The Biological Function and Clinical Utilization of CD147 in Human Diseases: A Review of the Current Scientific Literature
Abstract
:1. Introduction
2. Discovery and Molecular Characterization of CD147
2.1. Discovery and Molecular Structure of CD147
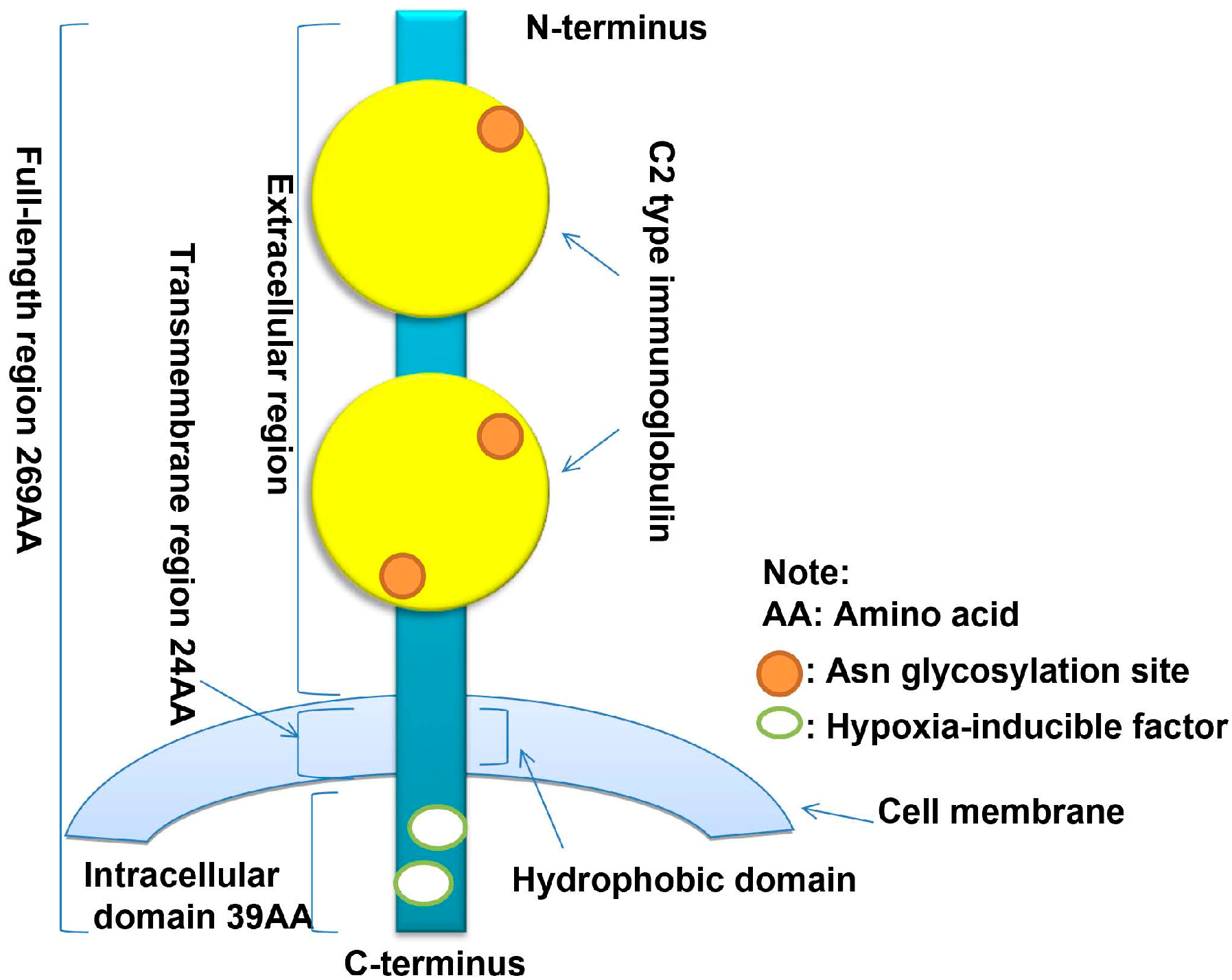
2.2. Other Isoforms of CD147
3. The Expression and Role of CD147 in Tumor Cells
3.1. CD147 Is Over-Expressed in Common Tumors
| Cancer | Regulatory Functions of CD147 | Investigators (References) |
|---|---|---|
| Brain Cancer (Gliomas) | CD147 expression contributes to glioma invasion and metastasis via stimulating MMPs. | Riethdorf et al. 2006 [56], Tian et al. 2013 [57]. |
| Breast Cancer | CD147 stimulates MMP-2 from fibroblast and regulates breast cell Invasiveness through interacting with P-glycoprotein, CD44, or EGFR. | Taylor et al. 2002 [41], Yang et al. 2006 [58], Wang et al.2008 [59], Grass et al. 2013 [60]. |
| Cervical Cancer/Carcinoma | CD147 expression correlated with MCT1 and MCT4 regulates invasion and metastasis and chemosensitivity in human cervical cancer cells. | Ju et al. 2008 [61], Pinheiro et al. 2009 [62], Zhang et al. 2013 [63]. |
| Colon Cancer | CD147-mediated tumor-host interactions regulate colon cancer growth. | Abraham et al. 2008 [64]. |
| Endometrial Cancer | CD147 may reduce e-cadherin level and increase vimentin and snail levels in endometrial cancer. | Nakamura et al. 2012 [65]. |
| Head and Neck Squamous Cell Carcinoma | CD147 expression mediated by FGFR promotes HNSCC proliferation and metastasis. | Rosenthal et al. 2005 [66], Liu et al. 2011 [67], Sweeny et al. 2012 [68], Knowles et al. 2012 [69], Sweeny et al. 2013 [70]. |
| Lymphoma | CD147 and LYVE-1 may cooperate to regulate chemoresistance in primary effusion lymphoma. | Qin et al. 2011 [71]. |
| Liver Cancer (Hepatocellular Carcinoma) | CD147 overexpression stimulates MMP production, modulates HCC growth and promotes invasion and metastasis; upregulates anoikis resistance of HCCs via interacting with GnT-Iva or Sp1 or Annexin A2. | Mamori et al. 2007 [72], Kong et al. 2011 [73], Fan et al. 2012 [74], Ke et al. 2012 [75], Feng et al. 2013 [76], Zhang et al. 2013 [77], Zhu et al. 2014 [78]. |
| Lung Cancer | CD147 regulates the invasion and metastasis of human lung cancer and correlates with HO-1 or Sp1 in NSCLC. | Kong et al. 2010 [79], Ke et al. 2012 [10], Tsai et al. 2012 [80], Xu et al. 2013 [81]. |
| Melanoma | CD147 regulates calcium signaling and hypoxia-induced MMP-2 activities via interacting with calcium-modulating cyclophilin ligand for human melanoma progression. | Long et al. 2013 [82], Zeng et al. 2014 [83]. |
| Oral Squamous Cell Carcinoma | CD147 promotes epithelial-to-mesenchymal transition by activating MMPs for OSCC invasion and progression associated with oxidative stress marker Keap1. | Huang et al. 2013 [84], Richard et al. 2013 [85], Siu et al. 2013 [86]. |
| Ovarian Cancer | CD147 as a partner of MCT1 is overexpressed under the hypoxic microenvironment and mediates cell proliferation and cycling, apoptosis, migration and invasion via activating VEGF and MMP-9 secretion, and vesicles shed from ovarian cancer cells to induce proangiogenic activities of HUVECs. | Millimaggi et al. 2007 [87], Fukuoka et al. 2012 [9], Yang et al. 2013 [11,88], Zhao et al. 2013 [89]. |
| Pancreatic Cancer | CD147 as a novel upstream activator of STAT3 interacting with CD44s is highly expressed and plays a critical role in pancreatic cancer development. | Riethdorf et al. 2006 [56], Li et al. 2013 [90], Sugyo et al. 2013 [91]. |
| Retinoblastoma | CD147 plays a role in the up-regulation of MMP-2 in invasive retinoblastoma. | Mӓӓttӓ et al. 2006 [42], Adithi et al. 2007 [43]. |
| Urothelial Carcinoma of the Bladder | CD147 expression regulates UCB invasion by affecting MMP-2, MMP-9, MMP14 and VEGF secretion. MCT1 and MCT4 may take part in this process. | Wittschieber et al. 2011 [92], Xue et al. 2011 [93], Bhagirath et al. 2012 [94], Choi et al. 2014 [95]. |
| Stomach/Gastric Cancer | CD147 expression mediates gastric cancer cell proliferation and invasion via the ERK1/2 signaling pathway and is up-regulated in gastric cancer lesions in correlation with ADAM17. | Shou et al. 2012 [96], Chen et al. 2013 [97]. |
3.2. The Roles of CD147 in the Invasion, Growth and Metastasis of Different Tumors
3.3. CD147 Always Associates with Other Proteins in Tumors
4. The Expression and Role of CD147 in Tissues and Diseases Other than Cancer
4.1. CD147 Expression in Healthy Tissues and Other Diseases
4.2. Roles of CD147 in Tissue Systems or Other Diseases to Promote MMP Production
4.3. CD147 Interacts with Other Proteins to Regulate Physiological and Pathological Processes
5. Recent Studies on the Regulatory Mechanism of CD147
5.1. CD147 Takes Part in the Regulatory Mechanism of Chemoresistance

5.2. CD147 Regulates MMP and VEGF Production or Signals for Tumor Cell Invasion and Metastasis
5.3. Some New Findings on CD147 Regulatory Mechanisms in Other Diseases via Signal Pathways
6. Additional Regulatory Mechanisms Controlling CD147 Expression and Function
7. Clinical Applications of CD147
7.1. Potential Diagnostic Markers of Disease
7.2. CD147 as a Potential Therapeutic Target
7.3. Anti-CD147 Antibody Therapeutics
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simon-Chazottes, D.; Matsubara, S.; Miyauchi, T.; Muramatsu, T.; Guenet, J.L. Chromosomal localization of two cell surface-associated molecules of potential importance in development: Midkine (Mdk) and basigin (Bsg). Mamm. Genome 1992, 2, 269–271. [Google Scholar] [CrossRef]
- Kasinrerk, W.; Fiebiger, E.; Stefanova, I.; Baumruker, T.; Knapp, W.; Stockinger, H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J. Immunol. 1992, 149, 847–854. [Google Scholar]
- Saxena, D.K.; Oh-Oka, T.; Kadomatsu, K.; Muramatsu, T.; Toshimori, K. Behaviour of a sperm surface transmembrane glycoprotein basigin during epididymal maturation and its role in fertilization in mice. Reproduction 2002, 123, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Zhang, Y.; DeCastro, R.; Guo, H.; Nakamura, T.; Kataoka, H.; Nabeshima, K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995, 55, 434–439. [Google Scholar] [PubMed]
- Miyauchi, T.; Masuzawa, Y.; Muramatsu, T. The basigin group of the immunoglobulin superfamily: Complete conservation of a segment in and around transmembrane domains of human and mouse basigin and chicken HT7 antigen. J. Biochem. 1991, 110, 770–774. [Google Scholar] [PubMed]
- Tyler, R.E.; Pearce, M.M.; Shaler, T.A.; Olzmann, J.A.; Greenblatt, E.J.; Kopito, R.R. Unassembled CD147 is an endogenous endoplasmic reticulum-associated degradation substrate. Mol. Biol. Cell 2012, 23, 4668–4678. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.W.R. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Hamasaki, M.; Koga, K.; Hayashi, H.; Aoki, M.; Kawarabayashi, T.; Miyamoto, S.; Nabeshima, K. Expression patterns of emmprin and monocarboxylate transporter-1 in ovarian epithelial tumors. Virchows Arch. 2012, 461, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Fei, F.; Chen, Y.; Xu, L.; Zhang, Z.; Huang, Q.; Zhang, H.; Yang, H.; Chen, Z.; Xing, J.; et al. Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis 2012, 3, 1598–1607. [Google Scholar]
- Yang, H.; Zou, W.; Chen, B. Overexpression of CD147 in ovarian cancer is initiated by the hypoxic microenvironment. Cell Biol. Int. 2013, 37, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fok, K.L.; Yu, S.; Jiang, J.; Chen, Z.; Gui, Y.; Cai, Z.; Chan, H.C. CD147 is required for matrix metalloproteinases-2 production and germ cell migration during spermatogenesis. Mol. Hum. Reprod. 2011, 17, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Fok, K.L.; Chen, H.; Ruan, Y.C.; Chan, H.C. Novel regulators of spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lam Fok, K.; Jiang, X.; Chan, H.C. New insights into germ cell migration and survival/apoptosis in spermatogenesis: Lessons from CD147. Spermatogenesis 2012, 2, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Chiampanichayakul, S.; Peng-in, P.; Khunkaewla, P.; Stockinger, H.; Kasinrerk, W. CD147 contains different bioactive epitopes involving the regulation of cell adhesion and lymphocyte activation. Immunobiology 2006, 211, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Wilson, M.C.; Heddle, C.; Brown, M.H.; Barclay, A.N.; Halestrap, A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000, 19, 3896–3904. [Google Scholar] [CrossRef] [PubMed]
- Burnett, L.A.; Light, M.M.; Mehrotra, P.; Nowak, R.A. Stimulation of GPR30 increases release of EMMPRIN-containing microvesicles in human uterine epithelial cells. J. Clin. Endocrinol. Metab. 2012, 97, 4613–4622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gou, X.; Ke, X.; Cui, H.; Chen, Z. Human tumor cells induce angiogenesis through positive feedback between CD147 and insulin-like growth factor-I. PLoS One 2012, 7, e40965. [Google Scholar]
- Tu, Y.; Fu, J.; Wang, J.; Fu, G.; Wang, L.; Zhang, Y. Extracellular matrix metalloproteinase inducer is associated with severity of brain oedema following experimental subarachnoid haemorrhage in rats. J. Int. Med. Res. 2012, 40, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Arendt, B.K.; Walters, D.K.; Wu, X.; Tschumper, R.C.; Jelinek, D.F. Multiple myeloma dell-derived microvesicles are enriched in CD147 expression and enhance tumor cell proliferation. Oncotarget 2014, 5, 5686–5699. [Google Scholar] [PubMed]
- Jiang, J.L.; Zhou, Q.; Yu, M.K.; Ho, L.S.; Chen, Z.N.; Chan, H.C. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J. Biol. Chem. 2001, 276, 46870–46877. [Google Scholar] [CrossRef] [PubMed]
- Fossum, S.; Mallett, S.; Barclay, A.N. The MRC OX-47 antigen is a member of the immunoglobulin superfamily with an unusual transmembrane sequence. Eur. J. Immunol. 1991, 21, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Seulberger, H.; Unger, C.M.; Risau, W. HT7, Neurothelin, Basigin, gp42 and OX-47—Many names for one developmentally regulated immuno-globulin-like surface glycoprotein on blood–brain barrier endothelium, epithelial tissue barriers and neurons. Neurosci. Lett. 1992, 140, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Major, T.; Bocan, T. Characterization of the promoter of human extracellular matrix metalloproteinase inducer (EMMPRIN). Gene 2002, 282, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Kaname, T.; Miyauchi, T.; Kuwano, A.; Matsuda, Y.; Muramatsu, T.; Kajii, T. Mapping basigin (BSG), a member of the immunoglobulin superfamily, to 19p13.3. Cytogenet. Cell Genet. 1993, 64, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Majmudar, G.; Jensen, T.C.; Biswas, C.; Toole, B.P.; Gordon, M.K. Characterization of the gene for human EMMPRIN, a tumor cell surface inducer of matrix metalloproteinases. Gene 1998, 220, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; Hu, T.; Du, J.M.; Ding, J.P.; Yang, X.M.; Zhang, J.; Yang, B.; Shen, X.; Zhang, Z.; Zhong, W.D.; et al. Crystal structure of HAb18G/CD147: Implications for immunoglobulin superfamily homophilic adhesion. J. Biol. Chem. 2008, 283, 18056–18065. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wu, Y.M.; Zhao, P.; Yang, X.M.; Jiang, J.L.; Chen, Z.N. Overexpression of HAb18G/CD147 promotes invasion and metastasis via α3β1 integrin mediated FAK–paxillin and FAK–PI3K–Ca2+ pathways. Cell Mol. Life Sci. 2008, 65, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.Y.; Dou, K.F.; Wang, C.H.; Zhao, P.; Lau, W.B.; Tao, L.; Wu, Y.M.; Tang, J.; Jiang, J.L.; Chen, Z.N.; et al. The interaction of HAb18G/CD147 with integrin α6β1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer 2009, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, J.; Yuan, S.Y. MCT1 and MCT4 expression during myocardial ischemic-reperfusion injury in the isolated rat heart. Cell. Physiol. Biochem. 2013, 32, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Sienel, W.; Polze, B.; Elshawi, K.; Lindner, M.; Morresi-Hauf, A.; Vay, C.; Eder, F.; Passlick, B.; Klein, C.A. Cellular localization of EMMPRIN predicts prognosis of patients with operable lung adenocarcinoma independent from MMP-2 and MMP-9. Mod. Pathol. 2008, 21, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Biegler, B.; Kasinrerk, W. Reduction of CD147 surface expression on primary T cells leads to enhanced cell proliferation. Asian Pac. J. Allergy Immunol. 2012, 30, 259–267. [Google Scholar] [PubMed]
- Caudroy, S.; Polette, M.; Nawrocki-Raby, B.; Cao, J.; Toole, B.P.; Zucker, S.; Birembaut, P. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin. Exp. Metastasis 2002, 19, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, S.; Zhou, H.; Cao, J.; Hu, Y.; Zhang, J. Caveolin-1 up-regulates CD147 glycosylation and the invasive capability of murine hepatocarcinoma cell lines. Int. J. Biochem. Cell Biol. 2006, 38, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.M.; Nabeshima, K.; Biswas, C. Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res. 1989, 49, 3385–3391. [Google Scholar] [PubMed]
- Cui, H.Y.; Guo, T.; Wang, S.J.; Zhao, P.; Dong, Z.S.; Zhang, Y.; Jiang, J.L.; Chen, Z.N.; Yu, X.L. Dimerization is essential for HAb18G/CD147 promoting tumor invasion via MAPK pathway. Biochem. Biophys. Res. Commun. 2012, 419, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.M.; Kirk, P.; Holt, O.J.; Puklavec, M.J.; Brown, M.H.; Barclay, A.N. A novel form of the membrane protein CD147 that contains an extra Ig-like domain and interacts homophilically. BMC Biochem. 2003, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Redzic, J.S.; Armstrong, G.S.; Isern, N.G.; Jones, D.N.; Kieft, J.S.; Eisenmesser, E.Z. The retinal specific CD147 Ig0 domain: From molecular structure to biological activity. J. Mol. Biol. 2011, 411, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, J.; Redzic, J.S.; Porter, C.C.; Yurchenko, V.; Bukrinsky, M.; Labeikovsky, W.; Armstrong, G.S.; Zhang, F.; Isern, N.G.; DeGregori, J.; et al. Solution characterization of the extracellular region of CD147 and its interaction with its enzyme ligand cyclophilin A. J. Mol. Biol. 2009, 391, 518–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhai, H.; Xu, Y.; Dong, Y.; Sun, Y.; Zang, X.; Zhao, J. Amniotic membrane traps and induces apoptosis of inflammatory cells in ocular surface chemical burn. Mol. Vis. 2012, 18, 2137–2146. [Google Scholar] [PubMed]
- Taylor, P.M.; Woodfield, R.J.; Hodgkin, M.N.; Pettitt, T.R.; Martin, A.; Kerr, D.J.; Wakelam, M.J. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene 2002, 21, 5765–5772. [Google Scholar] [CrossRef] [PubMed]
- Maatta, M.; Tervahartiala, T.; Kaarniranta, K.; Tang, Y.; Yan, L.; Tuukkanen, J.; Sorsa, T. Immunolocalization of EMMPRIN (CD147) in the human eye and detection of soluble form of EMMPRIN in ocular fluids. Curr. Eye Res. 2006, 31, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Adithi, M.; Nalini, V.; Kandalam, M.; Krishnakumar, S. Expression of matrix metalloproteinases and their inhibitors in retinoblastoma. J. Pediatr. Hematol. Oncol. 2007, 29, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tan, N.; Guo, W.; Wang, L.; Li, H.; Zhang, T.; Liu, X.; Xu, Q.; Li, J.; Guo, Z.; et al. Overexpression of EMMPRIN isoform 2 is associated with head and neck cancer metastasis. PLoS One 2014, 9, e91596. [Google Scholar]
- Li, H.M.; Zhu, P.; Fan, C.M.; Wang, Y.H.; Zheng, Z.H.; Li, X.Y.; Lu, N. Effects of PMA on CD147 expression in cultured THP-1 cells and monocytes of RA patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2006, 22, 333–335. (In Chinese) [Google Scholar] [PubMed]
- Hanata, K.; Yamaguchi, N.; Yoshikawa, K.; Mezaki, Y.; Miura, M.; Suzuki, S.; Senoo, H.; Ishikawa, K. Soluble EMMPRIN (extra-cellular matrix metalloproteinase inducer) stimulates the migration of HEp-2 human laryngeal carcinoma cells, accompanied by increased MMP-2 production in fibroblasts. Arch. Histol. Cytol. 2007, 70, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Li, Y.T.; Du, D.Y. Oxidized low-density lipoprotein-induced CD147 expression and its inhibition by high-density lipoprotein on platelets in vitro. Thromb. Res. 2013, 132, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, M.; Kurz, H.; Schachtele, C.; Marme, D.; Christ, B.; Prols, F.; Wilting, J. Induction of the blood–brain barrier marker neurothelin/HT7 in endothelial cells by a variety of tumors in chick embryos. Histochem. Cell Biol. 2000, 113, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.Y.; Huang, J.; Zhang, H.Z.; Jiang, Z.M.; Chen, J. Factors related to biologic behavior in giant cell tumor of bone. Zhonghua Bing Li Xue Za Zhi 2013, 42, 669–674. (In Chinese) [Google Scholar] [PubMed]
- Zhu, P.; Lu, N.; Shi, Z.G.; Zhou, J.; Wu, Z.B.; Yang, Y.; Ding, J.; Chen, Z.N. CD147 overexpression on synoviocytes in rheumatoid arthritis enhances matrix metalloproteinase production and invasiveness of synoviocytes. Arthritis Res. Ther. 2006, 8, R44. [Google Scholar]
- Kang, M.J.; Kim, H.P.; Lee, K.S.; Yoo, Y.D.; Kwon, Y.T.; Kim, K.M.; Kim, T.Y.; Yi, E.C. Proteomic analysis reveals that CD147/EMMPRIN confers chemoresistance in cancer stem cell-like cells. Proteomics 2013, 13, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.K.; Arendt, B.K.; Jelinek, D.F. CD147 regulates the expression of MCT1 and lactate export in multiple myeloma cells. Cell Cycle 2013, 12, 3175–3183. [Google Scholar] [PubMed]
- Chen, H.; Wang, L.; Beretov, J.; Hao, J.; Xiao, W.; Li, Y. Co-expression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clin. Exp. Metastasis 2010, 27, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Man, D.P.; Ma, S.M.; Cao, S.W.; Li, D.W. Expressions and significances of CD147, OPN and MMP-2 in oral squamous cell carcinoma. Sichuan Da Xue Xue Bao Yi Xue Bao 2012, 43, 683–686. (In Chinese) [Google Scholar]
- Pinheiro, C.; Penna, V.; Morais-Santos, F.; Abrahão-Machado, L.F.; Ribeiro, G.; Curcelli, E.C.; Olivieri, M.V.; Morini, S.; Valença, I.; Ribeiro, D.; et al. Characterization of monocarboxylate transporters (MCTs) expression in soft tissue sarcomas: Distinct prognostic impact of MCT1 sub-cellular localization. J. Transl. Med. 2014, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Riethdorf, S.; Reimers, N.; Assmann, V.; Kornfeld, J.W.; Terracciano, L.; Sauter, G.; Pantel, K. High incidence of EMMPRIN expression in human tumors. Int. J. Cancer 2006, 119, 1800–1810. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Chen, Y.; Cai, M.; Dong, H.; Xiong, L. EMMPRIN is an independent negative prognostic factor for patients with astrocytic glioma. PLoS One 2013, 8, e58069. [Google Scholar]
- Yang, J.M.; O’Neill, P.; Jin, W.; Foty, R.; Medina, D.J.; Xu, Z.; Lomas, M.; Arndt, G.M.; Tang, Y.; Nakada, M.; et al. Extracellular matrix metalloproteinase inducer (CD147) confers resistance of breast cancer cells to Anoikis through inhibition of Bim. J. Biol. Chem. 2006, 281, 9719–9727. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Li, Q.Q.; Xu, J.D.; Cao, X.X.; Li, H.X.; Tang, F.; Chen, Q.; Yang, J.M.; Xu, Z.D.; Liu, X.P.; et al. Interaction between CD147 and P-glycoprotein and their regulation by ubiquitination in breast cancer cells. Chemotherapy 2008, 54, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.D.; Tolliver, L.B.; Bratoeva, M.; Toole, B.P. CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness. J. Biol. Chem. 2013, 288, 26089–26104. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.Z.; Yang, J.M.; Zhou, X.Y.; Li, Z.T.; Wu, X.H. EMMPRIN expression as a prognostic factor in radiotherapy of cervical cancer. Clin. Cancer Res. 2008, 14, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Longatto-Filho, A.; Pereira, S.M.; Etlinger, D.; Moreira, M.A.; Jube, L.F.; Queiroz, G.S.; Schmitt, F.; Baltazar, F. Monocarboxylate transporters 1 and 4 are associated with CD147 in cervical carcinoma. Dis. Markers 2009, 26, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zeng, Y.L.; Zhang, X.G.; Chen, W.J.; Yang, R.; Li, S.J. RNA interference targeting extracellular matrix metalloproteinase inducer (CD147) inhibits growth and increases chemosensitivity in human cervical cancer cells. Eur. J. Gynaecol. Oncol. 2013, 34, 429–435. [Google Scholar] [PubMed]
- Abraham, D.; Zins, K.; Sioud, M.; Lucas, T.; Aharinejad, S. Host CD147 blockade by small interfering RNAs suppresses growth of human colon cancer xenografts. Front. Biosci. 2008, 13, 5571–5579. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kodama, J.; Hongo, A.; Hiramatsu, Y. Role of emmprin in endometrial cancer. BMC Cancer 2012, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Zhang, W.; Talbert, M.; Raisch, K.P.; Peters, G.E. Extracellular matrix metalloprotease inducer-expressing head and neck squamous cell carcinoma cells promote fibroblast-mediated type I collagen degradation in vitro. Mol. Cancer Res. 2005, 3, 195–202. [Google Scholar] [PubMed]
- Liu, Z.; Hartman, Y.E.; Warram, J.M.; Knowles, J.A.; Sweeny, L.; Zhou, T.; Rosenthal, E.L. Fibroblast growth factor receptor mediates fibroblast-dependent growth in EMMPRIN-depleted head and neck cancer tumor cells. Mol. Cancer Res. 2011, 9, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, L.; Liu, Z.; Bush, B.D.; Hartman, Y.; Zhou, T.; Rosenthal, E.L. CD147 and AGR2 expression promote cellular proliferation and metastasis of head and neck squamous cell carcinoma. Exp. Cell Res. 2012, 318, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.A.; Heath, C.H.; Saini, R.; Umphrey, H.; Warram, J.; Hoyt, K.; Rosenthal, E.L. Molecular targeting of ultrasonographic contrast agent for detection of head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, L.; Hartman, Y.E.; Zinn, K.R.; Prudent, J.R.; Marshall, D.J.; Shekhani, M.S.; Rosenthal, E.L. A novel extracellular drug conjugate significantly inhibits head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Dai, L.; Bratoeva, M.; Slomiany, M.G.; Toole, B.P.; Parsons, C. Cooperative roles for emmprin and LYVE-1 in the regulation of chemoresistance for primary effusion lymphoma. Leukemia 2011, 25, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Mamori, S.; Nagatsuma, K.; Matsuura, T.; Ohkawa, K.; Hano, H.; Fukunaga, M.; Matsushima, M.; Masui, Y.; Fushiya, N.; Onoda, H.; et al. Useful detection of CD147 (EMMPRIN) for pathological diagnosis of early hepatocellular carcinoma in needle biopsy samples. World J. Gastroenterol. 2007, 13, 2913–2917. [Google Scholar] [PubMed]
- Kong, L.M.; Liao, C.G.; Chen, L.; Yang, H.S.; Zhang, S.H.; Zhang, Z.; Bian, H.J.; Xing, J.L.; Chen, Z.N. Promoter hypomethylation up-regulates CD147 expression through increasing Sp1 binding and associates with poor prognosis in human hepatocellular carcinoma. J. Cell. Mol. Med. 2011, 15, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, S.; Yu, S.; He, J.; Zheng, W.; Zhang, J. N-Acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147. Glycoconj. J. 2012, 29, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Li, L.; Dong, H.L.; Chen, Z.N. Acquisition of anoikis resistance through CD147 upregulation: A new mechanism underlying metastasis of hepatocellular carcinoma cells. Oncol. Lett. 2012, 3, 1249–1254. [Google Scholar] [PubMed]
- Feng, L.; Zhu, S.; Zhang, Y.; Li, Y.; Gong, L.; Lan, M.; Han, X.; Yao, L.; Zhang, W. Expression and clinical significance of HAb18G/CD147 in malignant tumors. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2013, 29, 958–961. (In Chinese) [Google Scholar] [PubMed]
- Zhang, W.; Zhao, P.; Xu, X.L.; Cai, L.; Song, Z.S.; Cao, D.Y.; Tao, K.S.; Zhou, W.P.; Chen, Z.N.; Dou, K.F.; et al. Annexin A2 promotes the migration and invasion of human hepatocellular carcinoma cells in vitro by regulating the shedding of CD147-harboring microvesicles from tumor cells. PLoS One 2013, 8, e67268. [Google Scholar]
- Zhu, S.; Li, Y.; Zhang, Y.; Wang, X.; Gong, L.; Han, X.; Yao, L.; Lan, M.; Zhang, W. Expression and clinical implications of HAb18G/CD147 in hepatocellular carcinoma. Hepatol. Res. 2014. [Google Scholar] [CrossRef]
- Kong, L.M.; Liao, C.G.; Fei, F.; Guo, X.; Xing, J.L.; Chen, Z.N. Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. 2010, 101, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.R.; Wang, H.M.; Liu, P.L.; Chen, Y.H.; Yang, M.C.; Chou, S.H.; Cheng, Y.J.; Yin, W.H.; Hwang, J.J.; Chong, I.W.; et al. High expression of heme oxygenase-1 is associated with tumor invasiveness and poor clinical outcome in non-small cell lung cancer patients. Cell. Oncol. 2012, 35, 461–471. [Google Scholar] [CrossRef]
- Xu, X.Y.; Lin, N.; Li, Y.M.; Zhi, C.; Shen, H. Expression of HAb18G/CD147 and its localization correlate with the progression and poor prognosis of non-small cell lung cancer. Pathol. Res. Pract. 2013, 209, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Su, J.; Tang, W.; Luo, Z.; Liu, S.; Liu, Z.; Zhou, H.; Qi, M.; Zeng, W.; Zhang, J.; et al. A novel interaction between calcium-modulating cyclophilin ligand and Basigin regulates calcium signaling and matrix metalloproteinase activities in human melanoma cells. Cancer Lett. 2013, 339, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Su, J.; Wu, L.; Yang, D.; Long, T.; Li, D.; Kuang, Y.; Li, J.; Qi, M.; Zhang, J.; et al. CD147 promotes melanoma progression through hypoxia-induced MMP2 activation. Curr. Mol. Med. 2014, 14, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Zhang, L.; Ma, S.R.; Zhao, Z.L.; Wang, W.M.; He, K.F.; Zhao, Y.F.; Zhang, W.F.; Liu, B.; Sun, Z.J.; et al. Clinical significance of Keap1 and Nrf2 in oral squamous cell carcinoma. PLoS One 2013, 8, e83479. [Google Scholar]
- Richard, V.; Sebastian, P.; Nair, M.G.; Nair, S.N.; Malieckal, T.T.; Santhosh Kumar, T.R.; Pillai, M.R. Multiple drug resistant, tumorigenic stem-like cells in oral cancer. Cancer Lett. 2013, 338, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Siu, A.; Chang, J.; Lee, C.; Lee, S.; Lee, C.; Ramos, D.M. Expression of EMMPRIN modulates mediators of tumor invasion in oral squamous cell carcinoma. J. Calif. Dent. Assoc. 2013, 41, 831–838. [Google Scholar] [PubMed]
- Millimaggi, D.; Mari, M.; D’Ascenzo, S.; Carosa, E.; Jannini, E.A.; Zucker, S.; Carta, G.; Pavan, A.; Dolo, V. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 2007, 9, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, B. CD147 in ovarian and other cancers. Int. J. Gynecol. Cancer 2013, 23, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, S.; Gou, W.F.; Niu, Z.F.; Zhao, S.; Xiao, L.J.; Takano, Y.; Zheng, H.C. The role of EMMPRIN expression in ovarian epithelial carcinomas. Cell Cycle 2013, 12, 2899–2913. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, W.; Wu, X.; Karnak, D.; Meng, X.; Thompson, R.; Hao, X.; Li, Y.; Qiao, X.T.; Lin, J.; et al. HAb18G/CD147 promotes pSTAT3-mediated pancreatic cancer development via CD44s. Clin. Cancer Res. 2013, 19, 6703–6715. [Google Scholar] [CrossRef] [PubMed]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Nagatsu, K.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Zhang, M.R.; Kurosawa, Y.; Saga, T.; et al. Evaluation of (89)Zr-labeled human anti-CD147 monoclonal antibody as a positron emission tomography probe in a mouse model of pancreatic cancer. PLoS One 2013, 8, e61230. [Google Scholar]
- Wittschieber, D.; Stenzinger, A.; Klauschen, F.; Stephan, C.; Jung, K.; Erbersdobler, A.; Rabien, A. Decreased RECK and Increased EMMPRIN expression in urothelial carcinoma of the bladder are associated with tumor aggressiveness. Pathobiology 2011, 78, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.J.; Lu, Q.; Sun, Z.X. CD147 overexpression is a prognostic factor and a potential therapeutic target in bladder cancer. Med. Oncol. 2011, 28, 1363–1372. [Google Scholar] [PubMed]
- Bhagirath, D.; Abrol, N.; Khan, R.; Sharma, M.; Seth, A.; Sharma, A. Expression of CD147, BIGH3 and Stathmin and their potential role as diagnostic marker in patients with urothelial carcinoma of the bladder. Clin. Chim. Acta 2012, 413, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, Y.; Lee, J.H.; Kim, Y.S. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology 2014, 84, 245.e9–245.e15. [Google Scholar]
- Shou, Z.X.; Jin, X.; Zhao, Z.S. Upregulated expression of ADAM17 is a prognostic marker for patients with gastric cancer. Ann. Surg. 2012, 256, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pan, Y.; Gu, L.; Nie, Z.; He, B.; Song, G.; Li, R.; Xu, Y.; Gao, T.; Wang, S.; et al. ERK1/2 signalling pathway is involved in CD147-mediated gastric cancer cell line SGC7901 proliferation and invasion. Exp. Biol. Med. 2013, 238, 903–912. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, Y.; Zhang, H.; Yan, M.; Wang, S.; Feng, F.; Ji, P.; Li, Y.; Li, B.; Gao, G.; et al. Prognostic significance of CD147 in patients with glioblastoma. J. Neurooncol. 2013, 115, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gabison, E.E.; Mourah, S.; Steinfels, E.; Yan, L.; Hoang-Xuan, T.; Watsky, M.A.; de Wever, B.; Calvo, F.; Mauviel, A.; Menashi, S.; et al. Differential expression of extracellular matrix metalloproteinase inducer (CD147) in normal and ulcerated corneas: Role in epithelio–stromal interactions and matrix metalloproteinase induction. Am. J. Pathol. 2005, 166, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, P.J. Targeted therapy of hepatocellular cancer. Expert Opin. Investig. Drugs 2010, 19, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, H.Y.; Zhang, Q.; Song, F.; Jiang, J.L.; Yang, X.M.; Mi, L.; Wen, N.; Tian, R.; Wang, L.; et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol. Cancer Res. 2007, 5, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Cao, L.M. Inhibitory effect of arsenic trioxide on invasion in human hepatocellular carcinoma SMMC-7721 cells and its mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2012, 28, 1254–1257. (In Chinese) [Google Scholar]
- Gou, X.; Ru, Q.; Zhang, H.; Chen, Y.; Li, L.; Yang, H.; Xing, J.; Chen, Z. HAb18G/CD147 inhibits starvation-induced autophagy in human hepatoma cell SMMC7721 with an involvement of Beclin 1 down-regulation. Cancer Sci. 2009, 100, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Chen, H.; Jin, F.; Wu, W.; Li, Y.; Long, J.; Gong, X.; Luo, M.; Bi, T.; Li, Z.; et al. Expressions of CD147, MMP-2 and MMP-9 in laryngeal carcinoma and its correlation with poor prognosis. Pathol. Oncol. Res. 2014, 20, 475–481. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Lei, B.; Li, W.; Lin, N.; Sheng, W.; Huang, A.; Shen, H. Expression of HAb18G in non-small lung cancer and characterization of activation, migration, proliferation, and apoptosis in A549 cells following siRNA-induced downregulation of HAb18G. Mol. Cell. Biochem. 2013, 383, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ayva, S.K.; Karabulut, A.A.; Akatli, A.N.; Atasoy, P.; Bozdogan, O. Epithelial expression of extracellular matrix metalloproteinase inducer/CD147 and matrix metalloproteinase-2 in neoplasms and precursor lesions derived from cutaneous squamous cells: An immunohistochemical study. Pathol. Res. Pract. 2013, 209, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, C.; Wang, S.; Song, S.; Liu, X. Construction of a CD147 lentiviral expression vector and establishment of its stably transfected A549 cell line. Zhongguo Fei Ai Za Zhi 2012, 15, 694–700. (In Chinese) [Google Scholar] [PubMed]
- Szubert, S.; Szpurek, D.; Moszynski, R.; Nowicki, M.; Frankowski, A.; Sajdak, S.; Michalak, S. Extracellular matrix metalloproteinase inducer (EMMPRIN) expression correlates positively with active angiogenesis and negatively with basic fibroblast growth factor expression in epithelial ovarian cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Milia-Argeiti, E.; Mourah, S.; Vallée, B.; Huet, E.; Karamanos, N.K.; Theocharis, A.D.; Menashi, S. EMMPRIN/CD147-encriched membrane vesicles released from malignant human testicular germ cells increase MMP production through tumor-stroma interaction. Biochim. Biophys. Acta 2014, 1840, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, D.; Zhang, L.; Huang, P.; Li, Z. Effects of CD147 gene silencing on protein expression of ANXA2,MMP-2 and TIMP-2 by thyroid medullary carcinoma TT cells and biologic characteristics. Zhonghua Bing Li Xue Za Zhi 2014, 43, 103–108. (In Chinese) [Google Scholar] [PubMed]
- Dai, L.; Bai, L.; Lu, Y.; Xu, Z.; Reiss, K.; del Valle, L.; Kaleeba, J.; Toole, B.P.; Parsons, C.; Qin, Z.; et al. Emmprin and KSHV: New partners in viral cancer pathogenesis. Cancer Lett. 2013, 337, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qin, Z.; Defee, M.; Toole, B.P.; Kirkwood, K.L.; Parsons, C. Kaposi sarcoma-associated herpesvirus (KSHV) induces a functional tumor-associated phenotype for oral fibroblasts. Cancer Lett. 2012, 318, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Dai, L.; Slomiany, M.G.; Toole, B.P.; Parsons, C. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 2010, 70, 3884–3889. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Bratoeva, M.; Toole, B.P.; Qin, Z.; Parsons, C. KSHV activation of VEGF secretion and invasion for endothelial cells is mediated through viral upregulation of emmprin-induced signal transduction. Int. J. Cancer 2012, 131, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Madigan, M.C.; Khatri, A.; Power, C.A.; Hung, T.T.; Beretov, J.; Chang, L.; Xiao, W.; Cozzi, P.J.; Graham, P.H.; et al. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either CD44 or CD147. PLoS One 2012, 7, e40716. [Google Scholar]
- Yang, Q.; Liu, Y.; Huang, Y.; Huang, D.; Li, Y.; Wu, J.; Duan, M. Expression of COX-2, CD44v6 and CD147 and relationship with invasion and lymph node metastasis in hypopharyngeal squamous cell carcinoma. PLoS One 2013, 8, e71048. [Google Scholar]
- Grass, G.D.; Bratoeva, M.; Toole, B.P. Regulation of invadopodia formation and activity by CD147. J. Cell Sci. 2012, 125, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Spring, F.A.; Holmes, C.H.; Simpson, K.L.; Mawby, W.J.; Mattes, M.J.; Okubo, Y.; Parsons, S.F. The Oka blood group antigen is a marker for the M6 leukocyte activation antigen, the human homolog of OX-47 antigen, basigin and neurothelin, an immunoglobulin superfamily molecule that is widely expressed in human cells and tissues. Eur. J. Immunol. 1997, 27, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Joghetaei, N.; Stein, A.; Byrne, R.A.; Schulz, C.; King, L.; May, A.E.; Schmidt, R. The extracellular matrix metalloproteinase inducer (EMMPRIN, CD147)—A potential novel target in atherothrombosis prevention? Thromb. Res. 2013, 131, 474–480. [Google Scholar]
- Seizer, P.; Gawaz, M.; May, A.E. Cyclophilin A and EMMPRIN (CD147) in cardiovascular diseases. Cardiovasc. Res. 2014, 102, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kanyenda, L.J.; Verdile, G.; Martins, R.; Meloni, B.P.; Chieng, J.; Mastaglia, F.; Laws, S.M.; Anderton, R.S.; Boulos, S. Is cholesterol and amyloid-beta stress induced CD147 expression a protective response? Evidence that extracellular cyclophilin a mediated neuroprotection is reliant on CD147. J. Alzheimers Dis. 2014, 39, 545–556. [Google Scholar] [PubMed]
- Agrawal, S.M.; Williamson, J.; Sharma, R.; Kebir, H.; Patel, K.; Prat, A.; Yong, V.W. Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain 2013, 136, 1760–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Zhao, Y.X.; Wei, D.; Li, Y.L.; Zhang, Y.; Wu, J.; Xu, J.; Chen, C.; Tang, H.; Zhang, W.; et al. HAb18G/CD147 promotes activation of hepatic stellate cells and is a target for antibody therapy of liver fibrosis. J. Hepatol. 2012, 57, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, C.; Liu, S.; Zeng, W.; Su, J.; Wu, L.; Luo, Z.; Zhou, S.; Li, Q.; Zhang, J.; et al. CD147 promotes MTX resistance by immune cells through up-regulating ABCG2 expression and function. J. Dermatol. Sci. 2013, 70, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Lam, M.P.; Lam, K.K.; Leung, C.O.; Pang, R.T.; Chu, I.K.; Wan, T.H.; Chai, J.; Yeung, W.S.; Chiu, P.C.; et al. Identification of CD147 (basigin) as a mediator of trophoblast functions. Hum. Reprod. 2013, 28, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Richard, V.; Pillai, M.R. The stem cell code in oral epithelial tumorigenesis: “The cancer stem cell shift hypothesis”. Biochim. Biophys. Acta 2010, 1806, 146–162. [Google Scholar] [PubMed]
- Jin, A.; Chen, H.; Wang, C.; Tsang, L.L.; Jiang, X.; Cai, Z.; Chan, H.C.; Zhou, X. Elevated expression of CD147 in patients with endometriosis and its role in regulating apoptosis and migration of human endometrial cells. Fertil. Steril. 2014, 101, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Pistol, G.; Matache, C.; Calugaru, A.; Stavaru, C.; Tanaseanu, S.; Ionescu, R.; Dumitrache, S.; Stefanescu, M. Roles of CD147 on T lymphocytes activation and MMP-9 secretion in systemic lupus erythematosus. J. Cell. Mol. Med. 2007, 11, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Wang, Y.; Li, J.M.; Mou, F.X.; Wu, H. Significance of serum MMP-3, TIMP-1, and monocyte CD147 in rheumatoid arthritis patients of damp-heat Bi-syndrome and of cold-damp Bi-syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013, 33, 770–773. (In Chinese) [Google Scholar] [PubMed]
- Maeda-Hori, M.; Kosugi, T.; Kojima, H.; Sato, W.; Inaba, S.; Maeda, K.; Nagaya, H.; Sato, Y.; Ishimoto, T.; Ozaki, T.; et al. Plasma CD147 reflects histological features in patients with lupus nephritis. Lupus 2014, 23, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, Z.X.; Pan, Y.F.; Zhang, F.C.; Zheng, B.R.; Deng, W.M.; Li, T.W.; Gu, J.R. Expressions of CD147 in peripheral monocytes and T lymphocytes of patients with ankylosing spondylitis. Zhonghua Yi Xue Za Zhi 2010, 90, 2902–2906. (In Chinese) [Google Scholar] [PubMed]
- Agrawal, S.M.; Silva, C.; Tourtellotte, W.W.; Yong, V.W. EMMPRIN: A novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurosci. 2011, 31, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, S.S.; Jiang, J.J.; Lu, X.B.; Xue, Y.S.; Wang, J.C.; Mi, Y.F.; Zhu, M.; Ge, W.L.; Tang, L.J.; et al. Expression of extracellular matrix metalloproteinase inducer in the unstable plaque of patients with acute coronary syndrome. Zhonghua Xin Xue Guan Bing Za Zhi 2012, 40, 416–420. (In Chinese) [Google Scholar] [PubMed]
- Zhou, J.; Song, B.; Duan, X.; Long, Y.; Lu, J.; Li, Z.; Zeng, S.; Zhan, Q.; Yuan, M.; Yang, Q.; et al. Association of BSG genetic polymorphisms with atherosclerotic cerebral infarction in the Han Chinese population. Int. J. Neurosci. 2014, 124, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Dohgu, S.; Koga, M.; Machida, T.; Watanabe, T.; Miura, T.; Tsumagari, K.; Terasawa, M.; Yamauchi, A.; Kataoka, Y.; et al. Cyclophilin A secreted from fibroblast-like synoviocytes is involved in the induction of CD147 expression in macrophages of mice with collagen-induced arthritis. J. Inflamm. 2012, 9, 44. [Google Scholar] [CrossRef]
- Geng, J.J.; Zhang, K.; Chen, L.N.; Miao, J.L.; Yao, M.; Ren, Y.; Fu, Z.G.; Chen, Z.N.; Zhu, P. Enhancement of CD147 on M1 macrophages induces differentiation of Th17 cells in the lung interstitial fibrosis. Biochim. Biophys. Acta 2014, 1842, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Philp, N.J.; Ochrietor, J.D.; Rudoy, C.; Muramatsu, T.; Linser, P.J. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1305–1311. [Google Scholar] [CrossRef]
- Payne, K.J.; Clyde, L.A.; Weldon, A.J.; Milford, T.A.; Yellon, S.M. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol. Reprod. 2012, 87, 106. [Google Scholar] [CrossRef] [PubMed]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T.; et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar] [PubMed]
- Williams, A.R.; Douglas, A.D.; Miura, K.; Illingworth, J.J.; Choudhary, P.; Murungi, L.M.; Furze, J.M.; Diouf, A.; Miotto, O.; Crosnier, C.; et al. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: Assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog. 2012, 8, e1002991. [Google Scholar]
- Muramatsu, T. Basigin: A multifunctional membrane protein with an emerging role in infections by malaria parasites. Expert Opin. Ther. Targets 2012, 16, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Wanaguru, M.; Liu, W.; Hahn, B.H.; Rayner, J.C.; Wright, G.J. RH5-Basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2013, 110, 20735–20740. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.C.; Simpson, N.; Join-Lambert, O.; Federici, C.; Laran-Chich, M.P.; Maïssa, N.; Bouzinba-Ségard, H.; Morand, P.C.; Chretien, F.; Taouji, S.; et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 2014, 20, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Bartholdson, S.J.; Crosnier, C.; Bustamante, L.Y.; Rayner, J.C.; Wright, G.J. Identifying novel Plasmodium falciparum erythrocyte invasion receptors using systematic extracellular protein interaction screens. Cell. Microbiol. 2013, 15, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liao, L.; Chen, C.; Zeng, W.; Liu, S.; Su, J.; Zhao, S.; Chen, M.; Kuang, Y.; Chen, X.; et al. CD147 mediates chemoresistance in breast cancer via ABCG2 by affecting its cellular localization and dimerization. Cancer Lett. 2013, 337, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, M.G.; Grass, G.D.; Robertson, A.D.; Yang, X.Y.; Maria, B.L.; Beeson, C.; Toole, B.P. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer. Res. 2009, 69, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P.; Slomiany, M.G. Hyaluronan, CD44 and Emmprin: Partners in cancer cell chemoresistance. Drug Resist. Updates 2008, 11, 110–121. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, L.; Wang, Y.; Zhuo, Y.; Li, H.; Chen, J.; Chen, W. Overexpression of CD147 contributes to the chemoresistance of head and neck squamous cell carcinoma cells. J. Oral Pathol. Med. 2013, 42, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, P.; Zhang, B.; Wang, A.; Yang, M. Role of STAT3 decoy oligodeoxynucleotides on cell invasion and chemosensitivity in human epithelial ovarian cancer cells. Cancer Genet. Cytogenet. 2010, 197, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Amit-Cohen, B.C.; Rahat, M.M.; Rahat, M.A. Tumor cell-macrophage interactions increase angiogenesis through secretion of EMMPRIN. Front. Physiol. 2013, 4, 178. [Google Scholar] [PubMed]
- Singh, M.; Kindelberger, D.; Nagymanyoki, Z.; Ng, S.W.; Quick, C.M.; Yamamoto, H.; Fichorova, R.; Fulop, V.; Berkowitz, R.S. Vascular endothelial growth factors and their receptors and regulators in gestational trophoblastic diseases and normal placenta. J. Reprod. Med. 2012, 57, 197–203. [Google Scholar] [PubMed]
- Mishra, B.; Kizaki, K.; Sato, T.; Ito, A.; Hashizume, K. The role of extracellular matrix metalloproteinase inducer (EMMPRIN) in the regulation of bovine endometrial cell functions. Biol. Reprod. 2012, 87, 149. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Kizaki, K.; Koshi, K.; Ushizawa, K.; Takahashi, T.; Hosoe, M.; Sato, T.; Ito, A.; Hashizume, K. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN) and its expected roles in the bovine endometrium during gestation. Domest. Anim. Endocrinol. 2012, 42, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bahmed, K.; Henry, C.; Holliday, M.; Redzic, J.; Ciobanu, M.; Zhang, F.; Weekes, C.; Sclafani, R.; Degregori, J.; Eisenmesser, E.; et al. Extracellular cyclophilin-A stimulates ERK1/2 phosphorylation in a cell-dependent manner but broadly stimulates nuclear factor kappa B. Cancer Cell Int. 2012, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Bultmann, A.; Fischel, S.; Gillitzer, A.; Cullen, P.; Walch, A.; Jost, P.; Ungerer, M.; Tolley, N.D.; Lindemann, S.; et al. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ. Res. 2008, 102, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, N.; Zhou, J.; Chen, Z.N.; Zhu, P. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology 2008, 47, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Reis, R.M.; Ricardo, S.; Longatto-Filho, A.; Schmitt, F.; Baltazar, F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J. Biomed. Biotechnol. 2010, 2010, 427694. [Google Scholar] [CrossRef]
- Klawitter, J.; Klawitter, J.; Schmitz, V.; Brunner, N.; Crunk, A.; Corby, K.; Bendrick-Peart, J.; Leibfritz, D.; Edelstein, C.L.; Thurman, J.M.; et al. Low-salt diet and cyclosporine nephrotoxicity: Changes in kidney cell metabolism. J. Proteome Res. 2012, 11, 5135–5144. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Schiene-Fischer, C. Functional aspects of extracellular cyclophilins. Biol. Chem. 2014, 395, 721–735. [Google Scholar] [PubMed]
- Venkatesan, B.; Valente, A.J.; Prabhu, S.D.; Shanmugam, P.; Delafontaine, P.; Chandrasekar, B. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-κB andMKK7/JNK/AP-1 signaling. J. Mol. Cell. Cardiol. 2010, 49, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Redzic, J.S.; Kendrick, A.A.; Bahmed, K.; Dahl, K.D.; Pearson, C.G.; Robinson, W.A.; Robinson, S.E.; Graner, M.W.; Eisenmesser, E.Z. Extracellular vesicles secreted from cancer cell lines stimulate secretion of MMP-9, IL-6, TGF-β1 and EMMPRIN. PLoS One 2013, 8, e71225. [Google Scholar]
- Wu, J.; Ru, N.Y.; Zhang, Y.; Li, Y.; Wei, D.; Ren, Z.; Huang, X.F.; Chen, Z.N.; Bian, H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-β signaling and is transcriptionally regulated by Slug. Oncogene 2011, 30, 4410–4427. [Google Scholar] [CrossRef] [PubMed]
- Menashi, S.; Serova, M.; Ma, L.; Vignot, S.; Mourah, S.; Calvo, F. Regulation of extracellular matrix metalloproteinase inducer and matrix metalloproteinase expression by amphiregulin in transformed human breast epithelial cells. Cancer Res. 2003, 63, 7575–7580. [Google Scholar] [PubMed]
- Capestrano, M.; Mariggio, S.; Perinetti, G.; Egorova, A.V.; Iacobacci, S.; Santoro, M.; di Pentima, A.; Iurisci, C.; Egorov, M.V.; di Tullio, G.; et al. Cytosolic phospholipase A2ε drives recycling through the clathrin-independent endocytic route. J. Cell Sci. 2014, 127, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Mauris, J.; Woodward, A.M.; Cao, Z.; Panjwani, N.; Argüeso, P. Molecular basis for MMP9 induction and disruption of epithelial cell–cell contacts by galectin-3. J. Cell Sci. 2014, 127, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, R.; Chiche, J.; Marchiq, I.; Naiken, T.; Ilc, K.; Murray, C.M.; Critchlow, S.E.; Roux, D.; Simon, M.P.; Pouyssegur, J.; et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 16663–16668. [Google Scholar] [CrossRef] [PubMed]
- Priglinger, C.S.; Szober, C.M.; Priglinger, S.G.; Merl, J.; Euler, K.N.; Kernt, M.; Gondi, G.; Behler, J.; Geerlof, A.; Kampik, A.; et al. Galectin-3 induces clustering of CD147 and integrin-β1 transmembrane glycoprotein receptors on the RPE cell surface. PLoS One 2013, 8, e70011. [Google Scholar]
- Feng, X.; Xiu, B.; Xu, L.; Yang, X.; He, J.; Leong, D.; He, F.; Zhang, H. Hepatitis C virus core protein promotes the migration and invasion of hepatocyte via activating transcription of extracellular matrix metalloproteinase inducer. Virus Res. 2011, 158, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Prabhu, S.D.; Mummidi, S.; Valente, A.J.; Venkatesan, B.; Shanmugam, P.; Delafontaine, P.; Chandrasekar, B. Interleukin-18 induces EMMPRIN expression in primary cardiomyocytes via JNK/Sp1 signaling and MMP-9 in part via EMMPRIN and through AP-1 and NF-κB activation. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1242–H1254. [Google Scholar]
- Kong, L.M.; Liao, C.G.; Zhang, Y.; Xu, J.; Li, Y.; Huang, W.; Zhang, Y.; Bian, H.; Chen, Z.N. A regulatory loop involving miR-22, Sp1 and c-Myc modulates CD147 expression in breast cancer invasion and metastasis. Cancer Res. 2014, 74, 3764–3778. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gao, X.; Pan, S. Effect of minocycline on carotid atherosclerotic plaques. Neurol. Res. 2013, 35, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Hemler, M.E. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J. Biol. Chem. 2004, 279, 11112–11118. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Simmons, M.N.; Seki, T.; Oh, S.P.; Sugrue, S.P. Change in gene expression subsequent to induction of Pnn/DRS/memA: Increase in p21 (cip1/waf1). Oncogene 2001, 20, 4007–4018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, X.; Liu, H.; Li, L.; Hou, Q.; Gao, J. Autophagy induced by baicalin involves downregulation of CD147 in SMMC-7721 cells in vitro. Oncol. Rep. 2012, 27, 1128–1134. [Google Scholar] [PubMed]
- Landskron, J.; Tasken, K. CD147 in regulatory T cells. Cell. Immunol. 2013, 282, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Zhi, C.; Li, Y.M.; Qi, W.J.; Mei, J.J.; Yan, Z.M.; Shen, H. Association of HAb18G with clinicopathologic features and prognosis in non-small cell carcinoma of lung. Zhonghua Bing Li Xue Za Zhi 2012, 41, 151–155. (In Chinese) [Google Scholar]
- Boye, K.; Nesland, J.M.; Sandstad, B.; Haugland Haugen, M.; Maelandsmo, G.M.; Flatmark, K. EMMPRIN is associated with S100A4 and predicts patient outcome in colorectal cancer. Br. J. Cancer 2012, 107, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Grupp, K.; Hohne, T.S.; Prien, K.; Hube-Magg, C.; Tsourlakis, M.C.; Sirma, H.; Pham, T.; Heinzer, H.; Graefen, M.; Michl, U.; et al. Reduced CD147 expression is linked to ERG fusion-positive prostate cancers but lacks substantial impact on PSA recurrence in patients treated by radical prostatectomy. Exp. Mol. Pathol. 2013, 95, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Wang, R.; Cheng, J.; Gao, S.; Liu, B. Treatment of (131)I-labeled anti-CD147 monoclonal antibody in VX2 carcinoma-induced liver tumors. Oncol. Rep. 2013, 30, 246–252. [Google Scholar] [PubMed]
- Agrawal, S.M.; Silva, C.; Wang, J.; Tong, J.P.; Yong, V.W. A novel anti-EMMPRIN function-blocking antibody reduces T cell proliferation and neurotoxicity: Relevance to multiple sclerosis. J. Neuroinflamm. 2012, 9, 64. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Chen, L.; Zhong, W.D.; Zhang, Z.; Mi, L.; Zhang, Y.; Liao, C.G.; Bian, H.J.; Jiang, J.L.; et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology 2009, 54, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Yang, H.; Hou, X.; Zhang, W.; Chen, B.; Xin, X. Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett. 2007, 248, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, V.; Constant, S.; Eisenmesser, E.; Bukrinsky, M. Cyclophilin-CD147 interactions: A new target for anti-inflammatory therapeutics. Clin. Exp. Immunol. 2010, 160, 305–317. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, L.; Edwards, C.K., III; Zhou, L. The Biological Function and Clinical Utilization of CD147 in Human Diseases: A Review of the Current Scientific Literature. Int. J. Mol. Sci. 2014, 15, 17411-17441. https://doi.org/10.3390/ijms151017411
Xiong L, Edwards CK III, Zhou L. The Biological Function and Clinical Utilization of CD147 in Human Diseases: A Review of the Current Scientific Literature. International Journal of Molecular Sciences. 2014; 15(10):17411-17441. https://doi.org/10.3390/ijms151017411
Chicago/Turabian StyleXiong, Lijuan, Carl K. Edwards, III, and Lijun Zhou. 2014. "The Biological Function and Clinical Utilization of CD147 in Human Diseases: A Review of the Current Scientific Literature" International Journal of Molecular Sciences 15, no. 10: 17411-17441. https://doi.org/10.3390/ijms151017411
APA StyleXiong, L., Edwards, C. K., III, & Zhou, L. (2014). The Biological Function and Clinical Utilization of CD147 in Human Diseases: A Review of the Current Scientific Literature. International Journal of Molecular Sciences, 15(10), 17411-17441. https://doi.org/10.3390/ijms151017411




