Characterization, Expression Profile, and Promoter Analysis of the Rhodeus uyekii Vitellogenin Ao1 Gene
Abstract
:1. Introduction
2. Results and Discussion
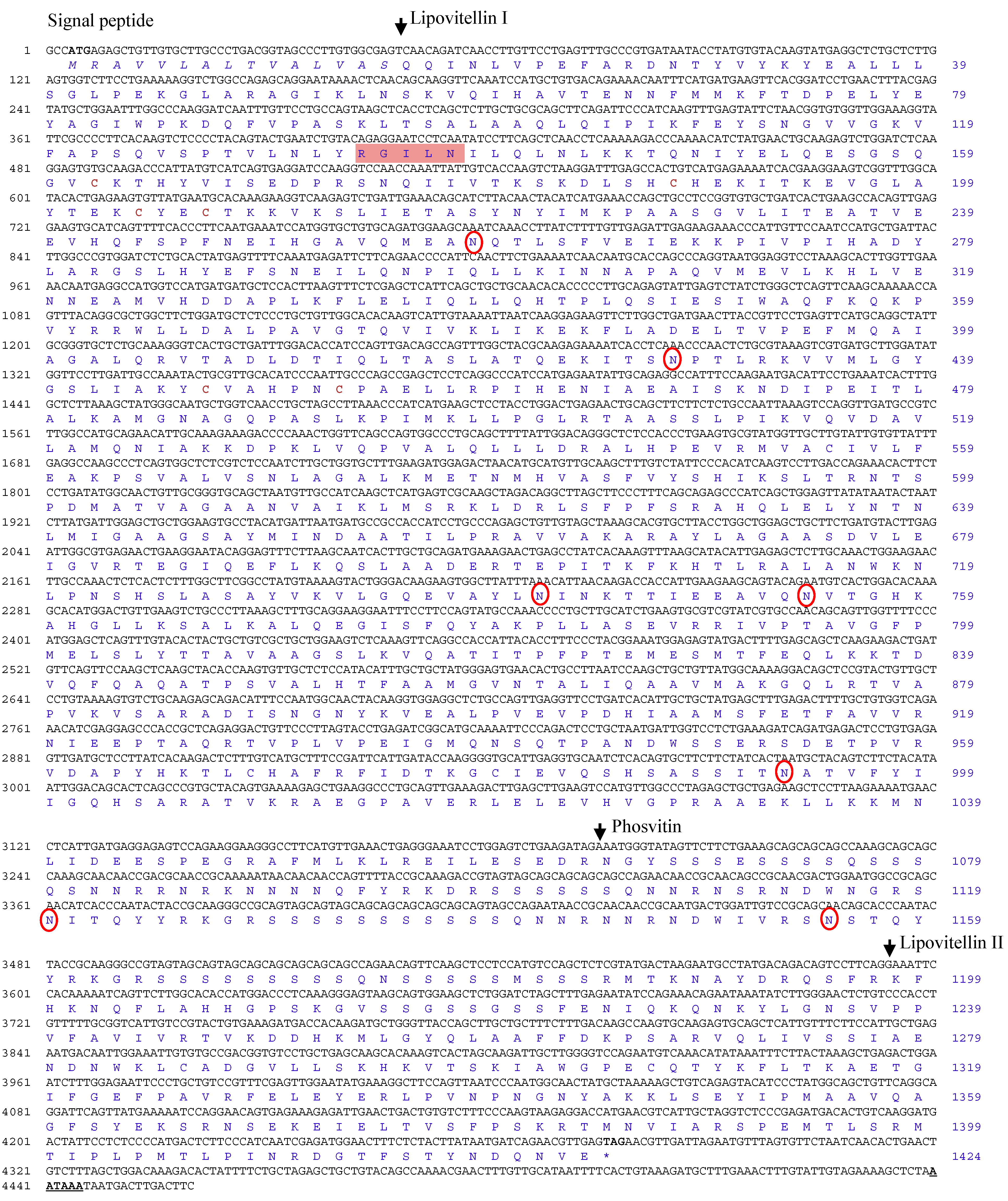
2.1. Molecular Characterization of the R. uyekii Vitellogenin Gene
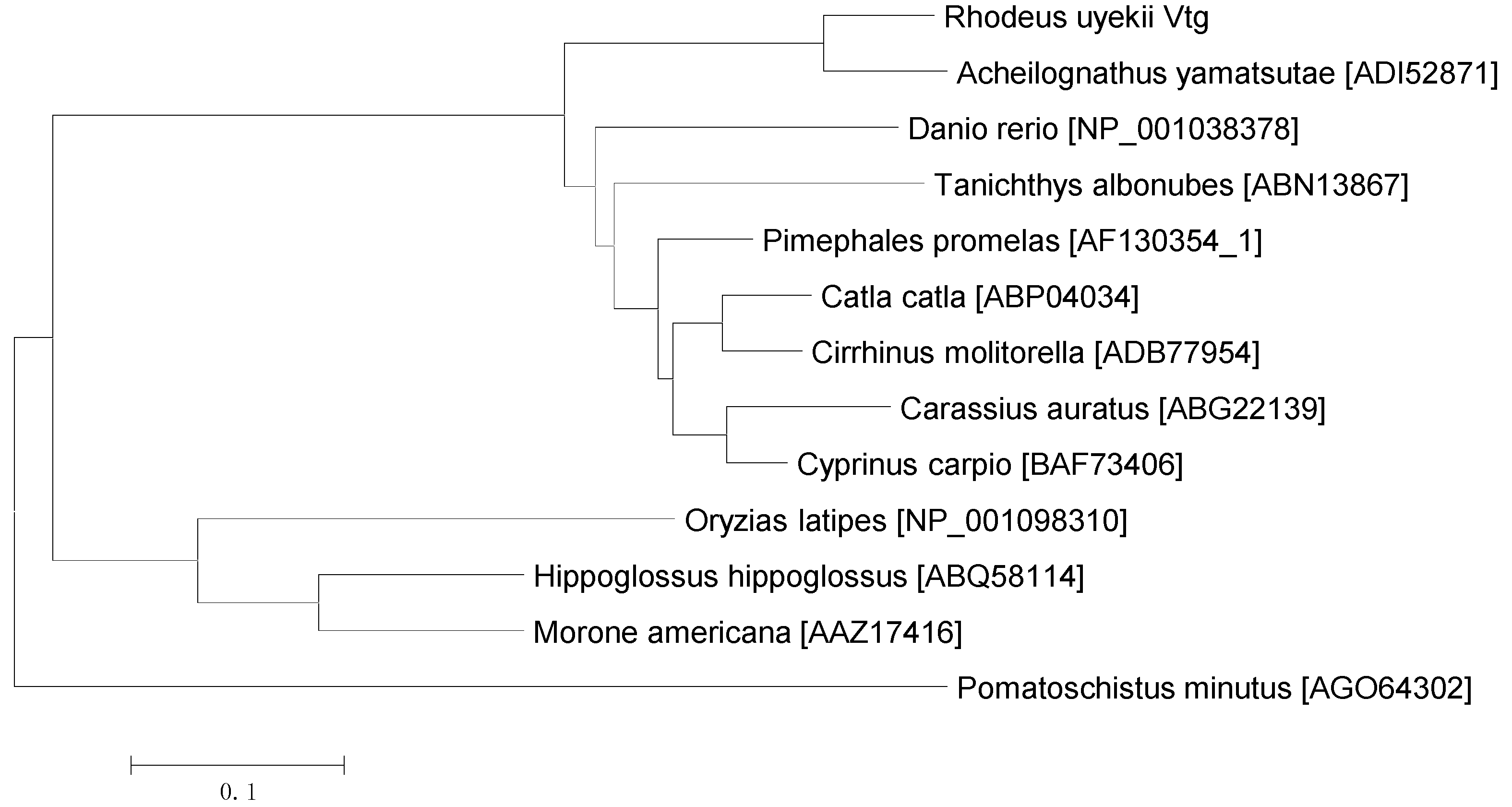
2.2. Pairwise Alignment and Phylogenetic Analysis of Vg Proteins
| Species | Accession Number | Identity (%) | Amino Acids |
|---|---|---|---|
| Acheilognathus yamatsutae | ADI52871 | 73.97 | 1550 |
| Cyprinus carpio | BAF73406 | 70.38 | 1353 |
| Pimephales promelas | AF130354_1 | 70.17 | 1340 |
| Cirrhinus molitorella | ADB77954 | 69.09 | 1342 |
| Catla catla | ABP04034 | 68.57 | 1339 |
| Carassius auratus | ABG22139 | 66.48 | 1348 |
| Tanichthys albonubes | ABN13867 | 64.06 | 1326 |
| Danio rerio | NP_001038378 | 54.58 | 1631 |
| Morone americana | AAZ17416 | 40.20 | 1682 |
| Hippoglossus hippoglossus | ABQ58114 | 40.05 | 1647 |
| Oryzias latipes | NP_001098310 | 38.04 | 1725 |
| Pomatoschistus minutes | AGO64302 | 32.17 | 1646 |
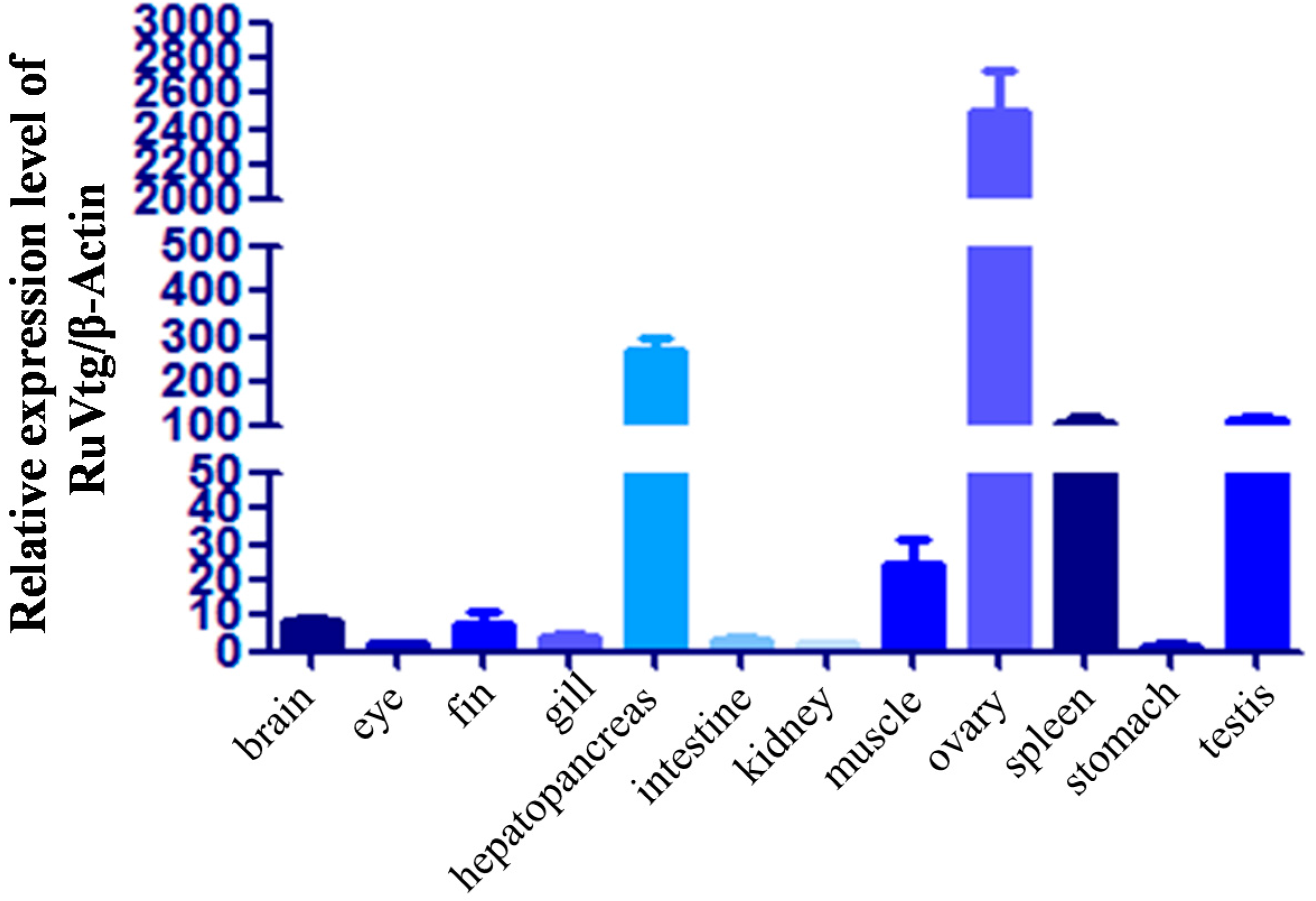
2.3. Expression of Ru-Vg mRNA in Tissues of R. uyekii
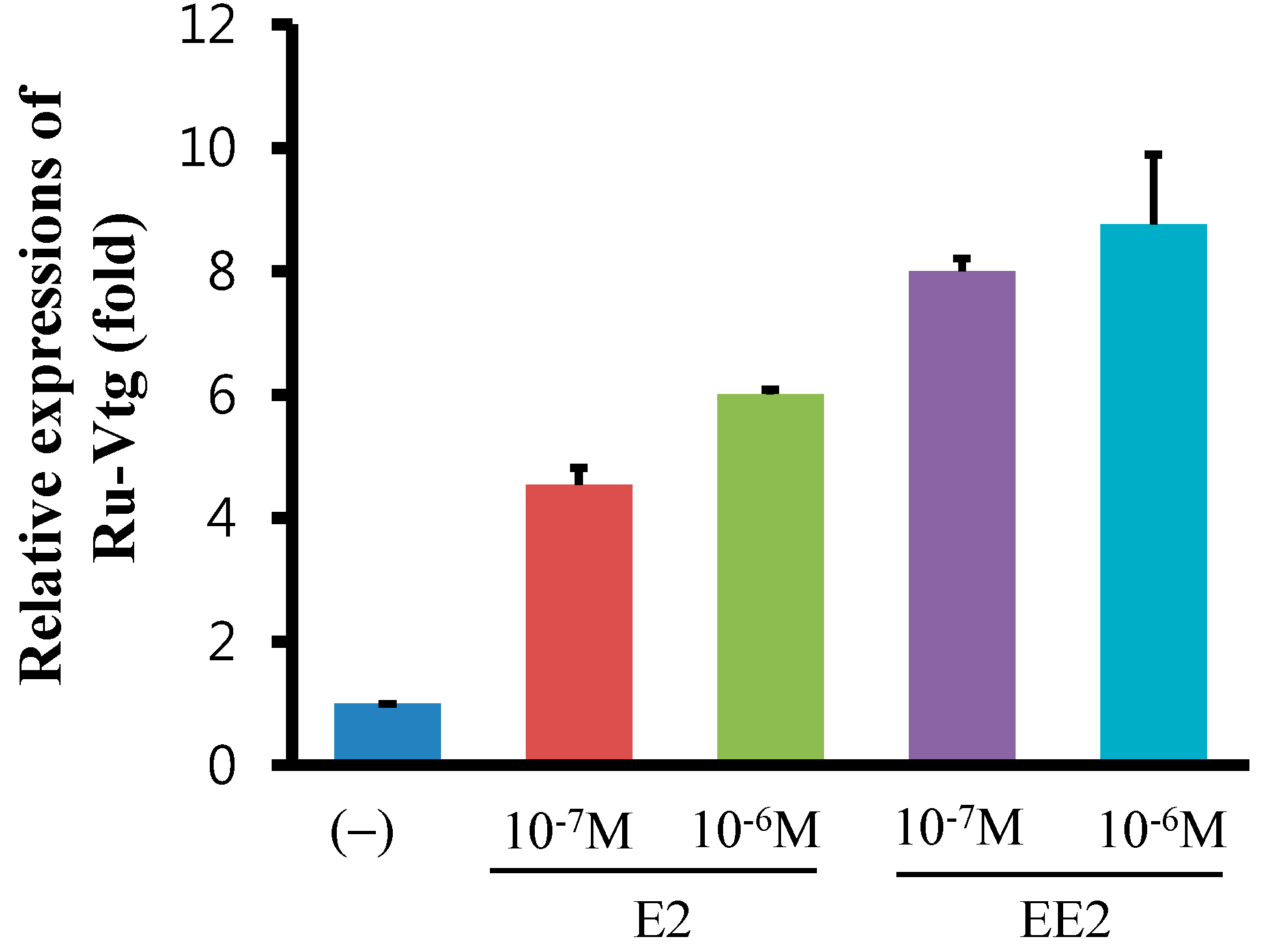
2.4. Regulation of Ru-Vg mRNA by 17β-Estradiol (E2) or 17α-Ethinylestradiol (EE2) in R. uyekii Hepatopancreas Cells
2.5. Sequence Analysis of the 5'-Flanking Regions of the Ru-Vg Gene
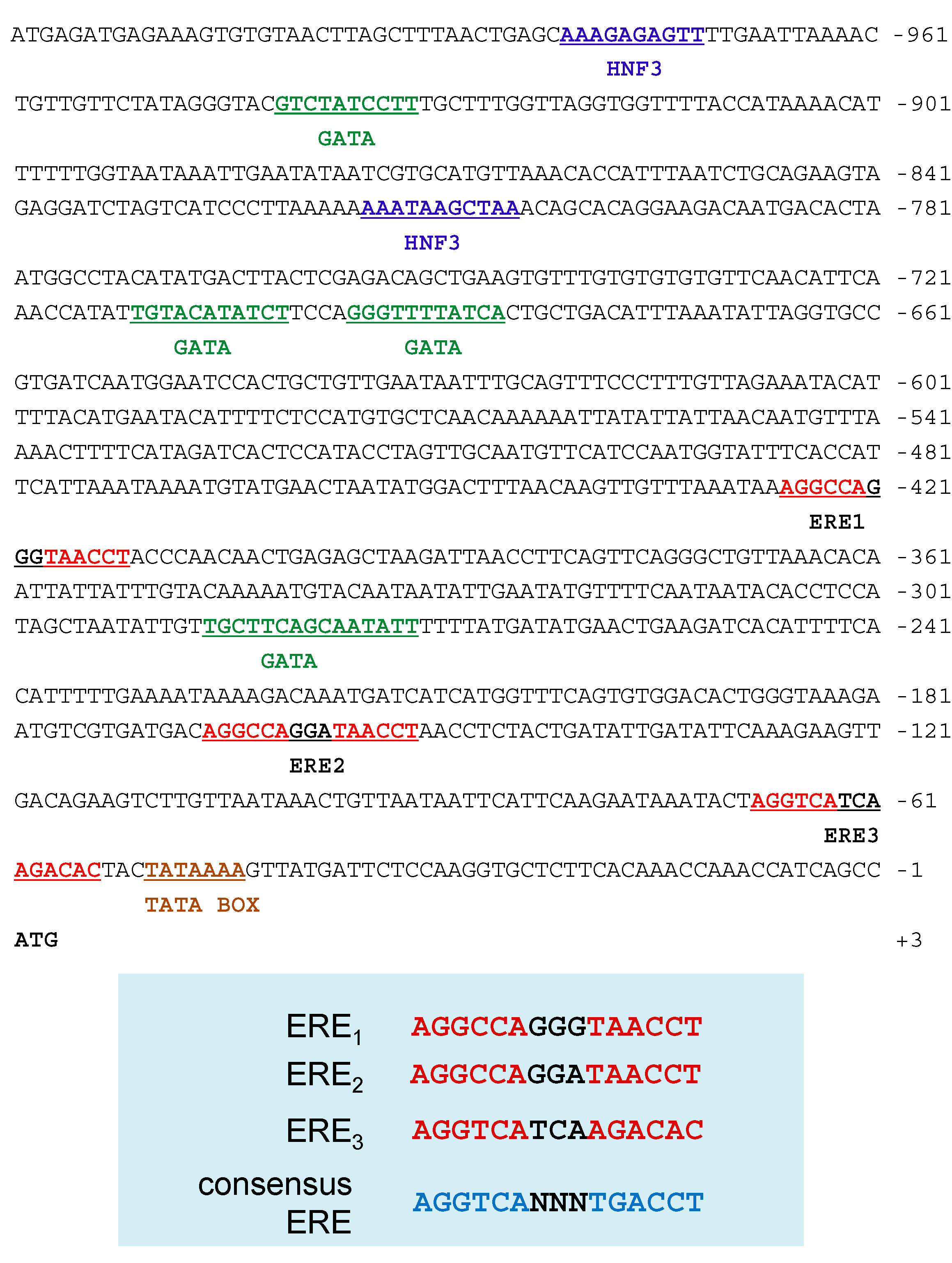
2.6. Functional Analysis of the 5'-Flanking Regions of the Ru-Vg Gene

3. Experimental Section
3.1. Fish Maintenance and Tissue Samples
3.2. Sequence Analyses and Phylogenetic Analyses
3.3. Quantitative Real-Time PCR
3.4. Isolation and Sequence Analysis of the Ru-Vg Gene
3.5. Cloning of the 5'-Flanking Region of the Ru-Vg Gene
3.6. Cell Culture, Transient Transfection, and Luciferase Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akasaka, M.; Kato, K.H.; Kitajima, K.; Sawada, H. Identification of novel isoforms of vitellogenin expressed in ascidian eggs. J. Exp. Zool. B Mol. Dev. Evol. 2013, 320, 118–128. [Google Scholar]
- Susca, V.; Corriero, A.; Bridges, C.R.; de Metrio, G. Study of the sexual maturity of female bluefin tuna: Purification and partial characterization of vitellogenin and its use in an enzyme-linked immunosorbent assay. J. Fish Biol. 2001, 58, 815–831. [Google Scholar]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar]
- Tyler, C.R.; Lancaster, P. Isolation and characterization of the receptor for vitellogenin from follicles of the rainbow trout, Oncorhynchus mykiss. J. Comp. Physiol. B 1993, 163, 225–233. [Google Scholar]
- Thompson, J.R.; Banaszak, L.J. Lipid-protein interactions in lipovitellin. Biochemistry 2002, 41, 9398–9409. [Google Scholar]
- Sawaguchi, S.; Ohkubo, N.; Matsubara, T. Identification of two forms of vitellogenin-derived phosvitin and elucidation of their fate and roles during oocyte maturation in the barfin flounder, Verasper moseri. Zool. Sci. 2006, 23, 1021–1029. [Google Scholar]
- Wahli, W. Evolution and expression of vitellogenin genes. Trends Genet. 1988, 4, 227–232. [Google Scholar]
- Mayadas, T.N.; Wagner, D.D. Vicinal cysteines in the prosequence play a role in von Willebrand factor multimer assembly. Proc. Natl. Acad. Sci. USA 1992, 89, 3531–3535. [Google Scholar]
- Mouchel, N.; Trichet, V.; Betz, A.; le Pennec, J.P.; Wolff, J. Characterization of vitellogenin from rainbow trout (Oncorhynchus mykiss). Gene 1996, 174, 59–64. [Google Scholar]
- Tyler, C.R.; Sumpter, J.P. The purification and partial characterization of carp, Cyprinus carpio, vitellogenin. Fish Physiol. Biochem. 1990, 8, 111–120. [Google Scholar]
- Heppell, S.A.; Denslow, N.D.; Folmar, L.C.; Sullivan, C.V. Universal assay of vitellogenin as a biomarker for environmental estrogens. Environ. Health Perspect. 1995, 103, 9–15. [Google Scholar]
- Kohn, Y.Y.; Lokman, P.M.; Lilimnik, A.; Symonds, J.E. Sex identification in captive hapuku (Polyprion oxygeneios) using ultrasound imagery and plasma levels of vitellogenin and sex steroids. Aquaculture 2013, 384–387, 87–93. [Google Scholar]
- Matozzo, V.; Gagné, F.; Marin, M.G.; Ricciardi, F.; Blaise, C. Vitellogenin as a biomarker of exposure to estrogenic compounds in aquatic invertebrates: A review. Environ. Int. 2008, 34, 531–545. [Google Scholar]
- Mommsen, T.P.; Walsh, P.J. Vitellogenesis and oocyte assembly. In Fish Physiology; Hoar, W.S., Randoll, D.J., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume 5, pp. 347–406. [Google Scholar]
- Jones, P.D.; de Coen, W.M.; Tremblay, L.; Giesy, J.P. Vitellogenin as a biomarkerfor environmental estrogens. Water Sci. Technol. 2000, 42, 1–14. [Google Scholar]
- Tiu, S.H.; Chan, S.M.; Tobe, S.S. The effects of farnesoic acid and 20-hydroxyecdysone on vitellogenin gene expression in the lobster, Homarus americanus, and possible roles in the reproductive process. Gen. Comp. Endocrinol. 2010, 166, 337–345. [Google Scholar]
- Chen, T.; Zhang, L.P.; Wong, N.K.; Zhong, M.; Ren, C.H.; Hu, C.Q. Pacific white shrimp (Litopenaeus vannamei) vitellogenesis-inhibiting hormone (VIH) is predominantly expressed in the brain and negatively regulates hepatopancreatic vitellogenin (Vg) gene expression. Biol. Reprod. 2014, 90, 47. [Google Scholar] [CrossRef]
- Baker, H.J.; Shapiro, D.J. Kinetics of estrogen induction of Xenopus laevis vitellogenin messenger RNA as measured by hybridization to complementary DNA. J. Biol. Chem. 1977, 252, 8428–8434. [Google Scholar]
- Shapiro, D. Steroid hormone regulation of vitellogenin gene expression. CRC Crit. Rev. Biochem. 1982, 12, 187–203. [Google Scholar]
- Robyr, D.; Gegonne, A.; Wolffe, A.P.; Wahli, W. Determinants of vitellogenin B1 promoter architecture. HNF3 and estrogen responsive transcription within chromatin. J. Biol. Chem. 2000, 275, 28291–28300. [Google Scholar]
- Teo, B.Y.; Tan, N.S.; Lam, T.J.; Ding, J.L. Synergistic effects of nuclear factors-GATA, VBP and ER in potentiating vitellogenin gene transcription. FEBS Lett. 1999, 459, 57–63. [Google Scholar]
- Dittmer, N.T.; Sun, G.; Wang, S.F.; Raikhel, A.S. CREB isoform represses yolk protein gene expression in the mosquito fat body. Mol. Cell. Endocrinol. 2003, 210, 39–49. [Google Scholar]
- Chan, S.F.; He, J.G.; Chu, K.H.; Sun, C.B. The Shrimp Heat Shock Cognate 70 Functions as a Negative Regulator in Vitellogenin Gene Expression. Biol. Reprod. 2014, 91, 1–11. [Google Scholar]
- Banarescu, P. Zoogeography of Fresh Waters: General Distribution and Dispersal of Freshwater Animals; Aula-Verlag: Berkeley, CA, USA, 1990. [Google Scholar]
- Kim, I.S.; Choi, Y.; Lee, C.L.; Lee, Y.J.; Kim, B.Y.; Kim, J.H. Illustrated Book of Korean Fishes; Kyohak Publishing: Seoul, Korea, 2005; p. 613. [Google Scholar]
- Kim, B.C.; Kang, T.W.; Kim, M.S.; Kim, C.B. The complete mitogenome of Rhodeus uyekii (Cypriniformes, Cyprinidae). DNA Seq. 2006, 17, 181–186. [Google Scholar]
- Tolar, J.F.; Mehollin, A.R.; Watson, R.D.; Angus, R.A. Mosquitofish (Gambusia affinis) vitellogenin: Identification, purification, and immunoassay. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 128, 237–245. [Google Scholar]
- MacLatchy, D.L.; Courtenay, S.C.; Rice, C.D.; van der Kraak, G.J. Development of a short-term reproductive endocrine bioassay using steroid hormone and vitellogenin end points in the estuarine mummichog (Fundulus heteroclitus). Environ. Toxicol. Chem. 2003, 22, 996–1008. [Google Scholar]
- Sawaguchi, S.; Kagawa, H.; Ohkubo, N.; Hiramatsu, N.; Sullivan, C.V.; Matsubara, T. Molecular characterization of three forms of vitellogenin and their yolk protein products during oocyte growth and maturation in red seabream (Pagrus major), a marine teleost spawning pelagic eggs. Mol. Reprod. Dev. 2006, 73, 719–736. [Google Scholar]
- Hiramatsu, N.; Hara, A.; Hiramatsu, K.; Fukada, H.; Weber, G.M.; Denslow, N.D.; Sullivan, C.V. Vitellogenin-derived yolk proteins of white perch, Morone americana: Purification, characterization, and vitellogenin-receptor binding. Biol. Reprod. 2002, 67, 655–667. [Google Scholar]
- Amano, H.; Fujita, T.; Hiramatsu, N.; Shimizu, M.; Sawaguchi, S.; Matsubara, T.; Kagawa, H.; Nagae, M.; Sullivan, C.V.; Hara, A.; et al. Egg yolk proteins in gray mullet (Mugil cephalus): purification and classification of multiple lipovitellins and other vitellogenin-derived yolk proteins and molecular cloning of the parent vitellogenin genes. J. Exp. Zool. A Ecol. Genet. Physiol. 2007, 307, 324–341. [Google Scholar]
- Finn, R.N.; Kristoffersen, B.A. Vertebrate vitellogenin gene duplication in relation to the “3R hypothesis”: Correlation to the pelagic egg and the oceanic radiation of teleosts. PLoS One 2007, 2, e169. [Google Scholar]
- Wang, H.; Tan, J.T.; Emelyanov, A.; Korzh, V.; Gong, Z. Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio. Gene 2005, 356, 91–100. [Google Scholar]
- Ma, L.; Li, D.; Wang, J.; He, J.; Yin, Z. Effects of adrenergic agonists on the extrahepatic expression of vitellogenin Ao1 in heart and brain of the Chinese rare minnow (Gobiocypris rarus). Aquat. Toxicol. 2009, 91, 19–25. [Google Scholar]
- Wang, R.; Gao, Y.; Zhang, L.; Zhang, Y.; Fang, Z.; He, J.; Zhang, W.; Ma, G. Cloning, expression, and induction by 17-beta estradiol (E2) of a vitellogenin gene in the white cloud mountain minnow Tanichthys albonubes. Fish Physiol. Biochem. 2010, 36, 157–164. [Google Scholar]
- Yin, N.; Jin, X.; He, J.; Yin, Z. Effects of adrenergic agents on the expression of zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2009, 238, 20–26. [Google Scholar]
- Jorieux, S.; Fressinaud, E.; Goudemand, J.; Gaucher, C.; Meyer, D.; Mazurier, C. Conformational changes in the D' domain of von Willebrand factor induced by CYS 25 and CYS 95 mutations lead to factor VIII binding defect and multimeric impairment. Blood 2000, 95, 3139–3145. [Google Scholar]
- Byrne, B.M.; Gruber, M.; Ab, G. The evolution of egg yolk proteins. Prog. Biophys. Mol. Biol. 1989, 53, 33–69. [Google Scholar]
- Sappington, T.W.; Raikhel, A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 1998, 28, 277–300. [Google Scholar]
- Nakamura, A.; Yasuda, K.; Adachi, H.; Sakurai, Y.; Ishii, N.; Goto, S. Vitellogenin-6 is a major carbonylated protein in aged nematode, Caenorhabditis elegans, Biochem. Biophys. Res. Commun. 1999, 264, 580–583. [Google Scholar]
- Jia, X.; Chen, Y.; Zou, Z.; Lin, P.; Wang, Y.; Zhang, Z. Characterization and expression profile of Vitellogenin gene from Scylla paramamosain. Gene 2013, 520, 119–130. [Google Scholar]
- Liang, Y.; Fang, Z. Molecular cloning and mRNA expression of the vitellogenin and nuclear receptor gene induced by 17β-estradiol in the mud carp, Cirrhinus molitorella. Ecotoxicology 2012, 21, 719–729. [Google Scholar]
- Kim, H.R.; Park, Y.J.; Kim, J.G.; Chung, K.H.; Oh, S.M. Molecular cloning of vitellogenin gene and mRNA expression by 17a-ethinylestradiol from slender bitterling. Gen. Comp. Endocrinol. 2010, 68, 484–495. [Google Scholar]
- Gruber, C.J.; Gruber, D.M.; Gruber, I.M.L.; Wieser, F.; Huber, J.C. Anatomy of the estrogen response element. Trends Endocrinol. MeTable 2004, 15, 73–78. [Google Scholar]
- Bouter, A.; Buisine, N.; le Grand, A.; Mouchel, N.; Chesnel, F.; le Goff, C.; le Tilly, V.; Wolff, J.; Sire, O. Control of vitellogenin genes expression by sequences derived from transposable elements in rainbow trout. Biochim. Biophys. Acta 2010, 1799, 546–554. [Google Scholar]
- Kaestner, K.H. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol. MeTable 2000, 11, 281–285. [Google Scholar]
- Davis, D.L.; Burch, J.B. The chicken vitellogenin II gene is flanked by a GATA factor-dependent estrogen response unit. Mol. Endocrinol. 1996, 10, 937–944. [Google Scholar]
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar]
- BLAST. Available online: http://www.ncbi.nlm.nih.gov/BLAST/ (accessed on 16 October 2014).
- ExPASy. Available online: http://web.expasy.org/compute_pi/ (accessed on 16 October 2014).
- SignalP. Available online: http://www.cbs.dtu.dk/services/SignalP/ (accessed on 16 October 2014).
- NetNGlyc1.0 Server. Available online: http://www.cbs.dtu.dk/services/NetNGlyc/ (accessed on 16 October 2014).
- ScanProsite Server. Available online: http://prosite.expasy.org/scanprosite (accessed on 16 October 2014).
- TFSEARCH. Available online: http://www.cbrc.jp/research/db/TFSEARCH.html (accessed on 16 October 2014).
- Mega 5 software. Available online: http://www.megasoftware.net/ (accessed on 16 October 2014).
- Chae, S.H.; Kim, J.W.; Cho, J.M.; Larkin, D.M.; Everts-van der Wi, A.; Park, H.S.; Yeo, J.S.; Choi, I. Chromosomal localization of Korean cattle (Hanwoo) BAC clones via BAC end sequence analysis. Asian Australas. J. Anim. Sci. 2007, 20, 316–327. [Google Scholar]
- Kong, H.J.; Nam, B.H.; Kim, Y.O.; Kim, W.J.; Cho, H.K.; Lee, C.H.; Lee, S.J.; Kim, K.K. Characterization of the flounder IL-6 promoter and its regulation by the p65 NF-κB subunit. Fish Shellfish Immunol. 2010, 28, 961–964. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.J.; Kim, J.L.; Moon, J.Y.; Kim, W.-J.; Kim, H.S.; Park, J.Y.; Cho, H.K.; An, C.M. Characterization, Expression Profile, and Promoter Analysis of the Rhodeus uyekii Vitellogenin Ao1 Gene. Int. J. Mol. Sci. 2014, 15, 18804-18818. https://doi.org/10.3390/ijms151018804
Kong HJ, Kim JL, Moon JY, Kim W-J, Kim HS, Park JY, Cho HK, An CM. Characterization, Expression Profile, and Promoter Analysis of the Rhodeus uyekii Vitellogenin Ao1 Gene. International Journal of Molecular Sciences. 2014; 15(10):18804-18818. https://doi.org/10.3390/ijms151018804
Chicago/Turabian StyleKong, Hee Jeong, Ju Lan Kim, Ji Young Moon, Woo-Jin Kim, Hyung Soo Kim, Jung Youn Park, Hyun Kook Cho, and Cheul Min An. 2014. "Characterization, Expression Profile, and Promoter Analysis of the Rhodeus uyekii Vitellogenin Ao1 Gene" International Journal of Molecular Sciences 15, no. 10: 18804-18818. https://doi.org/10.3390/ijms151018804
APA StyleKong, H. J., Kim, J. L., Moon, J. Y., Kim, W.-J., Kim, H. S., Park, J. Y., Cho, H. K., & An, C. M. (2014). Characterization, Expression Profile, and Promoter Analysis of the Rhodeus uyekii Vitellogenin Ao1 Gene. International Journal of Molecular Sciences, 15(10), 18804-18818. https://doi.org/10.3390/ijms151018804




