Melatonin Attenuates Intermittent Hypoxia-Induced Lipid Peroxidation and Local Inflammation in Rat Adrenal Medulla
Abstract
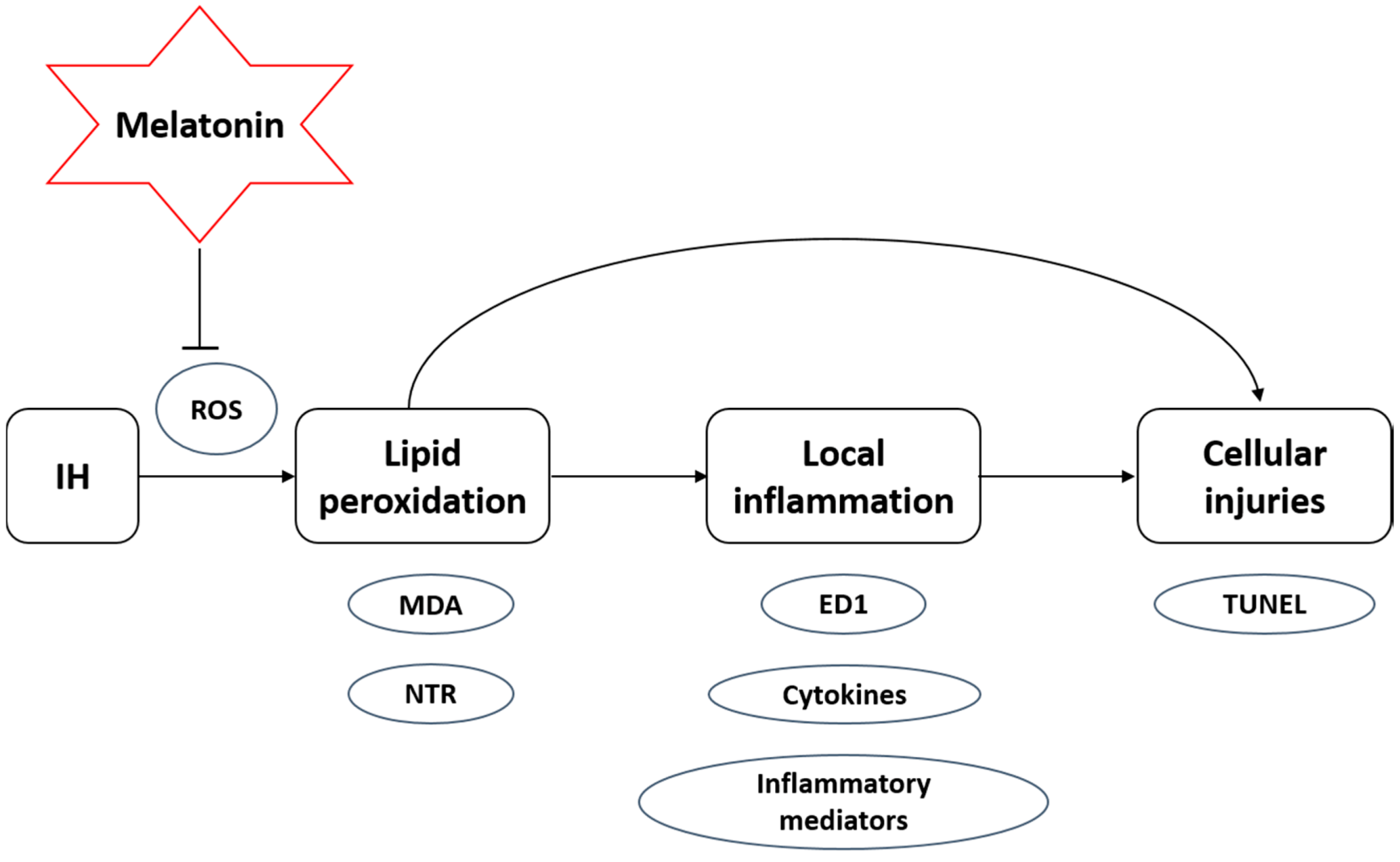
:1. Introduction
2. Results
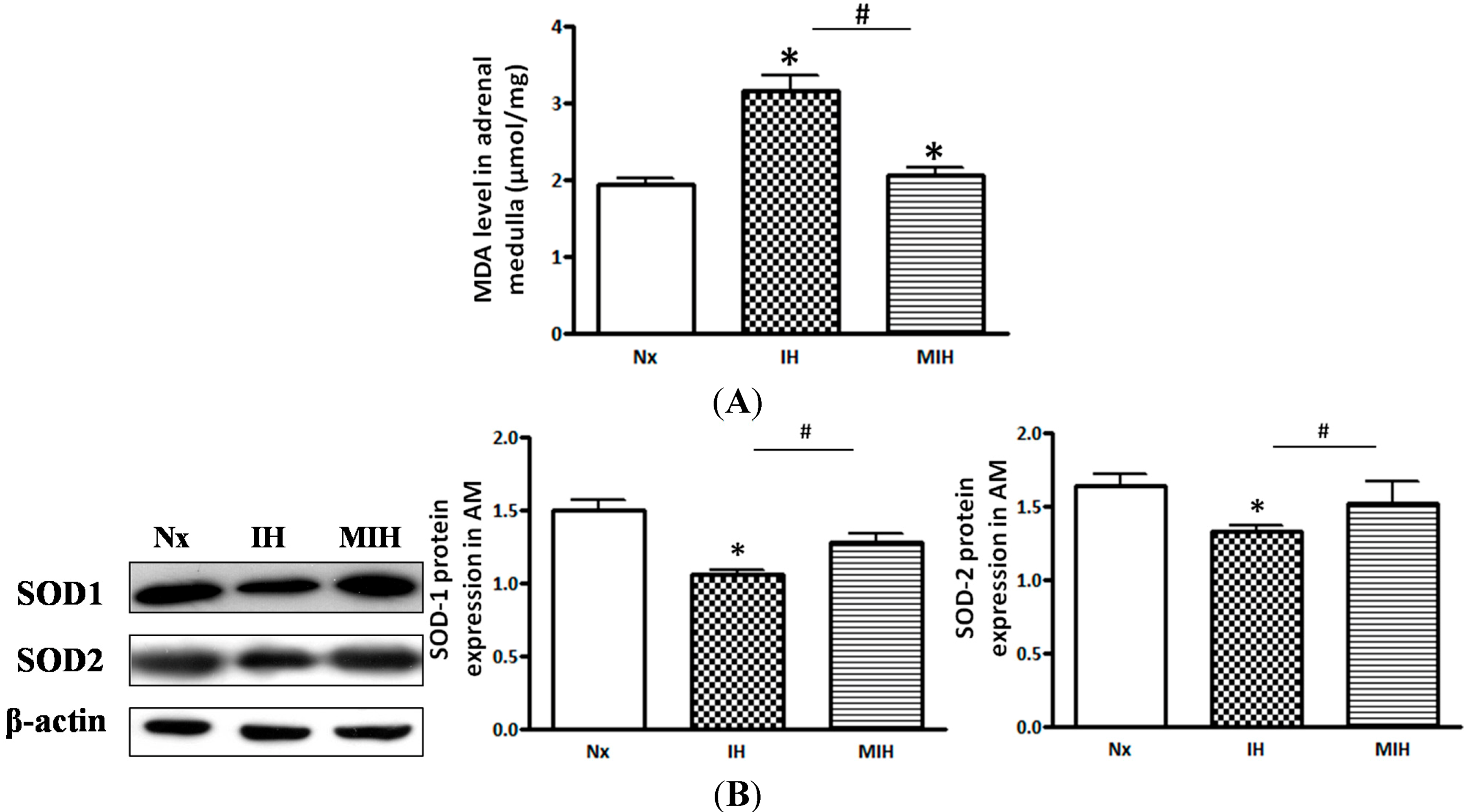
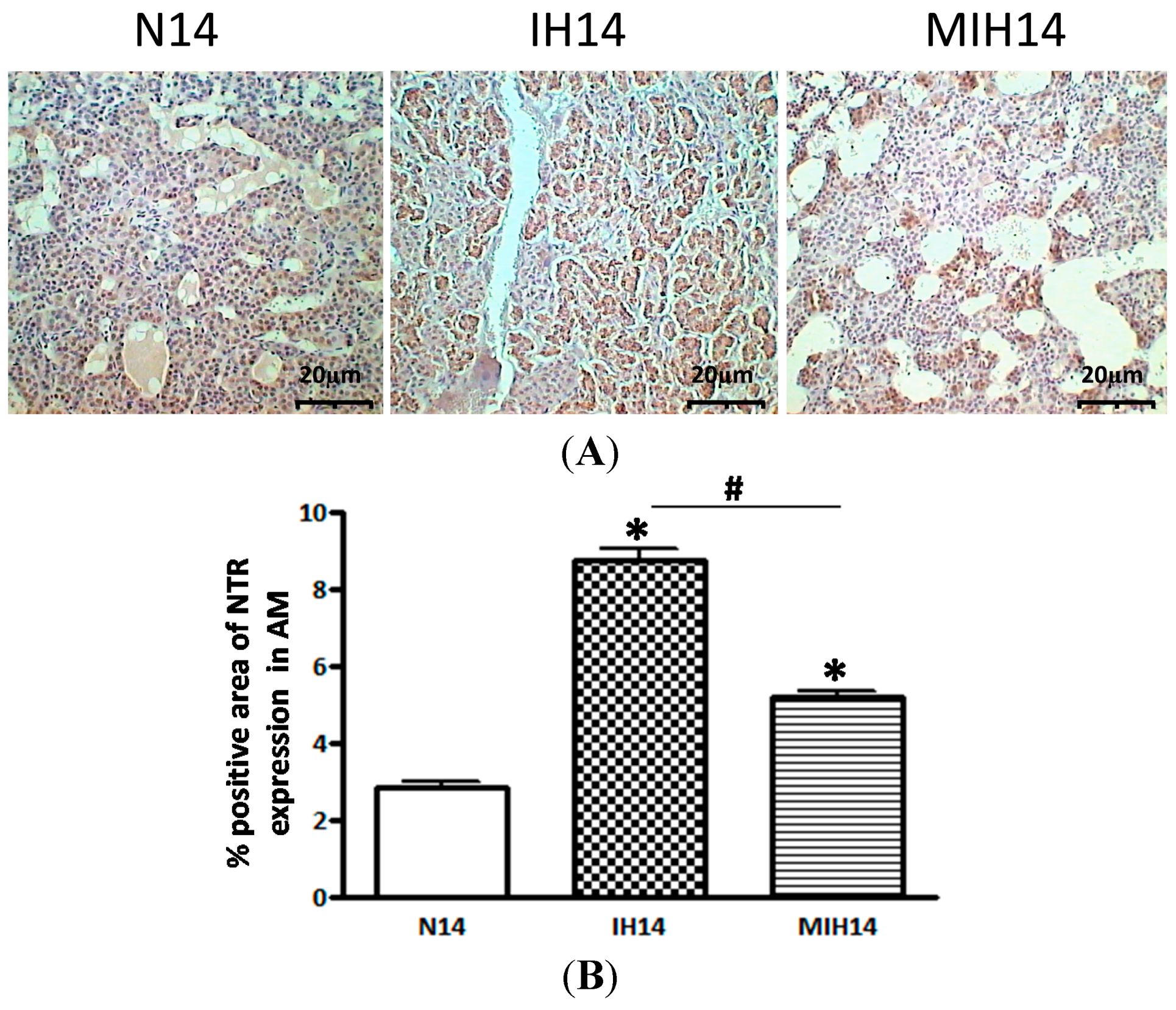
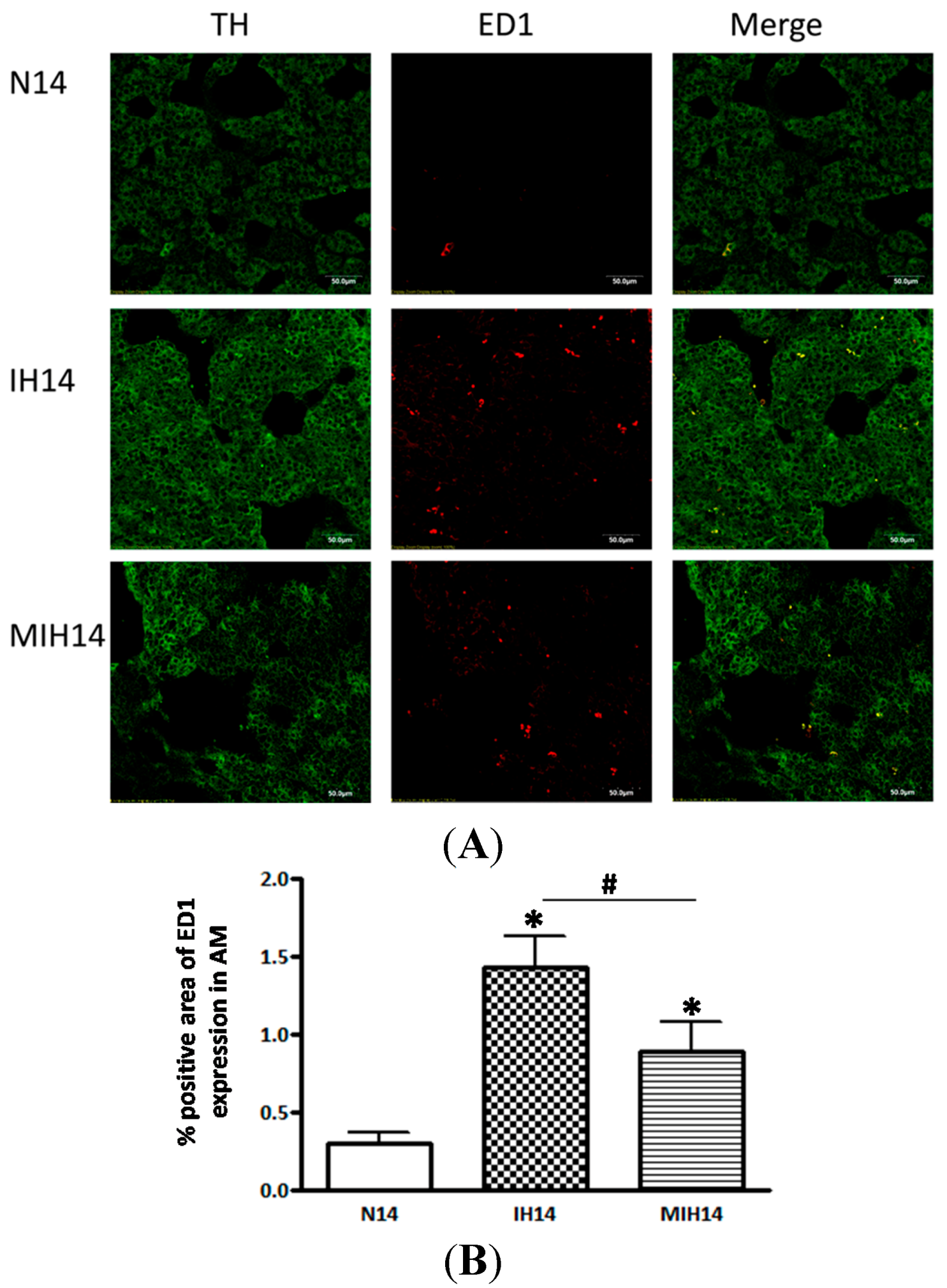

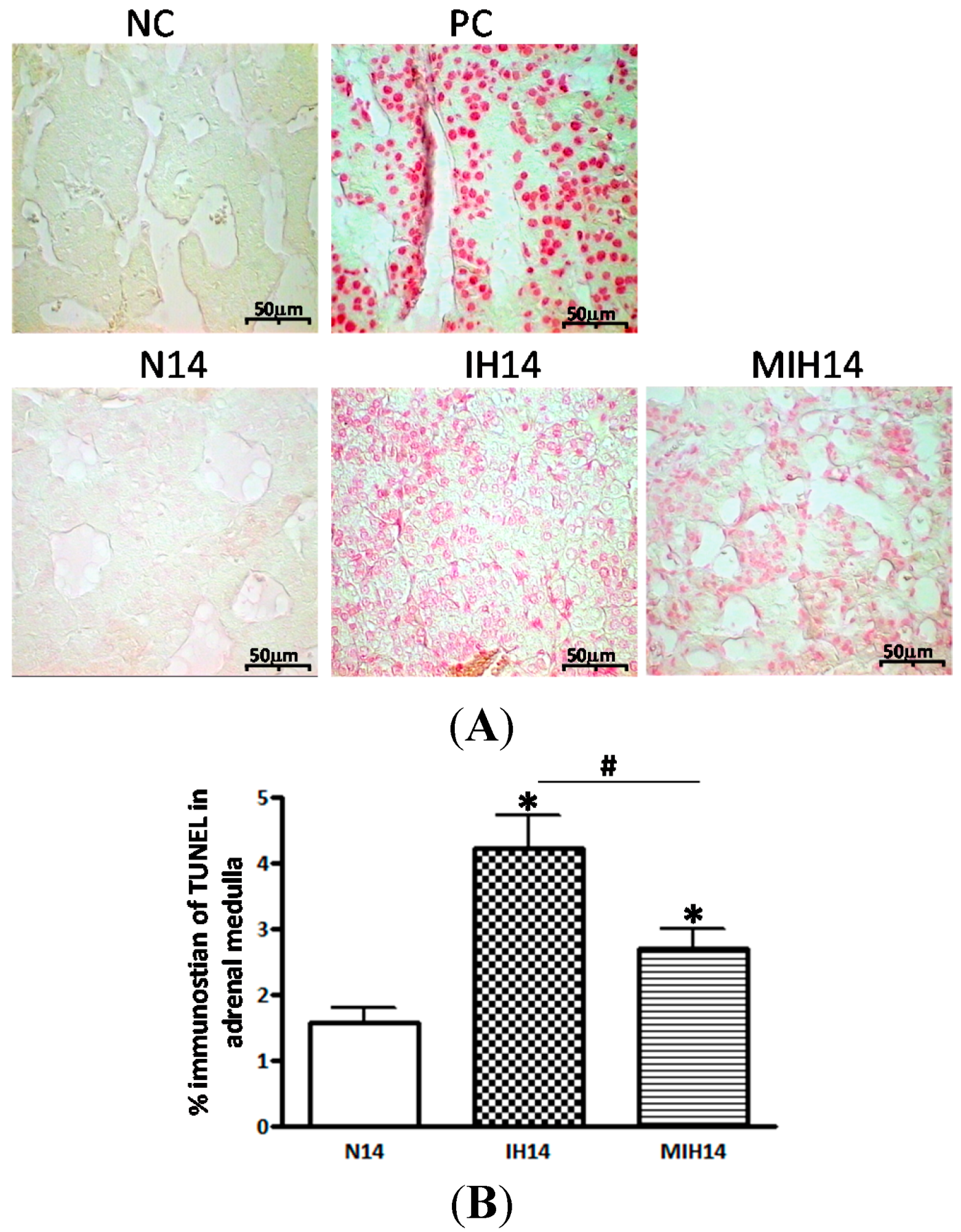
3. Discussion
4. Material and Methods
4.1. Treatment of Chronic Intermittent Hypoxia in Rats
4.2. Drug Preparation
4.3. Measurement of Malondialdehyde (MDA) Formation
4.4. Immunohistochemistry
4.5. Immunofluorescence Double Staining
4.6. Western Blotting
4.7. Enzyme-Linked Immunosorbent Assay (ELISA) Measurement
4.8. Apoptosis Measurement
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kent, B.D.; Ryan, S.; Mcnicholas, W.T. Obstructive sleep apnea and inflammation: Relationship to cardiovascular co-morbidity. Respir. Physiol. Neurobiol. 2011, 178, 475–481. [Google Scholar]
- Kasai, T.; Bradley, T.D. Obstructive sleep apnea and heart failure: Pathophysiologic and therapeutic implications. J. Am. Coll. Cardiol. 2011, 57, 119–127. [Google Scholar]
- Lavie, L.; Polotsky, V. Cardiovascular aspects in obstructive sleep apnea syndrome—Molecular issues, hypoxia and cytokine profiles. Respiration 2009, 78, 361–370. [Google Scholar]
- Ramirez, J.M.; Garcia, A.J.; Anderson, T.M.; Koschnitzky, J.E.; Peng, Y.-J.; Kumar, G.K.; Prabhakar, N.R. Central and peripheral factors contributing to obstructive sleep apneas. Respir. Physiol. Neurobiol. 2013, 189, 344–353. [Google Scholar]
- Pierola, J.; Alemany, A.; Yanez, A.; de-la-Peña, M.; Sánchez-de-la-Torre, M.; Esquinas, C.; Pérez-Gutierrez, C.; Burguera, B.; Barbé, F.; Barceló, A. NADPH oxidase p22phox polymorphisms and oxidative stress in patients with obstructive sleep apnoea. Respir. Med. 2011, 105, 1748–1754. [Google Scholar]
- Ramar, K.; Caples, S.M. Vascular changes, cardiovascular disease and obstructive sleep apnea. Future Cardiol. 2011, 7, 241–249. [Google Scholar]
- Yeung, H.M.; Hung, M.W.; Fung, M.L. Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J. Pineal Res. 2008, 45, 373–382. [Google Scholar]
- Hung, M.W.; Tipoe, G.L.; Poon, A.M.S.; Reiter, R.J.; Fung, M.-L. Protective effect of melatonin against hippocampal injury of rats with intermittent hypoxia. J. Pineal Res. 2008, 44, 214–221. [Google Scholar]
- Lavie, L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front. Biosci. 2012, 4, 1391–1403. [Google Scholar]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia—Revisited—The bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. Available online: http://www.sciencedirect.com/science/article/pii/S108707921400077X (accessed on 24 July 2014). [CrossRef]
- Lavie, L.; Dyugovskaya, L.; Polyakov, A. Biology of peripheral blood cells in obstructive sleep apnea—The tip of the iceberg. Arch. Physiol. Biochem. 2008, 114, 244–254. [Google Scholar]
- Tauman, R.; Lavie, L.; Greenfeld, M.; Sivan, Y. Oxidative stress in children with obstructive sleep apnea syndrome. J. Clin. Sleep Med. 2014, 10, 677–681. [Google Scholar]
- Sanchez-de-la-Torre, M.; Campos-Rodriguez, F.; Barbe, F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir. Med. 2013, 1, 61–72. [Google Scholar]
- He, Q.; Yang, Q.C.; Zhou, Q.; Zhu, H.; Niu, W.-Y.; Feng, J.; Wang, Y.; Cao, J.; Chen, B.-Y. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS One 2014, 9, e86326. [Google Scholar]
- Raghuraman, G.; Kalari, A.; Dhingra, R.; Prabhakar, N.R.; Kumar, G.K. Enhanced neuropeptide y synthesis during intermittent hypoxia in the rat adrenal medulla: Role of reactive oxygen species-dependent alterations in precursor Peptide processing. Antioxid. Redox Signal. 2011, 14, 1179–1190. [Google Scholar]
- Yuan, G.; Peng, Y.J.; Reddy, V.D.; Makarenko, V.V.; Nanduri, J.; Khan, S.A.; Garcia, J.A.; Kumar, G.K.; Semenza, G.L.; Prabhakar, N.R. Mutual antagonism between hypoxia-inducible factors 1α and 2α regulates oxygen sensing and cardio-respiratory homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E1788–E1796. [Google Scholar]
- Souvannakitti, D.; Kumar, G.K.; Fox, A.; Prabhakar, N.R. Contrasting effects of intermittent and continuous hypoxia on low O2 evoked catecholamine secretion from neonatal rat chromaffin cells. Adv. Exp. Med. Biol. 2009, 648, 345–349. [Google Scholar]
- Kuri, B.A.; Khan, S.A.; Chan, S.A.; Prabhakar, N.R.; Smith, C.B. Increased secretory capacity of mouse adrenal chromaffin cells by chronic intermittent hypoxia: Involvement of protein kinase C. J. Physiol. 2007, 584, 313–319. [Google Scholar]
- Salman, S.; Buttigieg, J.; Zhang, M.; Nurse, C.A. Chronic exposure of neonatal rat adrenomedullary chromaffin cells to opioids in vitro blunts both hypoxia and hypercapnia chemosensitivity. J. Physiol. 2013, 591, 515–529. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Maldonado, M.D. Melatonin as an antioxidant: Physiology versus pharmacology. J. Pineal Res. 2005, 39, 215–216. [Google Scholar]
- Reiter, R.J.; Sainz, R.M.; Lopez-Burillo, S.; Mayo, J.C.; Manchester, L.C.; Tan, D.X. Melatonin ameliorates neurologic damage and neurophysiologic deficits in experimental models of stroke. Ann. N. Y. Acad. Sci. 2003, 993, 35–47. [Google Scholar]
- Pei, Z.; Pang, S.F.; Cheung, R.T. Pretreatment with melatonin reduces volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. J. Pineal Res. 2002, 32, 168–172. [Google Scholar]
- Lee, M.Y.; Kuan, Y.H.; Chen, H.Y.; Chen, T.-Y.; Chen, S.-T.; Huang, C.-C. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res. 2007, 42, 297–309. [Google Scholar]
- Chen, H.Y.; Chen, T.Y.; Lee, M.Y.; Chen, S.T.; Hsu, Y.S.; Kuo, Y.L.; Chang, G.L.; Wu, T.S.; Lee, E.J. Melatonin decreases neurovascular oxidative/nitrosative damage and protects against early increases in the blood-brain barrier permeability after transient focal cerebral ischemia in mice. J. Pineal Res. 2006, 41, 175–182. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar]
- Wang, W.Z.; Fang, X.H.; Stephenson, L.L.; Khiabani, K.T.; Zamboni, W.A. Melatonin reduces ischemia/reperfusion-induced superoxide generation in arterial wall and cell death in skeletal muscle. J. Pineal Res. 2006, 41, 255–260. [Google Scholar]
- Haghjooy, J.S.; Ziaei, A.; Ziaei, S.; Mirmohammad-Sadeghi, M. The effect of preoperative melatonin on nuclear erythroid 2-related factor 2 activation in patients undergoing coronary artery bypass grafting surgery. Oxid. Med. Cell. Longev. 2013, 2013, 676829. [Google Scholar]
- Lee, E.J.; Lee, M.Y.; Chen, H.Y.; Hsu, Y.S.; Wu, T.S.; Chen, S.T.; Chang, G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005, 38, 42–52. [Google Scholar]
- EL-Sokkary, G.H.; Khidr, B.M.; Younes, H.A. Role of melatonin in reducing hypoxia-induced oxidative stress and morphological changes in the liver of male mice. Eur. J. Pharmacol. 2006, 540, 107–114. [Google Scholar]
- Warner, D.S.; Sheng, H.; Batinic-Haberle, I. Oxidants, antioxidants and the ischemic brain. J. Exp. Biol. 2004, 207, 3221–3231. [Google Scholar]
- Lewen, A.; Matz, P.; Chan, P.H. Free radical pathways in CNS injury. J. Neurotrauma 2000, 17, 871–890. [Google Scholar]
- Abd-Elsameea, A.A.; Mousta, A.A.; Mohamed, A.M. Modulation of the oxidative stress by metformin in the cerebrum of rats exposed to global cerebral ischemia and ischemia/reperfusion. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2387–2392. [Google Scholar]
- Cetinkaya, N.; Attar, R.; Yildirim, G.; Ficicioglu, C.; Ozkan, F.; Yilmaz, B.; Yesildaglar, N. The effects of different doses of melatonin treatment on endometrial implants in an oophorectomized rat endometriosis model. Arch. Gynecol. Obstet. Available online: http://link.springer.com/article/10.1007/s00404-014-3466-3 (accessed on 16 September 2014). [CrossRef]
- Ramesh, T.; Sureka, C.; Bhuvana, S.; Begum, V.H. Oxidative stress in the brain of cigarette smoke-induced noxiousness: Neuroprotective role of Sesbania grandiflora. Metab. Brain Dis. Available online: http://www.pubfacts.com/detail/25217401/Oxidative-stress-in-the-brain-of-cigarette-smoke-induced-noxiousness:-neuroprotective-role-of-Sesban (accessed on 9 September 2014). [CrossRef]
- Del, R.R.; Moya, E.A.; Iturriaga, R. Differential expression of pro-inflammatory cytokines, endothelin-1 and nitric oxide synthases in the rat carotid body exposed to intermittent hypoxia. Brain Res. 2011, 1395, 74–85. [Google Scholar]
- Savransky, V.; Reinke, C.; Jun, J.; Bevans-Fonti, S.; Nanayakkara, A.; Li, J.; Myers, A.C.; Torbenson, M.S.; Polotsky, V.Y. Chronic intermittent hypoxia and acetaminophen induce synergistic liver injury in mice. Exp. Physiol. 2009, 94, 228–239. [Google Scholar]
- Ramanathan, L.; Gozal, D.; Siegel, J.M. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J. Neurochem. 2005, 93, 47–52. [Google Scholar]
- Yang, C.; Ling, H.; Zhang, M.; Yang, Z.; Wang, X.; Zeng, F.; Wang, C.; Feng, J. Oxidative stress mediates chemical hypoxia-induced injury and inflammation by activating NF-κB-COX-2 pathway in HaCaT cells. Mol. Cells 2011, 31, 531–538. [Google Scholar]
- De Gonzalo-Calvo, D.; Neitzert, K.; Fernandez, M.; Vega-Naredo, I.; Caballero, B.; García-Macía, M.; Suárez, F.M.; Rodríguez-Colunga, M.J.; Solano, J.J.; Coto-Montes, A. Differential inflammatory responses in aging and disease: TNF-α and IL-6 as possible biomarkers. Free Radic. Biol. Med. 2010, 49, 733–737. [Google Scholar]
- Yuan, G.J.; Zhou, X.R.; Gong, Z.J.; Zhang, P.; Sun, X.M.; Zheng, S.H. Expression and activity of inducible nitric oxide synthase and endothelial nitric oxide synthase correlate with ethanol-induced liver injury. World J. Gastroenterol. 2006, 12, 2375–2381. [Google Scholar]
- Hampl, V.; Bibova, J.; Banasova, A.; Uhlík, J.; Miková, Dana; Hnilicková, O.; Lachmanová, V. Pulmonary vascular iNOS induction participates in the onset of chronic hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L11–L20. [Google Scholar]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar]
- Simonishvili, S.; Jain, M.R.; Li, H.; Levison, S.W.; Wood, T.L. Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia-ischemia. ASN Neuro 2013, 5, e00131. [Google Scholar]
- Fletcher, E.C. Sympathetic activity and blood pressure in the sleep apnea syndrome. Respiration 1997, 64, 1, 22–28. [Google Scholar]
- Fletcher, E.C.; Bao, G.; Li, R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 1999, 34, 309–314. [Google Scholar]
- Kumar, G.K.; Rai, V.; Sharma, S.D.; Ramakrishnan, D.P.; Peng, Y.J.; Souvannakitti, D.; Prabhakar, N.R. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J. Physiol. 2006, 575, 229–239. [Google Scholar]
- Prabhakar, N.R.; Kumar, G.K.; Peng, Y.J. Sympatho-adrenal activation by chronic intermittent hypoxia. J. Appl. Physiol. 2012, 113, 1304–1310. [Google Scholar]
- Peng, Y.J.; Yuan, G.; Khan, S.; Nanduri, J.; Makarenko, V.V.; Reddy, V.D.; Vasavda, C.; Kumar, G.K.; Semenza, G.L.; Prabhakar, N.R. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J. Physiol. 2014, 592, 3841–3858. [Google Scholar]
- Sopova, I.Y.; Zamorskii, I.I. Effect of melatonin on the relationship between lipid peroxidation and proteolytic activity in basal nuclei of rat brain during acute hypoxia. Bull. Exp. Biol. Med. 2006, 142, 83–85. [Google Scholar]
- Lin, L.; Huang, Q.X.; Yang, S.S.; Chu, J.; Wang, J.-Z.; Tian, Q. Melatonin in Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 14575–14593. [Google Scholar]
- Daglioglu, E.; Serdar, D.M.; Kilinc, K.; Erdogan, D.; Take, G.; Ergungor, F.; Okay, O.; Biyikli, Z. Neuroprotective effect of melatonin on experimental peripheral nerve injury: An electron microscopic and biochemical study. Cent. Eur. Neurosurg. 2009, 70, 109–114. [Google Scholar]
- Munoz-Casares, F.C.; Padillo, F.J.; Briceno, J.; Collado, J.A.; Muñoz-Castañeda, J.R.; Ortega, R.; Cruz, A.; Túnez, I.; Montilla, P.; Pera, C.; et al. Melatonin reduces apoptosis and necrosis induced by ischemia/reperfusion injury of the pancreas. J. Pineal Res. 2006, 40, 195–203. [Google Scholar]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar]
- Negi, G.; Kumar, A.; Sharma, S.S. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: Effects on NF-κB and Nrf2 cascades. J. Pineal Res. 2011, 50, 124–131. [Google Scholar]
- Maity, P.; Bindu, S.; Dey, S.; Goyal1, M.; Alam, A.; Pal, C.; Reiter, R.; Bandyopadhyay, U. Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J. Pineal Res. 2009, 46, 314–323. [Google Scholar]
- Chetsawang, B.; Putthaprasart, C.; Phansuwan-Pujito, P.; Govitrapong, P. Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: Involvement of nuclear factor κ B, Bax and Bcl-2. J. Pineal Res. 2006, 41, 116–123. [Google Scholar]
- Juknat, A.A.; Mendez, M.V.; Quaglino, A.; Fameli, C.I.; Mena, M.; Kotler, M.L. Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J. Pineal Res. 2005, 38, 84–92. [Google Scholar]
- Espino, J.; Bejarano, I.; Redondo, P.C.; Rosado, J.A.; Barriga, C.; Reiter, R.J.; Pariente, J.A.; Rodríguez, A.B. Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: Evidence for the involvement of mitochondria and Bax activation. J. Membr. Biol. 2010, 233, 105–118. [Google Scholar]
- Radogna, F.; Diederich, M.; Ghibelli, L. Melatonin: A pleiotropic molecule regulating inflammation. Biochem. Pharmacol. 2010, 80, 1844–1852. [Google Scholar]
- Radogna, F.; Cristofanon, S.; Paternoster, L.; D’Alessio, M.; de Nicola, M.; Cerella, C.; Dicato, M.; Diederich, M.; Ghibelli, L. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J. Pineal Res. 2008, 44, 316–325. [Google Scholar]
- Ling, S.; Sheng, J.Z.; Braun, J.E.; Braun, A.P. Syntaxin 1A co-associates with native rat brain and cloned large conductance, calcium-activated potassium channels in situ. J. Physiol. 2003, 553, 65–81. [Google Scholar]
- Liu, P.; Smith, P.F.; Appleton, I.; Darlington, C.L.; Bilkey, D.K. Regional variations and age-related changes in nitric oxide synthase and arginase in the sub-regions of the hippocampus. Neuroscience 2003, 119, 679–687. [Google Scholar]
- Kadekaro, M.; Su, G.; Chu, R.; Lei, Y.; Li, J; Fang, L. Effects of nitric oxide on expressions of nitrosocysteine and calcium-activated potassium channels in the supraoptic nuclei and neural lobe of dehydrated rats. Neurosci. Lett. 2007, 411, 117–122. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tipoe, G.L.; Fung, M.L. Melatonin Attenuates Intermittent Hypoxia-Induced Lipid Peroxidation and Local Inflammation in Rat Adrenal Medulla. Int. J. Mol. Sci. 2014, 15, 18437-18452. https://doi.org/10.3390/ijms151018437
Liu Y, Tipoe GL, Fung ML. Melatonin Attenuates Intermittent Hypoxia-Induced Lipid Peroxidation and Local Inflammation in Rat Adrenal Medulla. International Journal of Molecular Sciences. 2014; 15(10):18437-18452. https://doi.org/10.3390/ijms151018437
Chicago/Turabian StyleLiu, Yu, George Lim Tipoe, and Man Lung Fung. 2014. "Melatonin Attenuates Intermittent Hypoxia-Induced Lipid Peroxidation and Local Inflammation in Rat Adrenal Medulla" International Journal of Molecular Sciences 15, no. 10: 18437-18452. https://doi.org/10.3390/ijms151018437
APA StyleLiu, Y., Tipoe, G. L., & Fung, M. L. (2014). Melatonin Attenuates Intermittent Hypoxia-Induced Lipid Peroxidation and Local Inflammation in Rat Adrenal Medulla. International Journal of Molecular Sciences, 15(10), 18437-18452. https://doi.org/10.3390/ijms151018437



