CYP24A1 Expression Inversely Correlates with Melanoma Progression: Clinic-Pathological Studies
Abstract
:1. Introduction
2. Results
2.1. CYP24A1 Immunostaining in Melanocytic Lesions
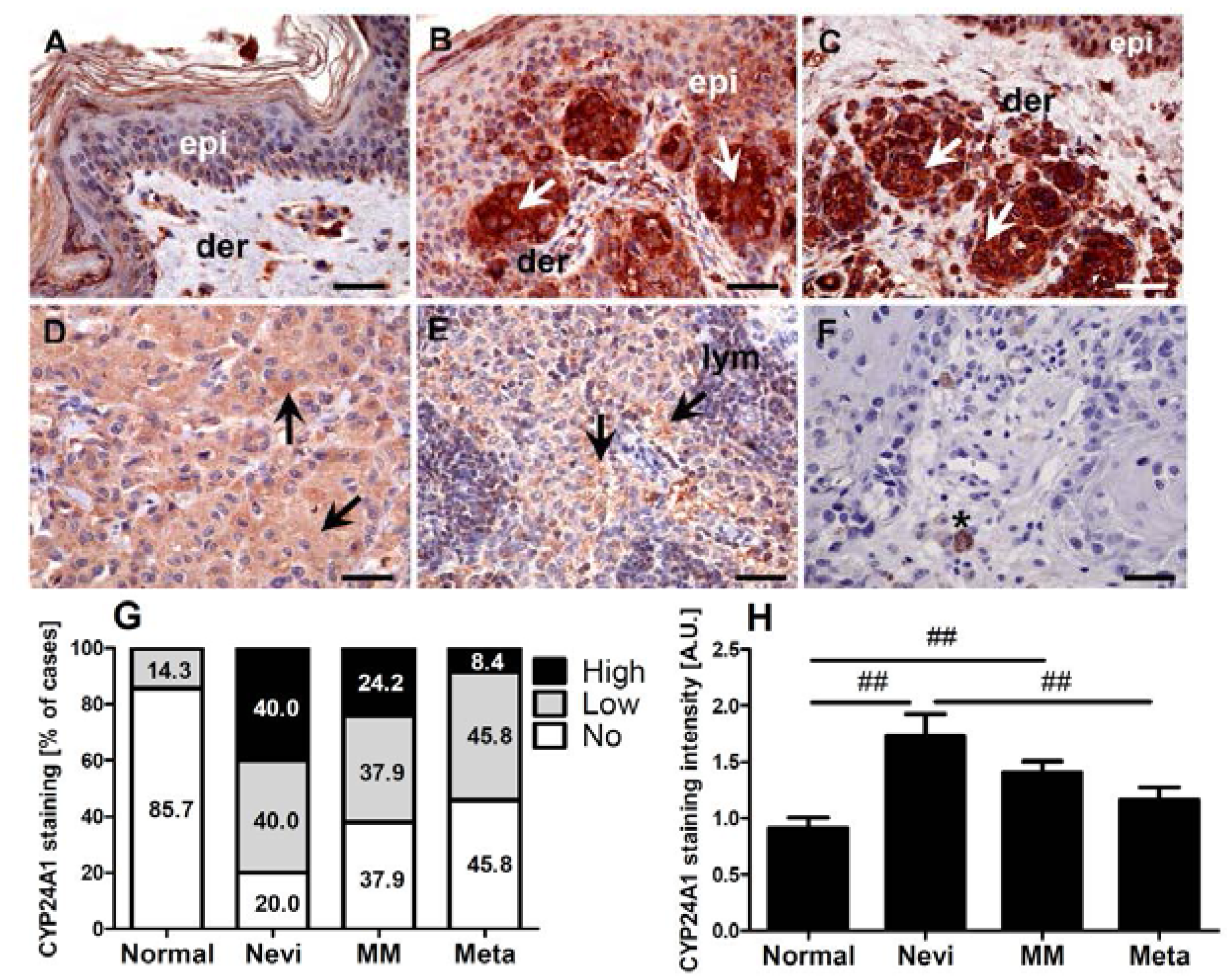
2.2. Expression of CYP24A1 in Cultured Melanoma Cells, Epidermal Melanocytes and Keratinocytes
| Identification | Cell Type | Mean Cp Value ± SD (Lower Number Means Higher Expression Level) | Statistical Significance (p Value) vs. | |||||
|---|---|---|---|---|---|---|---|---|
| HEMn | HEMa | HEKn | HEKa | HaCaT | ||||
| YUWERA | Human melanoma | 10.3 ± 0.2 | <0.001 | <0.01 | NS | NS | <0.001 | |
| YUTICA | Human melanoma | 12.0 ± 0.4 | <0.001 | <0.001 | NS | NS | <0.001 | |
| YUKSI | Human melanoma | 17.3 ± 0.3 | <0.001 | <0.001 | NS | NS | <0.001 | |
| YULAC | Human melanoma | 14.5 ± 0.3 | <0.001 | <0.001 | NS | NS | <0.001 | |
| YUSIV | Human melanoma | 15.2 ± 0.3 | <0.001 | <0.001 | NS | NS | <0.001 | |
| YUAME | Human melanoma | 4.6 ± 0.3 | NS | <0.01 | NS | NS | <0.001 | |
| YUROB | Human melanoma | 15.2 ± 0.1 | <0.001 | <0.001 | NS | NS | <0.001 | |
| YUCOT | Human melanoma | 8.3 ± 0.3 | <0.001 | NS | NS | NS | <0.01 | |
| YUKIM | Human melanoma | 6.7 ± 0.4 | <0.01 | NS | NS | NS | NS | |
| YUMUT | Human melanoma | 11.6 ± 0.2 | <0.001 | <0.001 | NS | NS | <0.001 | |
| YUKOLI | Human melanoma | 9.7 ± 0.1 | <0.001 | <0.01 | NS | NS | <0.001 | |
| SBCE2 | Human melanoma | 9.3 ± 0.5 | <0.001 | <0.05 | NS | NS | <0.01 | |
| WM1341 | Human melanoma, amelanotic | 11.3 ± 0.2 | <0.001 | <0.001 | NS | NS | <0.001 | |
| HEMn | Epidermal melanocytes, neonatal | 4.1 ± 0.1 | NA | <0.001 | <0.001 | <0.01 | <0.001 | |
| HEMa | Epidermal melanocytes, adult | 7.7 ± 0.4 | <0.001 | NA | NS | NS | <0.01 | |
| HEKn | Epidermal keratinocytes, neonatal | 9.3 ± 3.0 | <0.001 | NS | NA | NS | NS | |
| HEKa | Epidermal keratinocytes, adult | 8.0 ± 2.7 | <0.01 | NS | NS | NA | <0.01 | |
| HaCaT | Immortalised keratinocytes | 6.7 ± 0.07 | <0.001 | <0.01 | NS | <0.01 | NA | |
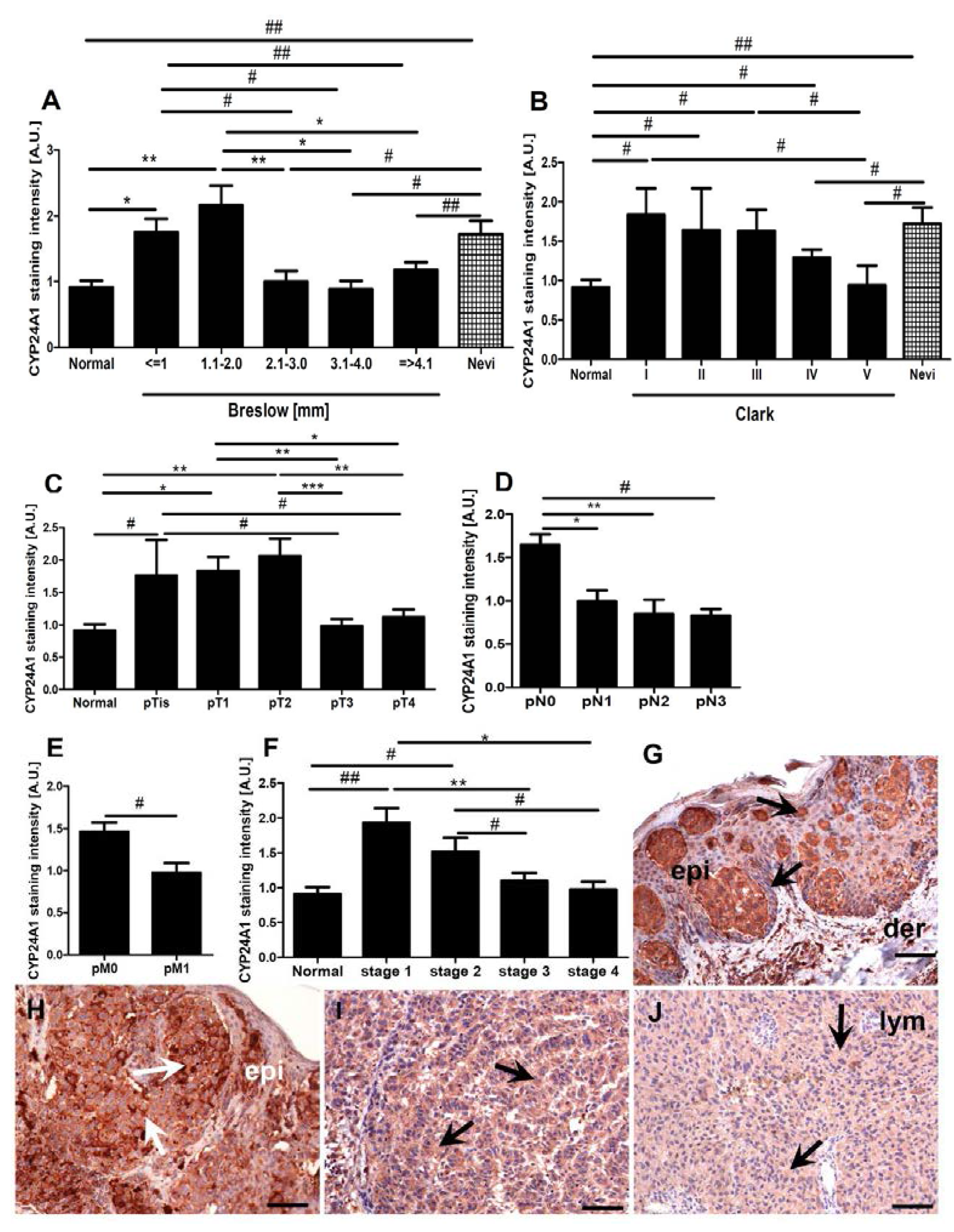
2.3. CYP24A1 Immunostaining in Relation to Melanoma Pathomorphological Features

2.4. Correlations between CYP24A1, Vitamin D Receptor (VDR) and CYP27B1 Immunostaining
2.5. Correlation between CYP24A1 Immunostaining and Overall Survival
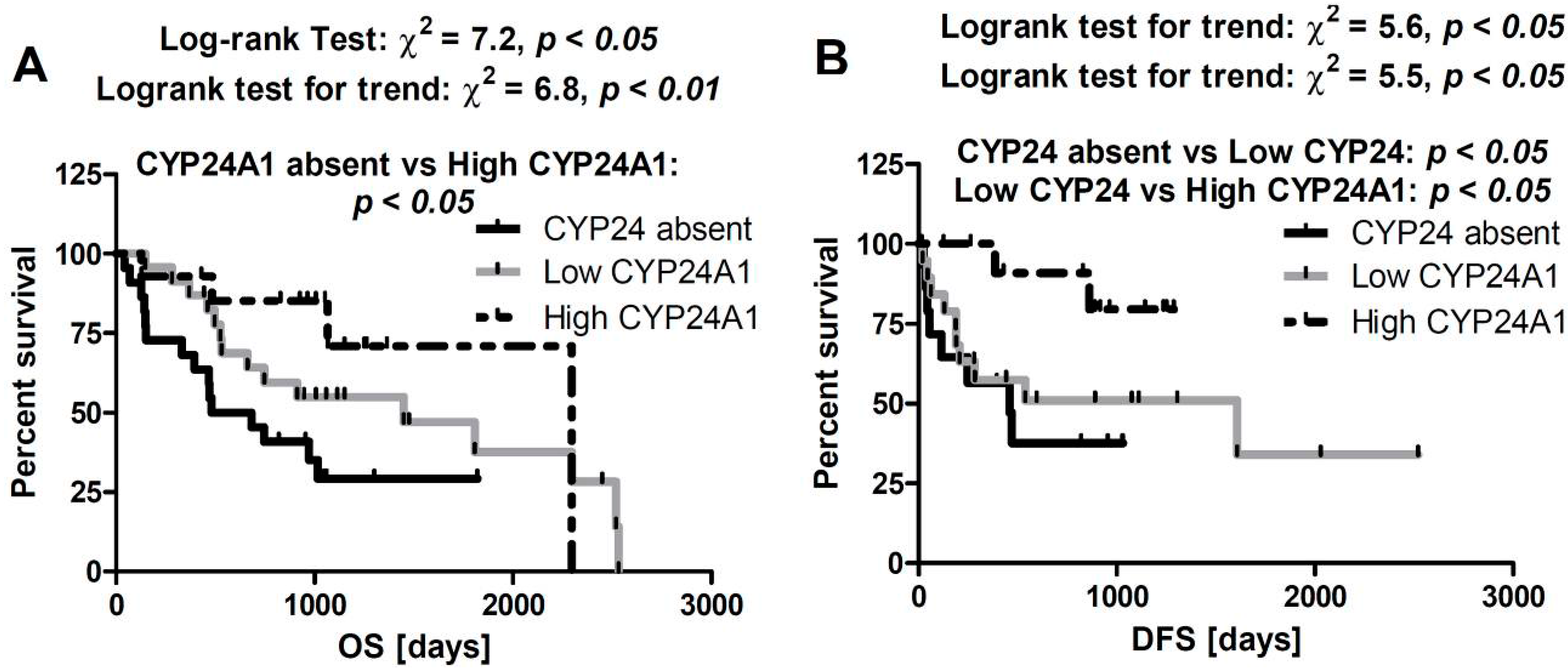
3. Discussion
4. Materials and Methods
4.1. Patients
| Clinicopathologic Features | No. | |
|---|---|---|
| Type of Lesions | All samples | 104 |
| Nevi | 15 | |
| Primary melanomas | 57 | |
| Nodular | 31 | |
| Superficial spreading | 26 | |
| Melanoma metastases | 25 | |
| Normal skin | 7 | |
| Age (year) | Nevi: mean = 43, median = 36; range = 20–85 | 15 |
| Melanomas: mean = 60, median = 57; range = 25–100 | 57 | |
| Patients’ Gender | Nevi: M/F | 5/10 |
| Melanomas: M/F | 28/29 | |
| Anatomical Site | Nevi | 15 |
| Extremity | 2 | |
| Head and neck | 3 | |
| Trunk | 10 | |
| Melanoma | 57 | |
| Acral | 2 | |
| Anogenital | 2 | |
| Extremity | 19 | |
| Head and neck | 12 | |
| Trunk | 22 | |
| Breslow Thickness (mm) | 0 (is) | 3 |
| 0–1 | 13 | |
| 1.1–2 | 6 | |
| 2.1–3 | 9 | |
| 3.1–4 | 4 | |
| >4.0 | 22 | |
| Clark Level | I | 7 |
| II | 3 | |
| III | 8 | |
| IV | 32 | |
| V | 7 | |
| pT | pT0 | 3 |
| pT1 | 13 | |
| pT2 | 7 | |
| pT3 | 14 | |
| pT4 | 20 | |
| pN | pN0 | 29 |
| pN1 | 13 | |
| pN2 | 5 | |
| pN3 | 9 | |
| pM | pM0 | 50 |
| pM1 | 7 | |
| Overall Stage | 0 | 3 |
| 1 | 13 | |
| 2 | 14 | |
| 3 | 20 | |
| 4 | 7 | |
4.2. Immunohistochemistry Staining and Evaluation
4.3. Cell Culture Studies
4.4. Reverse Transcription (RT) and Quantitative Polymerase Chain Reaction (qPCR)
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar]
- Holick, M.F. Vitamin D: Its role in cancer prevention and treatment. Prog. Biophys. Mol. Biol. 2006, 92, 49–59. [Google Scholar]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug. Discov. 2010, 9, 941–955. [Google Scholar]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar]
- Zehnder, D.; Bland, R.; Walker, E.A.; Bradwell, A.R.; Howie, A.J.; Hewison, M.; Stewart, P.M. Expression of 25-hydroxyvitamin D3-1α-hydroxylase in the human kidney. J. Am. Soc. Nephrol. 1999, 10, 2465–2473. [Google Scholar]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar]
- Tieu, E.W.; Tang, E.K.; Tuckey, R.C. Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. FEBS J. 2014, 281, 3280–3296. [Google Scholar]
- Schuster, I. Cytochromes p450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199. [Google Scholar]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome p450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar]
- Masuda, S.; Gao, M.; Zhang, A.; Kaufmann, M.; Jones, G. Importance of cytochrome p450-mediated metabolism in the mechanism of action of vitamin D analogs. Recent Results Cancer Res. 2003, 164, 189–202. [Google Scholar]
- King, A.N.; Beer, D.G.; Christensen, P.J.; Simpson, R.U.; Ramnath, N. The vitamin D/CYP24A1 story in cancer. Anticancer Agents Med. Chem. 2010, 10, 213–224. [Google Scholar]
- Sakaki, T.; Kagawa, N.; Yamamoto, K.; Inouye, K. Metabolism of vitamin D3 by cytochromes p450. Front. Biosci. 2005, 10, 119–134. [Google Scholar]
- Walentowicz-Sadlecka, M.; Grabiec, M.; Sadlecki, P.; Gotowska, M.; Walentowicz, P.; Krintus, M.; Mankowska-Cyl, A.; Sypniewska, G. 25(OH)D3 in patients with ovarian cancer and its correlation with survival. Clin. Biochem. 2012, 45, 1568–1572. [Google Scholar]
- Hauser, K.; Walsh, D.; Shrotriya, S.; Karafa, M. Low 25-hydroxyvitamin D levels in people with a solid tumor cancer diagnosis: The tip of the iceberg? Support. Care Cancer 2014, 22, 1931–1939. [Google Scholar]
- Robsahm, T.E.; Schwartz, G.G.; Tretli, S. The inverse relationship between 25-hydroxyvitamin D and cancer survival: Discussion of causation. Cancers 2013, 5, 1439–1455. [Google Scholar]
- Wang, D.; Velez de-la-Paz, O.I.; Zhai, J.X.; Liu, D.W. Serum 25-hydroxyvitamin D and breast cancer risk: A meta-analysis of prospective studies. Tumour Biol. 2013, 34, 3509–3517. [Google Scholar]
- Field, S.; Davies, J.; Bishop, D.T.; Newton-Bishop, J.A. Vitamin D and melanoma. Dermatoendocrinology 2013, 5, 121–129. [Google Scholar]
- Evans, K.N.; Taylor, H.; Zehnder, D.; Kilby, M.D.; Bulmer, J.N.; Shah, F.; Adams, J.S.; Hewison, M. Increased expression of 25-hydroxyvitamin D-1α-hydroxylase in dysgerminomas: A novel form of humoral hypercalcemia of malignancy. Am. J. Pathol. 2004, 165, 807–813. [Google Scholar]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. North Am. 2010, 39, 365–379. [Google Scholar]
- Lopes, N.; Sousa, B.; Martins, D.; Gomes, M.; Vieira, D.; Veronese, L.A.; Milanezi, F.; Paredes, J.; Costa, J.L.; Schmitt, F. Alterations in vitamin D signalling and metabolic pathways in breast cancer progression: A study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer 2010, 10, 483. [Google Scholar]
- Radermacher, J.; Diesel, B.; Seifert, M.; Tilgen, W.; Reichrath, J.; Fischer, U.; Meese, E. Expression analysis of CYP27B1 in tumor biopsies and cell cultures. Anticancer Res. 2006, 26, 2683–2686. [Google Scholar]
- Reichrath, J.; Rech, M.; Moeini, M.; Meese, E.; Tilgen, W.; Seifert, M. In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells. Cancer Biol. Ther. 2007, 6, 48–55. [Google Scholar]
- Tangpricha, V.; Flanagan, J.N.; Whitlatch, L.W.; Tseng, C.C.; Chen, T.C.; Holt, P.R.; Lipkin, M.S.; Holick, M.F. 25-hydroxyvitamin D-1α-hydroxylase in normal and malignant colon tissue. Lancet 2001, 357, 1673–1674. [Google Scholar]
- Brozyna, A.A.; Jozwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of the vitamin D-activating enzyme 1alpha-hydroxylase (CYP27B1) decreases during melanoma progression. Hum. Pathol. 2013, 44, 374–387. [Google Scholar]
- Fischer, D.; Thome, M.; Becker, S.; Cordes, T.; Diedrich, K.; Friedrich, M.; Thill, M. Expression of 25-hydroxyvitamin D3-24-hydroxylase in benign and malignant ovarian cell lines and tissue. Anticancer Res. 2009, 29, 3635–3639. [Google Scholar]
- Chen, T.C. 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) is a new class of tumor suppressor in the prostate. Anticancer Res. 2008, 28, 2015–2017. [Google Scholar]
- Fleet, J.C. Molecular actions of vitamin D contributing to cancer prevention. Mol. Aspects Med. 2008, 29, 388–396. [Google Scholar]
- Christakos, S.; DeLuca, H.F. Minireview: Vitamin D: Is there a role in extraskeletal health? Endocrinology 2011, 152, 2930–2936. [Google Scholar]
- Matusiak, D.; Murillo, G.; Carroll, R.E.; Mehta, R.G.; Benya, R.V. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1α-hydroxylase in normal and malignant human colon. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2370–2376. [Google Scholar]
- Chen, T.C.; Wang, L.; Whitlatch, L.W.; Flanagan, J.N.; Holick, M.F. Prostatic 25-hydroxyvitamin D-1α-hydroxylase and its implication in prostate cancer. J. Cell. Biochem. 2003, 88, 315–322. [Google Scholar]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar]
- Kosa, J.P.; Horvath, P.; Wolfling, J.; Kovacs, D.; Balla, B.; Matyus, P.; Horvath, E.; Speer, G.; Takacs, I.; Nagy, Z.; et al. CYP24A1 inhibition facilitates the anti-tumor effect of vitamin D3 on colorectal cancer cells. World J. Gastroenterol. 2013, 19, 2621–2628. [Google Scholar]
- Sakaki, T.; Yasuda, K.; Kittaka, A.; Yamamoto, K.; Chen, T.C. CYP24A1 as a potential target for cancer therapy. Anticancer Agents Med. Chem. 2014, 14, 97–108. [Google Scholar]
- Tannour-Louet, M.; Lewis, S.K.; Louet, J.F.; Stewart, J.; Addai, J.B.; Sahin, A.; Vangapandu, H.V.; Lewis, A.L.; Dittmar, K.; Pautler, R.G.; et al. Increased expression of CYP24A1 correlates with advanced stages of prostate cancer and can cause resistance to vitamin D3-based therapies. FASEB J. 2014, 28, 364–372. [Google Scholar]
- Brozyna, A.A.; Jozwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum. Pathol. 2011, 42, 618–631. [Google Scholar]
- Brozyna, A.A.; Jozwicki, W.; Slominski, A.T. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: New data and analyses. Anticancer Res. 2014, 34, 2735–2744. [Google Scholar]
- Janjetovic, Z.; Brozyna, A.A.; Tuckey, R.C.; Kim, T.K.; Nguyen, M.N.; Jozwicki, W.; Pfeffer, S.R.; Pfeffer, L.M.; Slominski, A.T. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br. J. Cancer 2011, 105, 1874–1884. [Google Scholar]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.K.; Wright, A.C.; Grese, L.N.; Riney, S.J.; Nguyen, M.N.; Tuckey, R.C. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012, 32, 3733–3742. [Google Scholar]
- Slominski, A.T.; Carlson, J.A. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin. Proc. 2014, 89, 429–433. [Google Scholar]
- Anderson, M.G.; Nakane, M.; Ruan, X.; Kroeger, P.E.; Wu-Wong, J.R. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother. Pharmacol. 2006, 57, 234–240. [Google Scholar]
- Urbschat, A.; Paulus, P.; von Quernheim, Q.F.; Bruck, P.; Badenhoop, K.; Zeuzem, S.; Ramos-Lopez, E. Vitamin D hydroxylases CYP2R1, CYP27B1 and CYP24A1 in renal cell carcinoma. Eur. J. Clin. Investig. 2012, 43, 1282–1290. [Google Scholar]
- Mimori, K.; Tanaka, Y.; Yoshinaga, K.; Masuda, T.; Yamashita, K.; Okamoto, M.; Inoue, H.; Mori, M. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann. Oncol. 2004, 15, 236–241. [Google Scholar]
- Chen, G.; Kim, S.H.; King, A.N.; Zhao, L.; Simpson, R.U.; Christensen, P.J.; Wang, Z.; Thomas, D.G.; Giordano, T.J.; Lin, L.; et al. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clin. Cancer Res. 2011, 17, 817–826. [Google Scholar]
- Horvath, H.C.; Lakatos, P.; Kosa, J.P.; Bacsi, K.; Borka, K.; Bises, G.; Nittke, T.; Hershberger, P.A.; Speer, G.; Kallay, E. The candidate oncogene CYP24A1: A potential biomarker for colorectal tumorigenesis. J. Histochem. Cytochem. 2010, 58, 277–285. [Google Scholar]
- Clinckspoor, I.; Hauben, E.; Verlinden, L.; van den Bruel, A.; Vanwalleghem, L.; Vander Poorten, V.; Delaere, P.; Mathieu, C.; Verstuyf, A.; Decallonne, B. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J. Histochem. Cytochem. 2012, 60, 502–511. [Google Scholar]
- Tieu, E.W.; Tang, E.K.; Chen, J.; Li, W.; Nguyen, M.N.; Janjetovic, Z.; Slominski, A.; Tuckey, R.C. Rat CYP24A1 acts on 20-hydroxyvitamin D3 producing hydroxylated products with increased biological activity. Biochem. Pharmacol. 2012, 84, 1696–1704. [Google Scholar]
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Li, W.; Szczesniewski, A.; Tuckey, R.C. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005, 272, 4080–4090. [Google Scholar]
- Tuckey, R.C.; Li, W.; Zjawiony, J.K.; Zmijewski, M.A.; Nguyen, M.N.; Sweatman, T.; Miller, D.; Slominski, A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008, 275, 2585–2596. [Google Scholar]
- Slominski, A.T.; Kim, T.-K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.Y.; Nguyen, M.N.; Benson, H.A.E.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar]
- Slominski, A.T.; Kim, T.K.; Li, W.; Yi, A.K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144, 28–39. [Google Scholar]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Tuckey, R.C.; Bieniek, R.; Yue, J.; Li, W.; Chen, J.; Nguyen, M.N.; Tang, E.K.; et al. 20-hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol. 2011, 300, C526–C541. [Google Scholar]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar]
- St-Arnaud, R.; Glorieux, F.H. 24,25-dihydroxyvitamin D-active metabolite or inactive catabolite? Endocrinology 1998, 139, 3371–3374. [Google Scholar]
- Lee, N.E.; Reddy, G.S.; Brown, A.J.; Williard, P.G. Synthesis, stereochemistry, and biological activity of 1α,23,25-trihydroxy-24-oxovitamin D3, a major natural metabolite of 1α,25-dihydroxyvitamin D3. Biochemistry 1997, 36, 9429–9437. [Google Scholar]
- Larsson, D.; Anderson, D.; Smith, N.M.; Nemere, I. 24,25-dihydroxyvitamin D3 binds to catalase. J. Cell. Biochem. 2006, 97, 1259–1266. [Google Scholar]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar]
- Slominski, A.; Kim, T.K.; Brozyna, A.A.; Janjetovic, Z.; Brooks, D.L.; Schwab, L.P.; Skobowiat, C.; Jozwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014. [Google Scholar] [CrossRef]
- Brozyna, A.A.; Jozwicki, W.; Carlson, J.A.; Slominski, A.T. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum. Pathol. 2013, 44, 2071–2074. [Google Scholar]
- Jozwicki, W.; Windorbska, W.; Brozyna, A.A.; Jochymski, C.; Basta, P.; Sikora, J.; Stasienko, E.; Dutsch-Wicherek, M.; Koper, K.; Wicherek, L. The analysis of receptor-binding cancer antigen expressed on siso cells (RCAS1) immunoreactivity within the microenvironment of the ovarian cancer lesion relative to the applied therapeutic strategy. Cell Tissue Res. 2011, 345, 405–414. [Google Scholar]
- Halaban, R.; Zhang, W.; Bacchiocchi, A.; Cheng, E.; Parisi, F.; Ariyan, S.; Krauthammer, M.; McCusker, J.P.; Kluger, Y.; Sznol, M. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of braf melanoma cells. Pigment Cell Melanoma Res. 2010, 23, 190–200. [Google Scholar]
- Janjetovic, Z.; Zmijewski, M.A.; Tuckey, R.C.; DeLeon, D.A.; Nguyen, M.N.; Pfeffer, L.M.; Slominski, A.T. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-κB activity by increasing IκBα levels in human keratinocytes. PLoS One 2009, 4, e5988. [Google Scholar]
- Slominski, A.; Moellmann, G.; Kuklinska, E.; Bomirski, A.; Pawelek, J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, l-tyrosine and l-dopa. J. Cell Sci. 1998, 89, 287–296. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brożyna, A.A.; Jochymski, C.; Janjetovic, Z.; Jóźwicki, W.; Tuckey, R.C.; Slominski, A.T. CYP24A1 Expression Inversely Correlates with Melanoma Progression: Clinic-Pathological Studies. Int. J. Mol. Sci. 2014, 15, 19000-19017. https://doi.org/10.3390/ijms151019000
Brożyna AA, Jochymski C, Janjetovic Z, Jóźwicki W, Tuckey RC, Slominski AT. CYP24A1 Expression Inversely Correlates with Melanoma Progression: Clinic-Pathological Studies. International Journal of Molecular Sciences. 2014; 15(10):19000-19017. https://doi.org/10.3390/ijms151019000
Chicago/Turabian StyleBrożyna, Anna A., Cezary Jochymski, Zorica Janjetovic, Wojciech Jóźwicki, Robert C. Tuckey, and Andrzej T. Slominski. 2014. "CYP24A1 Expression Inversely Correlates with Melanoma Progression: Clinic-Pathological Studies" International Journal of Molecular Sciences 15, no. 10: 19000-19017. https://doi.org/10.3390/ijms151019000
APA StyleBrożyna, A. A., Jochymski, C., Janjetovic, Z., Jóźwicki, W., Tuckey, R. C., & Slominski, A. T. (2014). CYP24A1 Expression Inversely Correlates with Melanoma Progression: Clinic-Pathological Studies. International Journal of Molecular Sciences, 15(10), 19000-19017. https://doi.org/10.3390/ijms151019000





