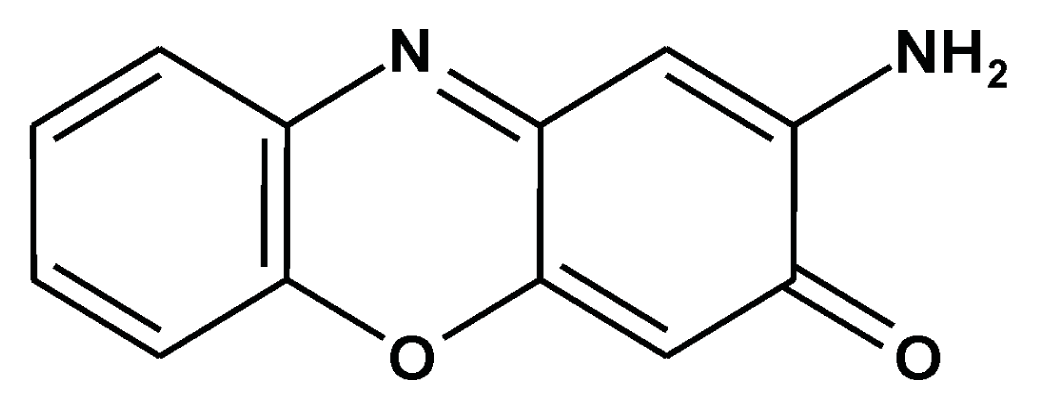
Prevention of Carcinogenesis and Development of Gastric and Colon Cancers by 2-Aminophenoxazine-3-one (Phx-3): Direct and Indirect Anti-Cancer Activity of Phx-3
Abstract
:1. Introduction
2. Direct Anticancer Activity of Phx-3 on Gastric and Colon Cancers
2.1. Cytotoxic and Proapoptotic Effects of Phx-3 on Gastric and Colon Cancer Cells in Vitro
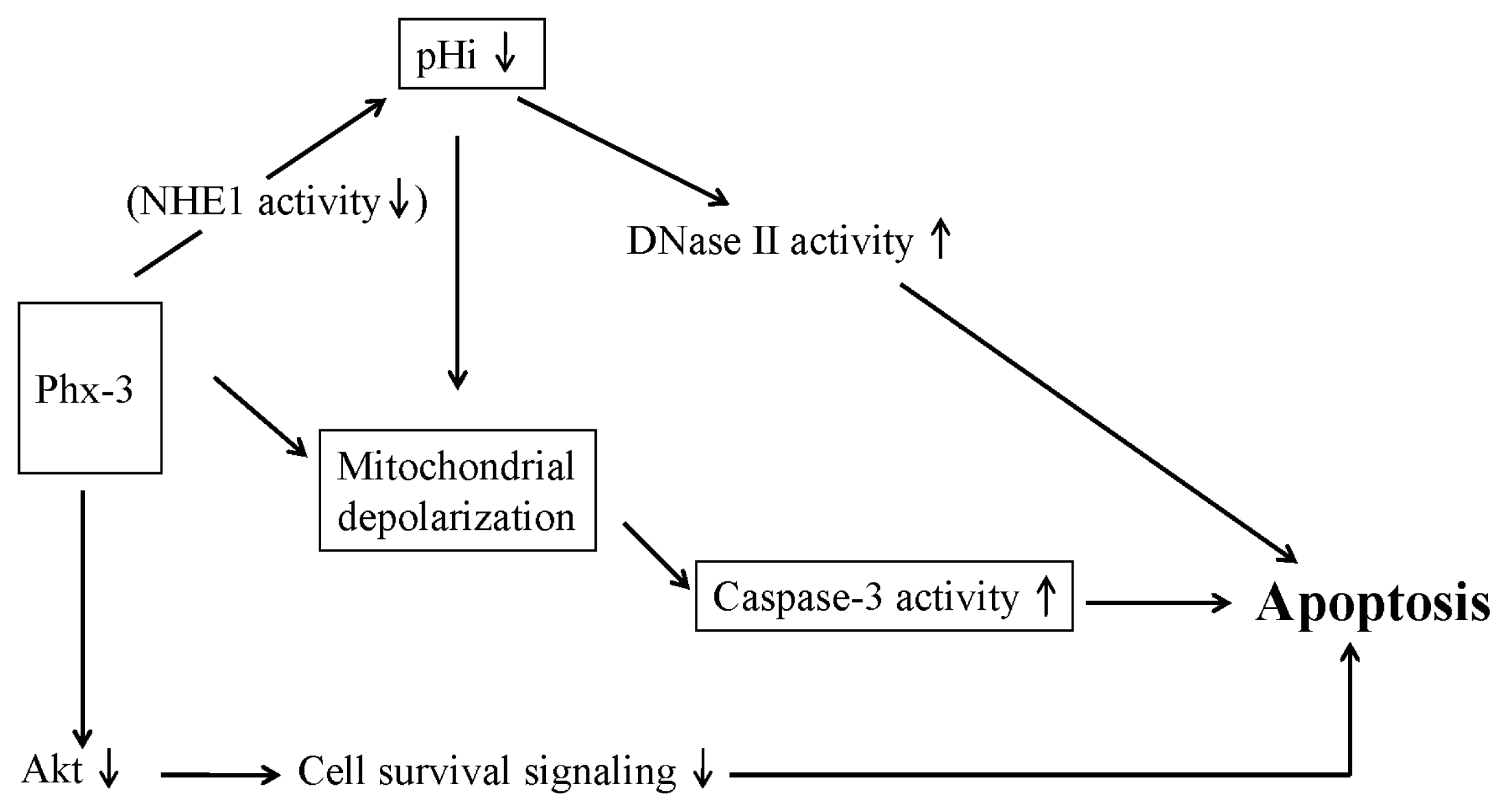
2.2. Phx-3 Induces the Apoptotic Cell Death of Gastric and Colon Cancer Cells by Reducing the Higher pHi of These Cells
2.3. Dysfunction of the Mitochondria in Gastric and Colon Cancer Cells by Phx-3
2.4. Plausible Mechanism for the Anticancer Effects of Phx-3 on Gastric and Colon Cancer Cells
3. Indirect Anticancer Activity of Phx-3 on Gastric and Colon Cancers
4. Future Aspect of Phx-3 as Anticancer Drug for the Treatment of Gastric and Colon Cancers
Acknowledgments
Conflicts of Interest
References
- Anzai, K.; Isono, K.; Okuma, K.; Suzuki, S. The new antibiotics, questiomycins A and B. J. Antibiotics 1960, 13, 125–132. [Google Scholar]
- Tomoda, A.; Yamaguchi, J.; Kojima, H.; Amemiya, H.; Yoneyama, Y. Mechanism of o-aminophenol metabolism in human erythrocytes. FEBS Lett 1986, 196, 44–48. [Google Scholar]
- Shimizu, S.; Suzuki, M.; Miyazawa, K.; Yokoyama, T.; Ohyashiki, K.; Miyazaki, K.; Abe, A.; Tomoda, A. Differentiation and apoptosis in human malignant melanoma G-361 cells induced by 2-aminophenoxazine-3-one. Oncol. Rep 2005, 14, 41–46. [Google Scholar]
- Kato, S.; Shirato, K.; Imaizumi, K.; Toyota, H.; Mizuguchi, J.; Odawara, M.; Che, X-F.; Akiyama, S.; Abe, A.; Tomoda, A. Anticancer effects of phenoxazine derivatives combined with tumor necrosis factor-related apoptosis-inducing ligand on pancreatic cancer cell lines, KLM-1 and MIA-PaCa-2. Oncol. Rep. 2006, 15, 843–848. [Google Scholar]
- Shirato, K.; Imaizumi, K.; Miyazawa, K.; Takasaki, A.; Mizuguchi, J.; Che, XF.; Akiyama, S.; Tomoda, A. Apoptosis induction preceded by mitochondrial depolarization in multiple myeloma cell line U266 by 2-aminophenoxazine-3-one. Biol. Pharm. Bull. 2008, 31, 62–67. [Google Scholar]
- Takasaki, A.; Hanyu, H.; Iwamoto, T.; Shirato, K.; Izumi, R.; Toyota, H.; Mizuguchi, J.; Miyazawa, K.; Tomoda, A. Mitochondrial depolarization and apoptosis associated with sustained activation of c-jun-N-terminal kinase in the human multiple myeloma cell line U266 induced by 2-aminophenoxazine-3-one. Mol. Med. Rep 2009, 2, 199–203. [Google Scholar]
- Zheng, C.L.; Che, X.-F.; Akiyama, S.; Miyazawa, K.; Tomoda, A. 2-Aminophenoxazine-3-one induces cellular apoptosis by causing rapid intracellular acidification and generating reactive oxygen species in human lung adenocarcinoma cells. Int. J. Oncol 2010, 36, 641–650. [Google Scholar]
- Che, X.F.; Zheng, C.L.; Akiyama, S.; Tomoda, A. 2-Aminophenoxazine-3-one and 2-amino- 4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one cause cellular apoptosis by reducing higher intracellular pH in cancer cells. Proc. Jpn. Acad. Ser. B 2011, 87, 199–213. [Google Scholar]
- Miyano-Kurosaki, N.; Kurosaki, K.; Hayashi, M.; Takaku, H.; Hayafune, M.; Shirato, K.; Kasuga, T.; Endo, T.; Tomoda, A. 2-Aminophenoxazine-3-one suppresses the growth of mouse malignant melanoma B16 cells transplanted into C57BL/6Cr Slc mice. Biol. Pharm. Bull 2006, 29, 2197–2201. [Google Scholar]
- Hongo, T.; Miyano-Kurosaki, N.; Kurosaki, K.; Hata, A.; Tomoda, A. 2-Aminophenoxazine-3-one prevents pulmonary metastasis of mouse B16 melanoma cells in mice. J. Pharmacol. Sci 2010, 114, 63–68. [Google Scholar]
- Hanawa, T.; Osaki, T.; Manzoku, T.; Fukuda, M.; Kawakami, H.; Tomoda, A.; Kamiya, S. In vitro antibacterial activity of Phx-3 against Helicobacter pylori. Biol. Pharm. Bull 2010, 33, 188–191. [Google Scholar]
- Uruma, T.; Yamaguchi, H.; Fukuda, M.; Kawakami, H.; Goto, H.; Kishimoto, T.; Yamamoto, Y.; Tomoda, A.; Kamiya, S. Chlamydia pneumoniae growth inhibition in human monocytic THP-1 cells and human epithelial HEp-2 cells by a novel phenoxazine derivative. J. Med. Microbiol 2005, 54, 1143–1149. [Google Scholar]
- Shimizu, S.; Suzuki, M.; Tomoda, A.; Arai, S.; Taguchi, H.; Hanawa, T.; Kamiya, S. Phenoxazine compounds produced by the reactions with bovine hemoglobin show antimicrobial activity against non-tuberculosis mycobacteria. Tohoku J. Exp. Med 2004, 203, 47–52. [Google Scholar]
- Hayashi, K.; Hayashi, T.; Tomoda, A. Phenoxazine derivatives inactivate human cytomegalovirus, herpes simplex virus-1, and herpes simplex virus-2 in vitro. J. Pharmacol. Sci 2008, 106, 369–375. [Google Scholar]
- Kasuga, T.; Tabuchi, T.; Shirato, K.; Imaizumi, K.; Tomoda, A. Caspase-independent cell death revealed in human gastric cancer cell lines, MKN45 and KATO III treated with phenoxazine derivatives. Oncol. Rep 2007, 17, 400–415. [Google Scholar]
- Nagata, H.; Che, X.-F.; Miyazawa, K.; Tomoda, A.; Konishi, M.; Ubukata, H.; Tabuchi, T. Rapid decrease of intracellular pH associated with inhibition of Na+/H+ exchanger precedes apoptotic events in the MNK45 and MNK74 gastric cancer cell lines treated with 2-aminophenoxazine-3-one. Oncol. Rep 2011, 25, 341–346. [Google Scholar]
- Nakachi, T.; Tabuchi, T.; Takasaki, A.; Arai, S.; Miyazawa, K.; Tomoda, A. Anticancer activity of phenoxazines produced by bovine erythrocytes on colon cancer cells. Oncol. Rep 2010, 23, 1517–1522. [Google Scholar]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 37, 539–545. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 430, 860–867. [Google Scholar]
- Richards, C.H.; Roxburgh, C.S.; Anderson, J.H.; McKee, R.F.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br. J. Surg 2012, 99, 287–294. [Google Scholar]
- Gregori, A.; Houghton, A. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res 2011, 71, 2411–2416. [Google Scholar]
- Tabuchi, T.; Soma, T.; Yonekawa, M.; Komai, T.; Hashimoto, T.; Adachi, M. Reduction of VX2 transplanted tumor by granulocyte depletion using extracorporeal circulation on rabbit models. Anticancer Res 1992, 12, 795–798. [Google Scholar]
- Tabuchi, T.; Ubukata, H.; Sato, S.; Nakata, I.; Goto, Y.; Watanabe, Y.; Hashimoto, T.; Mizuta, T.; Adachi, M.; Soda, T. Granulocytapheresis as a possible cancer treatment. Anticancer Res 1995, 15, 985–990. [Google Scholar]
- Tabuchi, T.; Ubukata, H.; Saniabadi, A.; Soma, T. Granulocyte apheresis as a possible new approach in cancer therapy: A pilot study involving two cases. Cancer Detect. Prev 1999, 23, 417–421. [Google Scholar]
- Satomi, A.; Murakami, S.; Ishida, K.; Matsuri, M.; Hashimoto, T.; Sonoda, M. Significance of increased neutrophils in patients with advance colorectal cancer. Acta Oncol 1995, 34, 69–73. [Google Scholar]
- Chua, W.; Charles, K.A.; Baracos, V.E.; Clarke, S.J. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Brit. J. Cancer 2011, 104, 1288–1295. [Google Scholar]
- Rao, H.L.; Chem, J.W.; Fu, X.J.; Zeng, Y.X.; Cai, M.Y.; Xie, D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One 2012, 7, e30806. [Google Scholar]
- Stratton, J.; Swanson, P.E.; Upton, M.P. Ulcerative colitis pathology. Available online: http://emedicine.medscape.com/article/2005396-overview (accessed on 26 May 2011).
- Shang, K.; Bai, U.P.; Wang, C.; Wang, Z.; Yu, H.; Du, X.; Zhou, X.Y.; Chi, Y.U.; Mukaida, N.; Li, Y.Y. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One 2012, 7, e51848. [Google Scholar]
- Tabuchi, T.; Che, X.F.; Hiraishi, K.; Adachi, M.; Miyano, K.; Sumimoto, H.; Tabuchi, T.; Miyazawa, K.; Tomoda, A. Selectively induced apoptosis in human neutrophils in the presence of oxidative phenoxazines, 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one and 2-aminophenoxazine-3-one, preceded by decrease of intracellular pH, depolarization of the mitochondria, and inhibition of superoxide generation. J. Pharmacol. Sci 2011, 117, 139–148. [Google Scholar]
- Kohno, K.; Miyake, M.; Sano, O.; Tanaka-Kataoka, M.; Yamamoto, S.; Koya-Miyata, S.; Arai, N.; Fujii, M.; Watanabe, H.; Ushio, S.; et al. Anti-inflammatory and immunomodulatory properties of 2-amino-3-phenoxazine-3-one. Biol. Pharm. Bull 2008, 31, 1938–1943. [Google Scholar]
- Bonin, S.; Schwarz, R.E.; Blanke, C.D. Gastric Cancer. In Cancer Management: A Multidisciplinary Approach; Pazdru, R., Coia, L.R., Hoskins, W., Wagman, L.D., Eds.; PPR Inc.: Melville, NY, USA, 2002; pp. 235–247. [Google Scholar]
- Sepulveda, A.R. Helicobacter, inflammation, and gastric cancer. Curr. Pathobiol. Rep 2013, 1, 9–18. [Google Scholar]
- Crew, K.N.; Neugut, A.L. Epidemiology of gastric cancer. World J. Gastroenterol 2006, 12, 354–362. [Google Scholar]
- Saliz, L.B.; Cox, J.V.; Blanke, C.; Rosen, L.S.; Fehrenbachr, L.; Moore, M.J.; Maroun, J.A.; Ackland, S.P.; Locker, P.K.; Pirotta, N.; et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N. Engl. J. Med 2000, 343, 905–914. [Google Scholar]
- Smith, M.G.; Hold, G.L.; Tahara, E.; El-Omar, E.M. Cellular and molecular aspects of gastric cancer. World J. Gastroenterol 2006, 12, 2979–2990. [Google Scholar]
- Dulabh, K.; Monga, M.D.; O’Connell, M.J. Surgical adjuvant therapy for colorectal cancer: Current approaches and future directions. Anal. Surg. Oncol 2006, 13, 1021–1034. [Google Scholar]
- Osaki, M.; Tanabe, S.; Goto, A.; Hayashi, H.; Oshimura, M.; Ito, H. 5-Fluorouracil (5-FU) induced apoptosis in gastric cancer cell lines: Role of the p53 gene. Apoptosis 1997, 2, 221–226. [Google Scholar]
- Fukuda, G.; Yoshitake, N.; Khan, Z.A.; Kanazawa, M.; Notoya, Y.; Che, X.F.; Akiyama, S.; Tomoda, A.; Chakrabarti, S.; Odawara, M. 2-Aminophenoxazine-3-one attenuates glucose-induced augmentation of embryonic form of myosin heavy chain, endothelin-l and plasminogen activator inhibitor-1 in human umbilical vein endothelial cells. Biol. Pharm. Bull 2005, 28, 797–801. [Google Scholar]
- Izumi, H.; Torigoe, T.; Ishiguchi, H.; Urano, H.; Yoshida, Y.; Tanabe, M.; Ise, T.; Murakami, T.; Yoshida, T.; Nomoto, M.; et al. Cellular pH regulators: Potentially promising molecular targets for cancer therapy. Cancer Treat. Rev 2003, 29, 541–549. [Google Scholar]
- Yamagata, M.; Tannock, I.F. The chronic administration of drugs that inhibit the regulation of intracellular pH in vitro and anti-tumor effects. Brit. J. Cancer 1996, 73, 1328–1334. [Google Scholar]
- Wahl, M.L.; Owen, J.A.; Burd, R.; Herlands, R.A.; Nogami, S.S.; Rodeck, U.; Beerd, D.; Leeper, D.B.; Owen, C.S. Regulation of intracellular pH in human melanoma: Potential therapeutic implications. Mol. Cancer Ther 2002, 1, 617–628. [Google Scholar]
- Tse, C.M.; Levine, S.A.; Yun, C.H.C.; Brant, S.R.; Nath, S.; Pouyssegur, J.; Donowitz, M. Molecular properties, kinetics and regulation of mammalian Na+/H+ exchangers. Cell. Physiol. Biochem 1994, 4, 282–300. [Google Scholar]
- Putney, L.K.; Denker, S.P.; Barber, D.L. The changing face of the Na+/H+ exchanger, NHE1: Structure, regulation, and cellular actions. Ann. Rev. Pharmacol. Toxicol 2002, 42, 527–552. [Google Scholar]
- Rich, I.N.; Worthington-White, D.; Garden, O.A.; Musk, P. Apopotosis of leukemic cells accompanies reduction in intracellular pH after targeted inhibition of the Na+/H+ exchanger. Blood 2000, 95, 1427–1434. [Google Scholar]
- Reshkin, S.J.; Cardone, R.; Harguindey, S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat. Anti-Cancer Drug Discovery 2013, 8, 85–99. [Google Scholar]
- Che, X.F.; Akiyama, S.; Tomoda, A. Suppression of the proliferation of cancer cell lines, KB-3-1 and K562 cells preceded by a decrease in intracellular pH caused by phenoxazine derivative. Oncol. Rep 2008, 19, 1253–1258. [Google Scholar]
- Lagadic-Gossmann, D.; Huc, L.; Lecureur, V. Alteration of intracellular pH homeostasis in apoptosis: Origin and roles. Cell Death Differ 2004, 11, 953–961. [Google Scholar]
- Shrode, L.D.; Tapper, H.; Grinstein, S. Role of intracellular pH in proliferation, transformation and apoptosis. J. Bioenerg. Biomembr. 1979, 29, 293–299. [Google Scholar]
- Harguindey, S.; Orive, G.; Pedraz, J.L.; Paradioso, A.; Reshkins, S.J. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—One single nature. BBA-Rev. Cancer 2005, 1756, 1–24. [Google Scholar]
- Harguindey, S.; Arranz, H.; Wahl, M.L.; Orive, G.; Reshkin, S.J. Proton transport inhibitors as potentially selective anticancer drugs. Anticancer Res 2009, 29, 2127–2136. [Google Scholar]
- Matsuyama, S.; Reed, J. Mitochondria dependent apoptosis and cellular pH regulation. Cell Death Differ 2000, 7, 1155–1165. [Google Scholar]
- Matsuyama, S.; Liopis, J.; Deveraux, Q.L.; Tsien, R.Y.; Reed, J.C. Changes in intramitochondrial and cytosolic pH homeostasis in apoptosis: Early events that modulate caspase activation during apoptosis. Nat. Cell Biol 2000, 2, 315–325. [Google Scholar]
- Perez-Sala, D.; Collado-Escobar, D.; Mollinedo, F. Intracellular alkalinization suppresses lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J. Biol. Chem 1995, 270, 6235–6242. [Google Scholar]
- Hendrich, A.B.; Stanczak, K.; Komorowska, M.; Motohashi, N.; Kawase, M.; Kichalak, K. A study on the perturbation of model lipid membranes by phenoxazines. Bioorg. Med. Chem 2006, 14, 5948–5954. [Google Scholar]
- Azuine, M.A.; Tokuda, H.; Takayasu, J.; Enjyo, F.; Mukainaka, T.; Konoshima, T.; Nishino, H.; Kapadia, G.J. Cancer chemopreventive effect of phenothiazines and related tri-heterocyclic analogues in the 12-O-tetradecanoylphorbol-13-acetate promoted Epstein-Barr virus early antigen activation and the mouse skin two-stage carcinogenesis models. Pharmacol. Res 2004, 49, 161–169. [Google Scholar]
- Enoki, E.; Sada, K.; Qu, X.; Kyo, S.; Miah, S.M.S.; Hatani, T.; Tomoda, A.; Yamamura, H. The phenoxazine derivative Phx-1 suppresses IgE-mediated degranulation in rat basophilic leukemia RBL-2H3 cells. J. Pharm. Sci 2004, 94, 329–333. [Google Scholar]
- Hara, K.; Okamoto, M.; Aki, T.; Yagita, H.; Tanaka, H.; Mizukami, Y.; Nakamura, H.; Tomoda, A.; Hamasaki, N.; Kang, D. Synergistic enhancement of TRAIL- and tumor necrosis factor α-induced cell death by a phenoxazine derivative. Mol. Cancer Ther 2005, 4, 1121–1127. [Google Scholar]
- Thimmaiah, K.N.; Easton, J.B.; Germain, G.S.; Morton, C.L.; Kamath, S.; Buolamwinini, J.K.; Houghton, P.J. Identification of N10 substituted phenoxazines as potential and specific inhibitors of Akt signaling. J. Biol. Chem 2005, 280, 31924–31935. [Google Scholar]
- Fan, X.M.; Jiang, X.H.; Gu, G.; Ching, Y.P.; He, H.; Xia, H.H.X.; Lin, M.C.M.; Chan, A.O.O.; Yen, M.F.; Kung, H.F.; et al. Inhibition of Akt/PKB by a COX-2 inhibitor induces apoptosis in gastric cancer cells. Digestion 2006, 73, 75–82. [Google Scholar]
- Shimazaki, J.; Goo, Y.; Nishida, K.; Tabuchi, T.; Motohashi, G.; Ubukata, H.; Tabuchi, T. In patients with colorectal cancer, preoperative serum interleukin-6 level and granulocyte/lymphocyte ratio are clinically relevant biomarkers of long-term cancer progression. Oncology 2013, 84, 356–361. [Google Scholar]
- Rossi, F. The O2−-forming NADPH oxidase of the phagocytes: Nature, mechanism of activation and function. Biochim. Biophys. Acta 1986, 833, 65–69. [Google Scholar]
- Cross, A.R.; Jones, O.T.G. Enzymic mechanisms of superoxide production. Biochim. Biophys. Acta 1991, 1057, 281–298. [Google Scholar]
- Miyake, M.; Kohno, K.; Sano, O. Antiinflammatory agent comprising 2-aminophenol or derivative thereof as active ingredient. Eur. Patents Appl. WO 2008/047758, 24 April 2008. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tomoda, A.; Miyazawa, K.; Tabuchi, T. Prevention of Carcinogenesis and Development of Gastric and Colon Cancers by 2-Aminophenoxazine-3-one (Phx-3): Direct and Indirect Anti-Cancer Activity of Phx-3. Int. J. Mol. Sci. 2013, 14, 17573-17583. https://doi.org/10.3390/ijms140917573
Tomoda A, Miyazawa K, Tabuchi T. Prevention of Carcinogenesis and Development of Gastric and Colon Cancers by 2-Aminophenoxazine-3-one (Phx-3): Direct and Indirect Anti-Cancer Activity of Phx-3. International Journal of Molecular Sciences. 2013; 14(9):17573-17583. https://doi.org/10.3390/ijms140917573
Chicago/Turabian StyleTomoda, Akio, Keisuke Miyazawa, and Takafumi Tabuchi. 2013. "Prevention of Carcinogenesis and Development of Gastric and Colon Cancers by 2-Aminophenoxazine-3-one (Phx-3): Direct and Indirect Anti-Cancer Activity of Phx-3" International Journal of Molecular Sciences 14, no. 9: 17573-17583. https://doi.org/10.3390/ijms140917573
APA StyleTomoda, A., Miyazawa, K., & Tabuchi, T. (2013). Prevention of Carcinogenesis and Development of Gastric and Colon Cancers by 2-Aminophenoxazine-3-one (Phx-3): Direct and Indirect Anti-Cancer Activity of Phx-3. International Journal of Molecular Sciences, 14(9), 17573-17583. https://doi.org/10.3390/ijms140917573



