sema7A is a potent monocyte stimulator. Scand. J. Immunol. 2002, 56, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okuno, T.; Yamamoto, M.; Pasterkamp, R.J.; Takegahara, N.; Takamatsu, H.; Kitao, T.; Takagi, J.; Rennert, P.D.; Kolodkin, A.L.; et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through α1β1 integrin. Nature 2007, 446, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Lazova, R.; Gould, R.; Bonnie, E.; Rimm, D.; Scott, G. The semaphorin 7A receptor Plexin C1 is lost during melanoma metastasis. Am. J. Dermatopathol. 2009, 31, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, R.J.; Peschon, J.J.; Melanie, M.K.; Kolodkin, A.L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003, 424, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Morote-Garcia, J.C.; Napiwotzky, D.; Kohler, D.; Rosenberger, P. Endothelial Semaphorin 7A promotes neutrophil migration during hypoxia. Proc. Natl. Acad. Sci. USA 2012, 109, 14146–14151. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhao, Y.; Li, B.; Wang, Q.; Liu, X.; Chen, X.; Nan, Y.; Liang, L.; Chang, R.; Liang, L.; et al. 3,5,4'-Tri-O-acetylresveratrol attenuates seawater aspiration-induced lung injury by inhibiting activation of nuclear factor-kappa B and hypoxia-inducible factor-1α. Respir. Physiol. Neurobiol. 2013, 185, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, M.; Fan, Q.; Xie, X.; Zhang, Y.; Mu, D.; Zhao, P.; Zhang, B.; Cao, F.; Wang, Y.; et al. Tanshinone IIA ameliorates seawater exposure-induced lung injury by inhibiting aquaporins (AQP) 1 and AQP5 expression in lung. Respir. Physiol. Neurobiol. 2011, 176, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Zhao, P.; Li, J.; Xie, X.; Xu, M.; Zhang, Y.; Mu, D.; Li, W.; Sun, R.; Liu, W.; et al. 17β-Estradiol administration attenuates seawater aspiration-induced acute lung injury in rats. Pulm. Pharmacol. Ther. 2011, 24, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Luo, Y.; Li, Y.; Liu, Z.; Xu, D.; Jin, F.; Li, Z. Seawater induces apoptosis in alveolar epithelial cells via the Fas/FasL-mediated pathway. Respir. Physiol. Neurobiol. 2012, 182, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Matsuura, M.; Suzuki, K.; Yamamoto, N. Cooperative activity of multiple upper layer proteins for thalamocortical axon growth. Dev. Neurobiol. 2008, 68, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Czopik, A.K.; Bynoe, M.S.; Palm, N.; Raine, C.S.; Medzhitov, R. Semaphorin 7A is a negative regulator of T cell responses. Immunity 2006, 24, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Reilkoff, R.; Peng, X.; Russell, T.; Chen, Q.; Mathai, S.K.; Homer, R.; Gulati, M.; Siner, J.; Elias, J.; et al. Role of semaphorin 7a signaling in transforming growth factor β1-induced lung fibrosis and scleroderma-related interstitial lung disease. Arthritis Rheumatol. 2011, 63, 2484–2494. [Google Scholar] [CrossRef]
- Ecklund, M.M.; Wahl, G.; Yamshchikov, A.V.; Smith, M.S. Journey of a survivor of near drowning, polymicrobial pneumonia, and acute respiratory distress syndrome. Crit. Care Nurs. Clin. N. Am. 2012, 24, 601–623. [Google Scholar] [CrossRef]
- Chen, S.F.; Fei, X.; Li, S.H. A new simple method for isolation of microvascular endothelial cells avoiding both chemical and mechanical injuries. Microvasc. Res. 1995, 50, 119–128. [Google Scholar] [CrossRef] [PubMed]
- You, Q.-H.; Sun, G.-Y.; Wang, N.; Shen, J.-L.; Wang, Y. Interleukin-17F-induced pulmonary microvascular endothelial monolayer hyperpermeability via the protein kinase C pathway. J. Surg. Res. 2010, 162, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pendyala, S.; Natarajan, V.; Garcia, J.G.N.; Jacobson, J.R. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am. J. Physiol. 2008, 295, L575–L583. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, L.; Dong, M.; Li, Z.; Jin, F. Endothelial Semaphorin 7A Promotes Inflammation in Seawater Aspiration-Induced Acute Lung Injury. Int. J. Mol. Sci. 2014, 15, 19650-19661. https://doi.org/10.3390/ijms151119650
Zhang M, Wang L, Dong M, Li Z, Jin F. Endothelial Semaphorin 7A Promotes Inflammation in Seawater Aspiration-Induced Acute Lung Injury. International Journal of Molecular Sciences. 2014; 15(11):19650-19661. https://doi.org/10.3390/ijms151119650
Chicago/Turabian StyleZhang, Minlong, Li Wang, Mingqing Dong, Zhichao Li, and Faguang Jin. 2014. "Endothelial Semaphorin 7A Promotes Inflammation in Seawater Aspiration-Induced Acute Lung Injury" International Journal of Molecular Sciences 15, no. 11: 19650-19661. https://doi.org/10.3390/ijms151119650
APA StyleZhang, M., Wang, L., Dong, M., Li, Z., & Jin, F. (2014). Endothelial Semaphorin 7A Promotes Inflammation in Seawater Aspiration-Induced Acute Lung Injury. International Journal of Molecular Sciences, 15(11), 19650-19661. https://doi.org/10.3390/ijms151119650



