The Protective Effect of MicroRNA-320 on Left Ventricular Remodeling after Myocardial Ischemia-Reperfusion Injury in the Rat Model
Abstract
:1. Introduction
2. Results
2.1. Differences in Cardiac Function of Three Groups
| Time Point | LVEF (%) | LVFS (%) | LVSP (mmHg) | |||||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||||||||||
| day 1 | 83.5 ± 2.7 | 72.2 ± 2.4 * | 80.6 ± 2.9 # | 47.3 ± 4.2 | 36.3 ± 1.9 * | 42.6 ± 3.1 # | 128 ± 4 | 111 ± 8 * | 119 ± 7 # | |||||||||
| day 3 | 84.3 ± 2.7 | 63.2 ± 1.0 * | 77.2 ± 2.2 # | 47.9 ± 4.2 | 30.1 ± 1.2 * | 40.4 ± 3.3 # | 130 ± 8 | 100 ± 8 * | 111 ± 7 # | |||||||||
| day 7 | 85.3 ± 2.6 | 56.7 ± 1.1 * | 70.7 ± 2.5 # | 48.3 ± 4.5 | 25.5 ± 1.3 * | 35.26 ± 2.2 # | 131 ± 8 | 96 ± 8 * | 104 ± 7 # | |||||||||
| day 15 | 85.2 ± 2.5 | 52.6 ± 1.3 * | 64.7 ± 2.2 # | 48.2 ± 3.9 | 23.4 ± 1.0 * | 30.8 ± 2.1 # | 129 ± 7 | 92 ± 5 * | 100 ± 6 # | |||||||||
| day 30 | 84.7 ± 2.7 | 46.9 ± 1.1 * | 59.3 ± 2.1 # | 48.4 ± 3.5 | 21.2 ± 1.4 * | 29.7 ± 1.9 # | 128 ± 6 | 88 ± 7 * | 97 ± 6 # | |||||||||
| Time Point | LVEDP (mmHg) | +dp/dtmax (mmHg/s) | −dp/dtmax (mmHg/s) | |||||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||||||||||
| day 1 | 4.5 ± 0.8 | 6.5 ± 1.1 * | 5.3 ± 0.9 # | 12,003 ± 789 | 9258 ± 753 * | 11,113 ± 719 # | 8367 ± 693 | 7442 ± 653 * | 8004 ± 598 # | |||||||||
| day 3 | 4.6 ± 0.6 | 6.8 ± 0.7 * | 5.9 ± 0.9 # | 11,936 ± 823 | 8843 ± 672 * | 9656 ± 679 # | 8438 ± 703 | 6982 ± 637 * | 7689 ± 655 # | |||||||||
| day 7 | 4.3 ± 0.6 | 7.1 ± 0.7 * | 6.8 ± 0.8 # | 12,538 ± 815 | 8121 ± 648 * | 9100 ± 772 # | 8257 ± 654 | 6478 ± 623 * | 7339 ± 641 # | |||||||||
| day 15 | 4.3 ± 0.7 | 7.8 ± 1.1 * | 7.1 ± 0.8 # | 12,784 ± 891 | 7934 ± 711 * | 8807 ± 707 # | 8354 ± 697 | 6002 ± 605 * | 6891 ± 646 # | |||||||||
| day 30 | 4.5 ± 0.7 | 8.9 ± 1.3 * | 7.9 ± 0.9 # | 12,034 ± 901 | 7453 ± 618 * | 7887 ± 710 # | 8369 ± 598 | 5840 ± 579 * | 6331 ± 622 # | |||||||||
2.2. Degree of Damage in Myocardial Cells
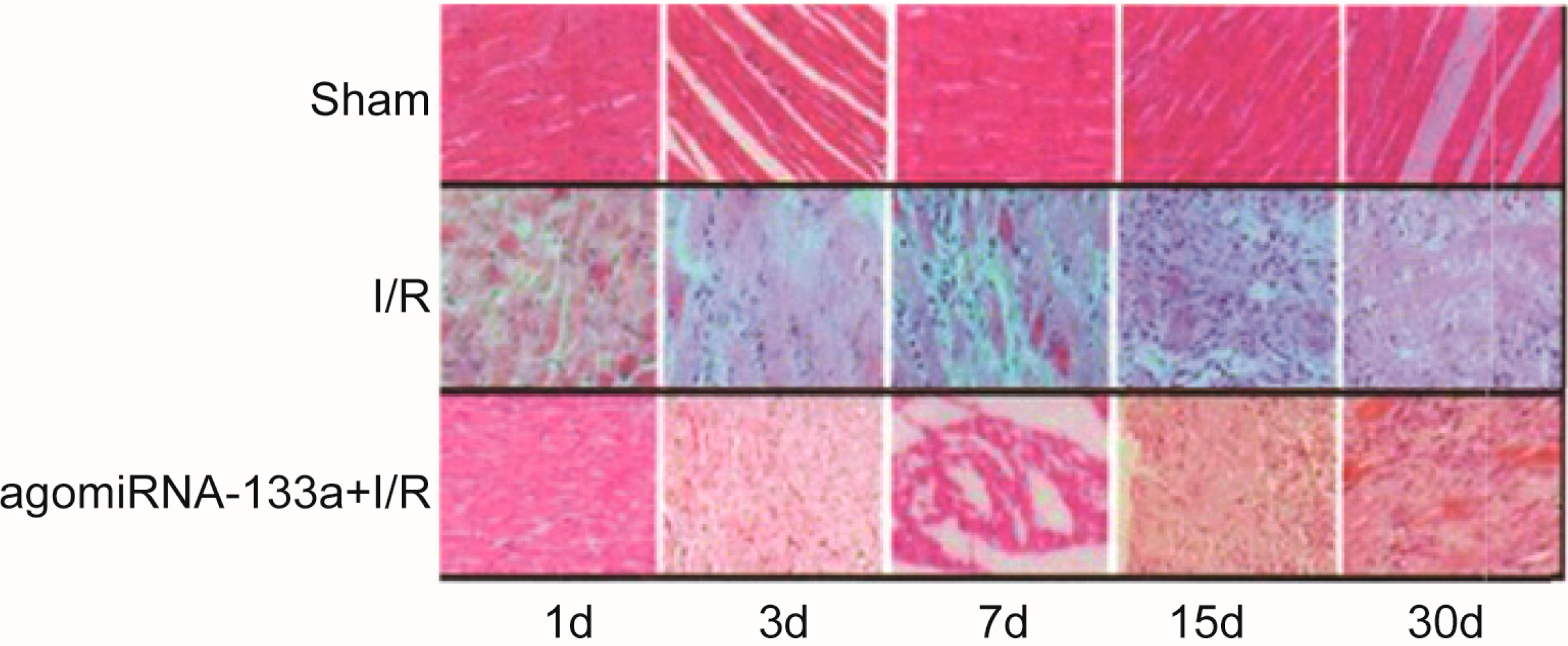
2.3. Evaluation of Myocardial Fibrosis of Three Groups
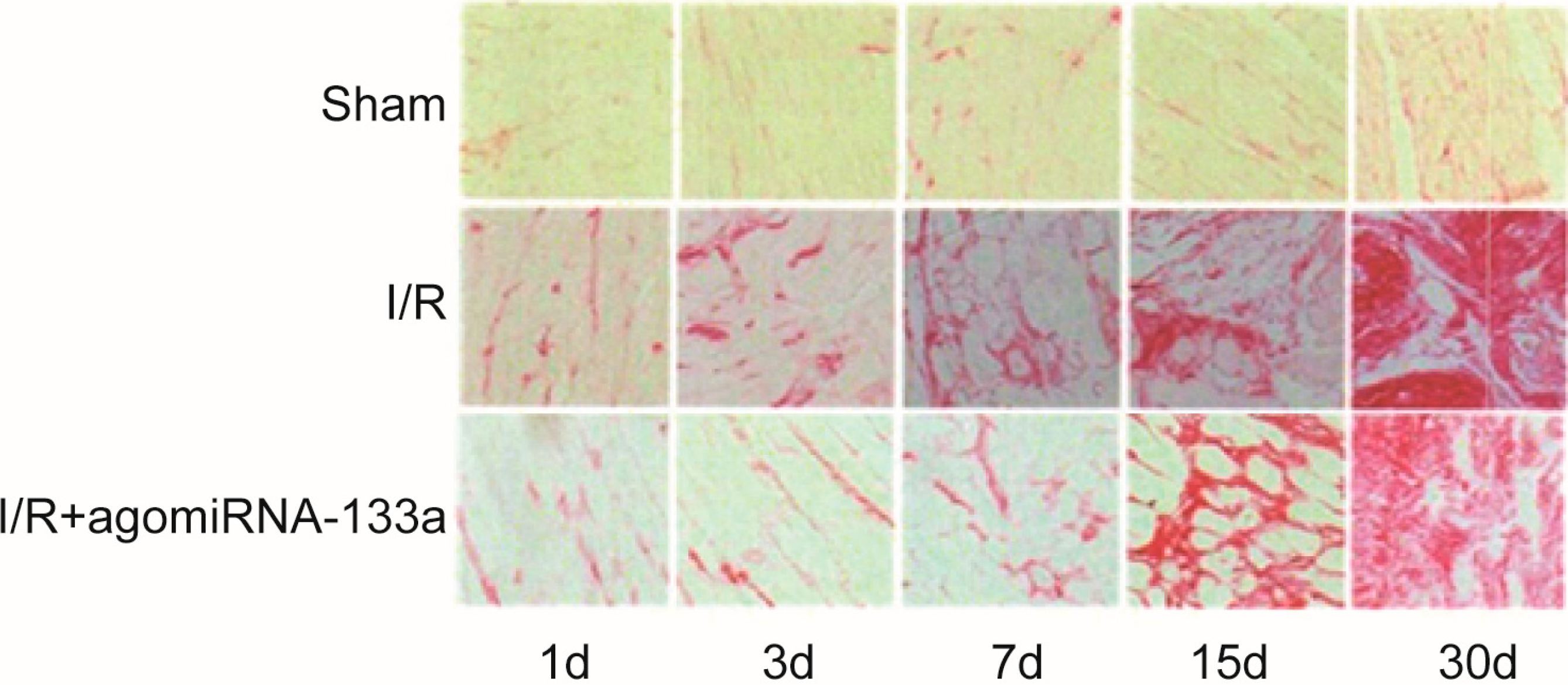
| Group | Degree of Myocardial Fibrosis (%) | ||||
|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 15 | Day 30 | |
| Sham | 1.83 ± 0.47 | 1.64 ± 0.41 | 1.57 ± 0.37 | 1.64 ± 0.38 | 1.74 ± 0.35 |
| I/R | 2.03 ± 0.81 | 5.60 ± 2.30 * | 28.54 ± 7.12 * | 36.21 ± 7.50 | 37.54 ± 5.71 |
| I/R + antagomir-320 | 1.96 ± 0.66 | 4.68 ± 1.55 # | 19.42 ± 6.11 # | 25.10 ± 8.30 | 29.81 ± 6.86 |
2.4. Detection of Apoptosis by Terminal dUTP Nick End-Labeling (TUNEL) Staining
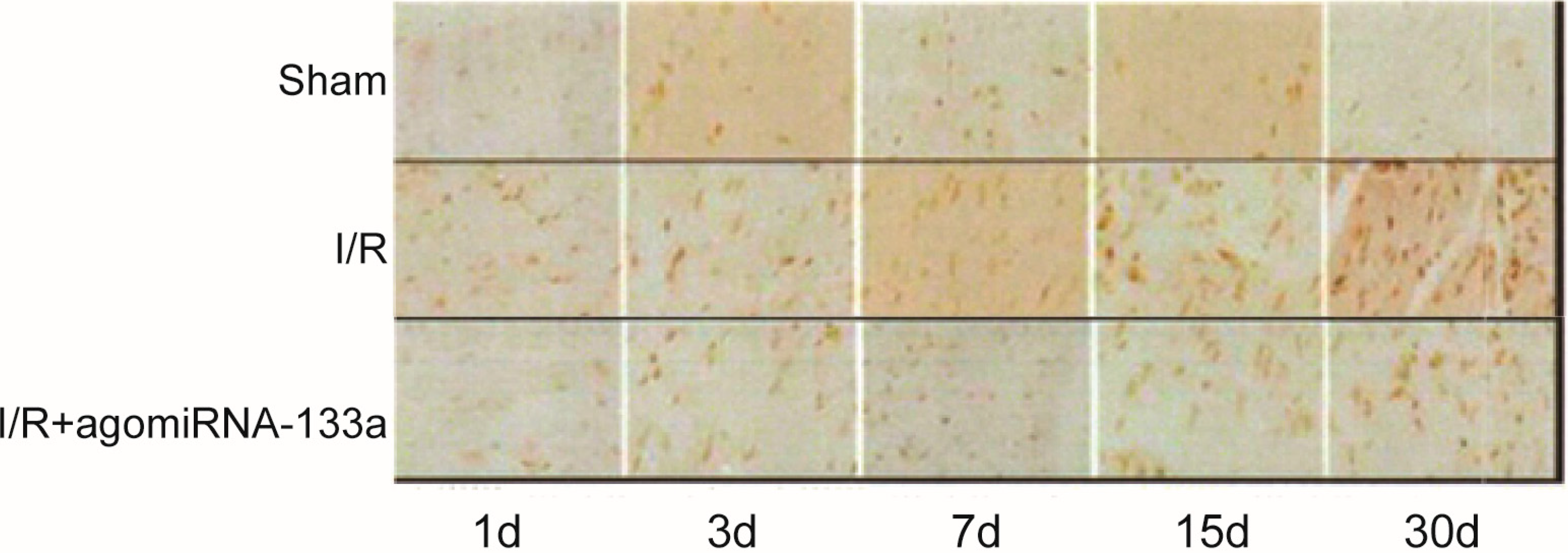
| Group | Apoptosis Rate (%) | ||||
|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 15 | Day 30 | |
| Sham | 5.6 ± 2.7 | 4.8 ± 2.4 | 5.4 ± 1.8 | 6.3 ± 2.2 | 6.4 ± 1.6 |
| I/R | 32.6 ± 5.4 * | 54.6 ± 7.3 * | 42.7 ± 6.8 * | 38.2 ± 7.5 * | 33.4 ± 5.3 * |
| I/R + antagomir-320 | 18.7 ± 3.3 # | 30.5 ± 6.4 # | 33.3 ± 8.7 # | 29.5 ± 8.7 # | 22.4 ± 4.8 # |
2.5. miR-320 Expression in Myocardial Tissue
| Group | miR-320 (2−∆∆Ct) | ||||
|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 15 | Day 30 | |
| Sham | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| I/R | 0.60 ± 0.13 * | 0.52 ± 0.13 * | 0.63 ± 0.10 * | 0.76 ± 0.09 * | 0.72 ± 0.10 * |
| I/R + agomiR-133a | 0.86 ± 0.33 | 1.55 ± 0.14 # | 1.73 ± 0.27 # | 1.63 ± 0.11 # | 1.91 ± 0.95 # |
3. Discussion
4. Materials and Methods
4.1. Animal Care
4.2. Ischemia-Reperfusion (I/R) Injury Rat Model
4.3. Transthoracic Echocardiography
4.4. Hemodynamic Examination
4.5. Histological Examination
4.6. Measurement of Myocardial Fibrosis
4.7. Determination of Myocardial Apoptosis
4.8. MicroRNA Extraction and qRT-PCR
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Santulli, G. Epidemiology of cardiovascular disease in the 21st century: Updated numbers and updated facts. J. Cardiovasc. Dis. 2013, 1, 1–2. [Google Scholar]
- Pagidipati, N.J.; Gaziano, T.A. Estimating deaths from cardiovascular disease: A review of global methodologies of mortality measurement. Circulation 2013, 127, 749–756. [Google Scholar]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B., Sr.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar]
- Konstam, M.A.; Kramer, D.G.; Patel, A.R.; Maron, M.S.; Udelson, J.E. Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment. JACC Cardiovasc. Imaging 2011, 4, 98–108. [Google Scholar]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 2013, 128, 388–400. [Google Scholar]
- Ito, Y.; Ito, K.; Shiroto, T.; Tsuburaya, R.; Yi, G.J.; Takeda, M.; Fukumoto, Y.; Yasuda, S.; Shimokawa, H. Cardiac shock wave therapy ameliorates left ventricular remodeling after myocardial ischemia-reperfusion injury in pigs in vivo. Coron. Artery Dis. 2010, 21, 304–311. [Google Scholar]
- Barallobre-Barreiro, J.; Didangelos, A.; Schoendube, F.A.; Drozdov, I.; Yin, X.; Fernandez-Caggiano, M.; Willeit, P.; Puntmann, V.O.; Aldama-Lopez, G.; Shah, A.M.; et al. Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury. Circulation 2012, 125, 789–802. [Google Scholar]
- Di, Y.; Lei, Y.; Yu, F.; Changfeng, F.; Song, W.; Xuming, M. MicroRNAs expression and function in cerebral ischemia reperfusion injury. J. Mol. Neurosci. 2014, 53, 242–250. [Google Scholar]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar]
- Olson, E.N. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci. Transl. Med. 2014, 6, 239ps3. [Google Scholar]
- Fic, P.; Kowalczuk, K.; Grabarska, A.; Stepulak, A. MicroRNA—A new diagnostic tool in coronary artery disease and myocardial infarction. Postepy Hig. Med. Dosw. Online 2014, 68, 410–418. [Google Scholar]
- Topkara, V.K.; Mann, D.L. Role of microRNAs in cardiac remodeling and heart failure. Cardiovasc. Drugs Ther. 2011, 25, 171–182. [Google Scholar]
- Ye, Y.; Perez-Polo, J.R.; Qian, J.; Birnbaum, Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol. Genomics 2011, 43, 534–542. [Google Scholar]
- Wang, X.; Zhang, X.; Ren, X.P.; Chen, J.; Liu, H.; Yang, J.; Medvedovic, M.; Hu, Z.; Fan, G.C. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 2010, 122, 1308–1318. [Google Scholar]
- Qin, Y.; Yu, Y.; Dong, H.; Bian, X.; Guo, X.; Dong, S. MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia-reperfusion injury by suppressing cell apoptosis. Int. J. Med. Sci. 2012, 9, 413–423. [Google Scholar]
- Li, B.; Li, R.; Zhang, C.; Bian, H.J.; Wang, F.; Xiao, J.; Liu, S.W.; Yi, W.; Zhang, M.X.; Wang, S.X.; et al. MicroRNA-7a/b protects against cardiac myocyte injury in ischemia/reperfusion by targeting poly(ADP-ribose) polymerase. PLoS One 2014, 9, e90096. [Google Scholar]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. hERG K(+) channels: Structure, function, and clinical significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar]
- White, R.E.; Giffard, R.G. MicroRNA-320 induces neurite outgrowth by targeting ARPP-19. Neuroreport 2012, 23, 590–595. [Google Scholar]
- Semin, B.K.; Ivanov, II; Rubin, A.B. Tetracaine inhibition of electron transport in pea chloroplasts is coupled to calcium displacement by the local anesthetic. Gen. Physiol. Biophys. 1990, 9, 65–70. [Google Scholar]
- Ren, X.P.; Wu, J.; Wang, X.; Sartor, M.A.; Qian, J.; Jones, K.; Nicolaou, P.; Pritchard, T.J.; Fan, G.C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar]
- Thum, T.; Galuppo, P.; Wolf, C.; Fiedler, J.; Kneitz, S.; van Laake, L.W.; Doevendans, P.A.; Mummery, C.L.; Borlak, J.; Haverich, A.; et al. MicroRNAs in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation 2007, 116, 258–267. [Google Scholar]
- Ikeda, S.; Kong, S.W.; Lu, J.; Bisping, E.; Zhang, H.; Allen, P.D.; Golub, T.R.; Pieske, B.; Pu, W.T. Altered microRNA expression in human heart disease. Physiol. Genomics 2007, 31, 367–373. [Google Scholar]
- Kim, A.S.; Johnston, S.C. Global variation in the relative burden of stroke and ischemic heart disease. Circulation 2011, 124, 314–323. [Google Scholar]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial ischemia reperfusion injury: From basic science to clinical bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar]
- Vilahur, G.; Juan-Babot, O.; Pena, E.; Onate, B.; Casani, L.; Badimon, L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell. Cardiol. 2011, 50, 522–533. [Google Scholar]
- Galiuto, L.; de Caterina, A.R.; Porfidia, A.; Paraggio, L.; Barchetta, S.; Locorotondo, G.; Rebuzzi, A.G.; Crea, F. Reversible coronary microvascular dysfunction: A common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur. Heart J. 2010, 31, 1319–1327. [Google Scholar]
- Herrmann, J.; Kaski, J.C.; Lerman, A. Coronary microvascular dysfunction in the clinical setting: From mystery to reality. Eur. Heart J. 2012, 33, 2771–2782. [Google Scholar]
- Vollmar, B.; Menger, M.D. Intestinal ischemia/reperfusion: Microcirculatory pathology and functional consequences. Langenbecks Arch. Surg. 2011, 396, 13–29. [Google Scholar]
- Santulli, G.; Cipolletta, E.; Sorriento, D.; del Giudice, C.; Anastasio, A.; Monaco, S.; Maione, A.S.; Condorelli, G.; Puca, A.; Trimarco, B.; et al. CaMK4 gene deletion induces hypertension. J. Am. Heart Assoc. 2012, 1, e001081. [Google Scholar]
- Jain, M.; Upadaya, S.; Zarich, S.W. Serial evaluation of microcirculatory dysfunction in patients with Takotsubo cardiomyopathy by myocardial contrast echocardiography. Clin. Cardiol. 2013, 36, 531–534. [Google Scholar]
- Oka, T.; Akazawa, H.; Naito, A.T.; Komuro, I. Angiogenesis and cardiac hypertrophy: Maintenance of cardiac function and causative roles in heart failure. Circ. Res. 2014, 114, 565–571. [Google Scholar]
- Miyazaki, Y.; Ikeda, Y.; Shiraishi, K.; Fujimoto, S.N.; Aoyama, H.; Yoshimura, K.; Inui, M.; Hoshijima, M.; Kasahara, H.; Aoki, H.; et al. Heart failure-inducible gene therapy targeting protein phosphatase 1 prevents progressive left ventricular remodeling. PLoS One 2012, 7, e35875. [Google Scholar]
- Samuel, S.M.; Thirunavukkarasu, M.; Penumathsa, S.V.; Koneru, S.; Zhan, L.; Maulik, G.; Sudhakaran, P.R.; Maulik, N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reducesventricular remodeling in infarcted myocardium of diabetic rats. Circulation 2010, 121, 1244–1255. [Google Scholar]
- Divakaran, V.; Mann, D.L. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ. Res. 2008, 103, 1072–1083. [Google Scholar]
- Weiss, J.B.; Eisenhardt, S.U.; Stark, G.B.; Bode, C.; Moser, M.; Grundmann, S. MicroRNAs in ischemia-reperfusion injury. Am. J. Cardiovasc. Dis. 2012, 2, 237–247. [Google Scholar]
- Takahama, H.; Shigematsu, H.; Asai, T.; Matsuzaki, T.; Sanada, S.; Fu, H.Y.; Okuda, K.; Yamato, M.; Asanuma, H.; Asano, Y.; et al. Liposomal amiodarone augments anti-arrhythmic effects and reduces hemodynamic adverse effects in an ischemia/reperfusion rat model. Cardiovasc. Drugs Ther. 2013, 27, 125–132. [Google Scholar]
- Santulli, G.; Iaccarino, G.; de Luca, N.; Trimarco, B.; Condorelli, G. Atrial fibrillation and microRNAs. Front. Physiol. 2014, 5, 15. [Google Scholar]
- Callis, T.E.; Pandya, K.; Seok, H.Y.; Tang, R.H.; Tatsuguchi, M.; Huang, Z.P.; Chen, J.F.; Deng, Z.; Gunn, B.; Shumate, J.; et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J. Clin. Investig. 2009, 119, 2772–2786. [Google Scholar]
- Shan, H.; Li, X.; Pan, Z.; Zhang, L.; Cai, B.; Zhang, Y.; Xu, C.; Chu, W.; Qiao, G.; Li, B.; et al. Tanshinone IIA protects against sudden cardiac death induced by lethal arrhythmias via repression of microRNA-1. Br. J. Pharmacol. 2009, 158, 1227–1235. [Google Scholar]
- Ali, N.; Rizwi, F.; Iqbal, A.; Rashid, A. Induced remote ischemic pre-conditioning on ischemia-reperfusion injury in patients undergoing coronary artery bypass. J. Coll. Physicians Surg. Pak. 2010, 20, 427–431. [Google Scholar]
- Toldo, S.; Seropian, I.M.; Mezzaroma, E.; van Tassell, B.W.; Salloum, F.N.; Lewis, E.C.; Voelkel, N.; Dinarello, C.A.; Abbate, A. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2011, 51, 244–251. [Google Scholar]
- Fan, Q.; Chen, M.; Zuo, L.; Shang, X.; Huang, M.Z.; Ciccarelli, M.; Raake, P.; Brinks, H.; Chuprun, K.J.; Dorn, G.W., 2nd.; et al. Myocardial ablation of G protein-coupled receptor kinase 2 (GRK2) decreases ischemia/reperfusion injury through an anti-intrinsic apoptotic pathway. PLoS One 2013, 8, e66234. [Google Scholar]
- Gidlöf, O.; Smith, J.G.; Miyazu, K.; Gilje, P.; Spencer, A.; Blomquist, S.; Erlinge, D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc. Disord. 2013, 13, 12. [Google Scholar]
- Zhu, H.; Fan, G.C. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc. Res. 2012, 94, 284–292. [Google Scholar]
- Ho, C.Y.; Lopez, B.; Coelho-Filho, O.R.; Lakdawala, N.K.; Cirino, A.L.; Jarolim, P.; Kwong, R.; Gonzalez, A.; Colan, S.D.; Seidman, J.G.; et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N. Engl. J. Med. 2010, 363, 552–563. [Google Scholar]
- Goldsmith, E.C.; Bradshaw, A.D.; Spinale, F.G. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: Moving beyond collagen expression. Am. J. Physiol. Cell Physiol. 2013, 304, C393–C402. [Google Scholar]
- Porter, K.E.; Turner, N.A. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol. Ther. 2009, 123, 255–278. [Google Scholar]
- Yu, S.; Li, G. MicroRNA expression and function in cardiac ischemic injury. J. Cardiovasc. Transl. Res. 2010, 3, 241–245. [Google Scholar]
- Schaar, D.G.; Medina, D.J.; Moore, D.F.; Strair, R.K.; Ting, Y. miR-320 targets transferrin receptor 1 (CD71) and inhibits cell proliferation. Exp. Hematol. 2009, 37, 245–255. [Google Scholar]
- Lorenzen, J.M.; Kielstein, J.T.; Hafer, C.; Gupta, S.K.; Kumpers, P.; Faulhaber-Walter, R.; Haller, H.; Fliser, D.; Thum, T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin. J. Am. Soc. Nephrol. 2011, 6, 1540–1546. [Google Scholar]
- Salloum, F.N.; Yin, C.; Kukreja, R.C. Role of microRNAs in cardiac preconditioning. J. Cardiovasc. Pharmacol. 2010, 56, 581–588. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.-L.; Liu, B.; Diao, H.-Y.; Shi, Y.-F.; Li, Y.-X.; Zhang, J.-C.; Lu, Y.; Wang, G.; Liu, J.; Yu, Y.-P.; et al. The Protective Effect of MicroRNA-320 on Left Ventricular Remodeling after Myocardial Ischemia-Reperfusion Injury in the Rat Model. Int. J. Mol. Sci. 2014, 15, 17442-17456. https://doi.org/10.3390/ijms151017442
Song C-L, Liu B, Diao H-Y, Shi Y-F, Li Y-X, Zhang J-C, Lu Y, Wang G, Liu J, Yu Y-P, et al. The Protective Effect of MicroRNA-320 on Left Ventricular Remodeling after Myocardial Ischemia-Reperfusion Injury in the Rat Model. International Journal of Molecular Sciences. 2014; 15(10):17442-17456. https://doi.org/10.3390/ijms151017442
Chicago/Turabian StyleSong, Chun-Li, Bin Liu, Hong-Ying Diao, Yong-Feng Shi, Yang-Xue Li, Ji-Chang Zhang, Yang Lu, Guan Wang, Jia Liu, Yun-Peng Yu, and et al. 2014. "The Protective Effect of MicroRNA-320 on Left Ventricular Remodeling after Myocardial Ischemia-Reperfusion Injury in the Rat Model" International Journal of Molecular Sciences 15, no. 10: 17442-17456. https://doi.org/10.3390/ijms151017442
APA StyleSong, C.-L., Liu, B., Diao, H.-Y., Shi, Y.-F., Li, Y.-X., Zhang, J.-C., Lu, Y., Wang, G., Liu, J., Yu, Y.-P., Guo, Z.-Y., Wang, J.-P., Zhao, Z., Liu, J.-G., Liu, Y.-H., Liu, Z.-X., Cai, D., & Li, Q. (2014). The Protective Effect of MicroRNA-320 on Left Ventricular Remodeling after Myocardial Ischemia-Reperfusion Injury in the Rat Model. International Journal of Molecular Sciences, 15(10), 17442-17456. https://doi.org/10.3390/ijms151017442




