A Nitric Oxide-Responsive Quorum Sensing Circuit in Vibrio harveyi Regulates Flagella Production and Biofilm Formation
Abstract
:1. Introduction
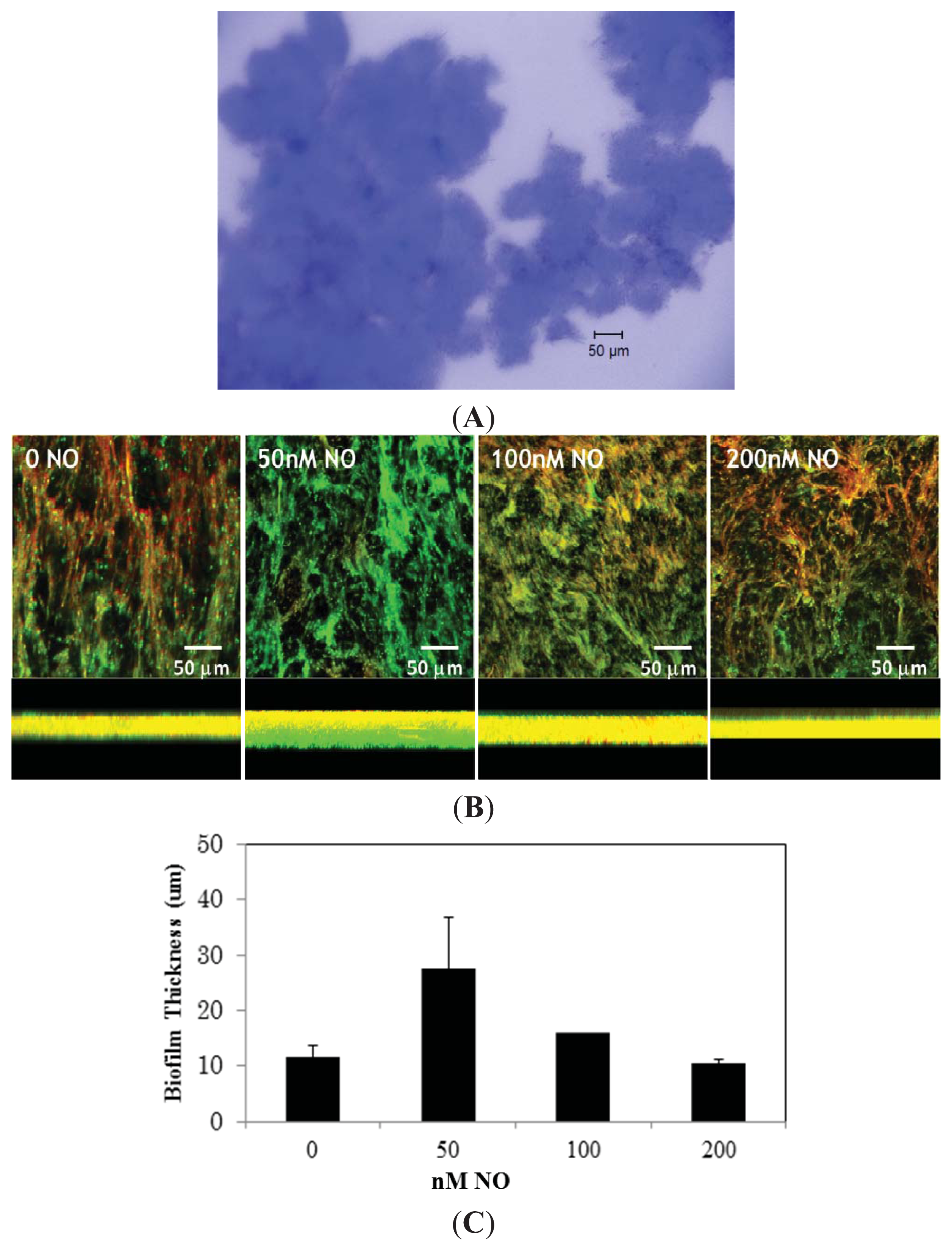
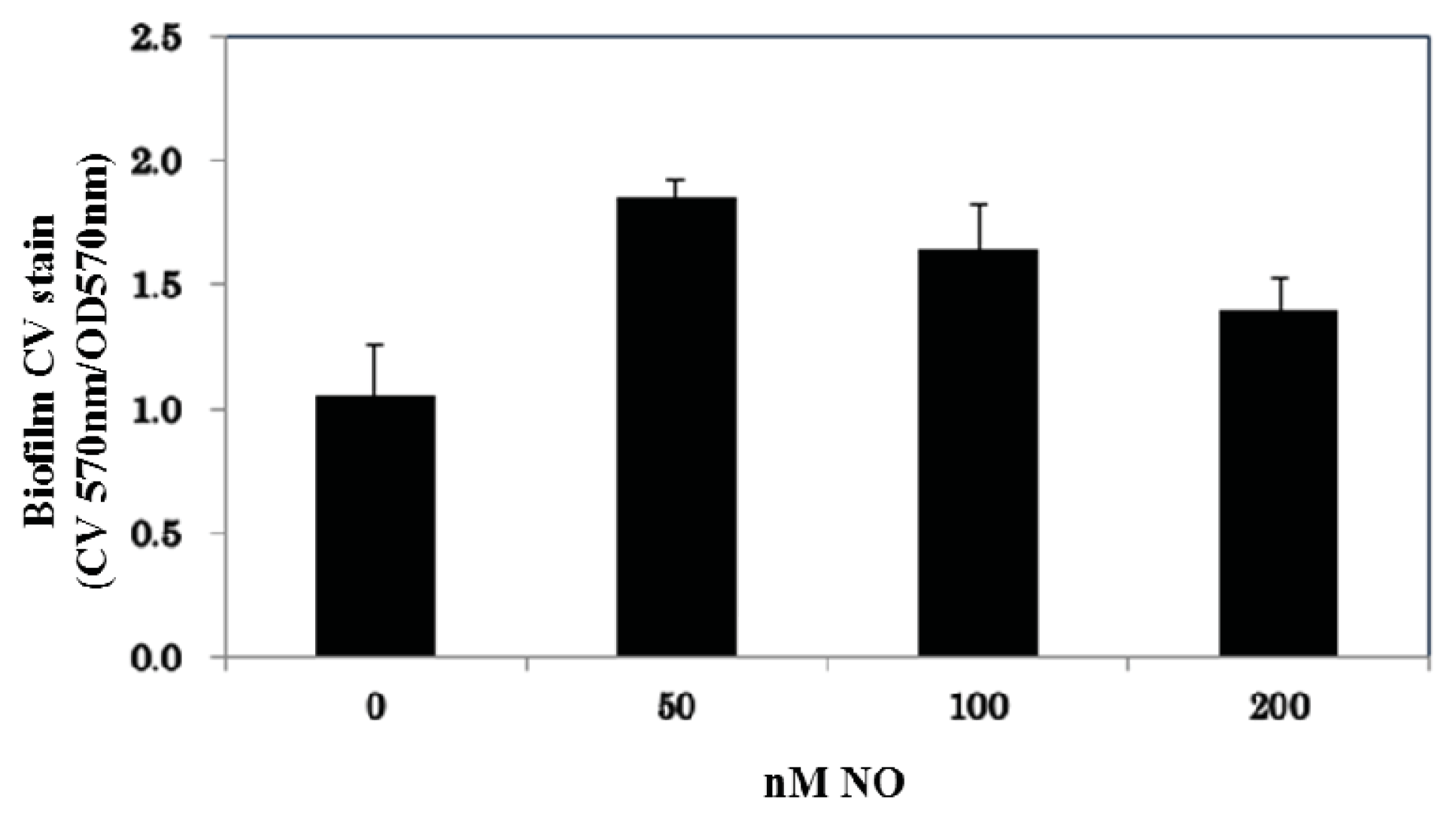
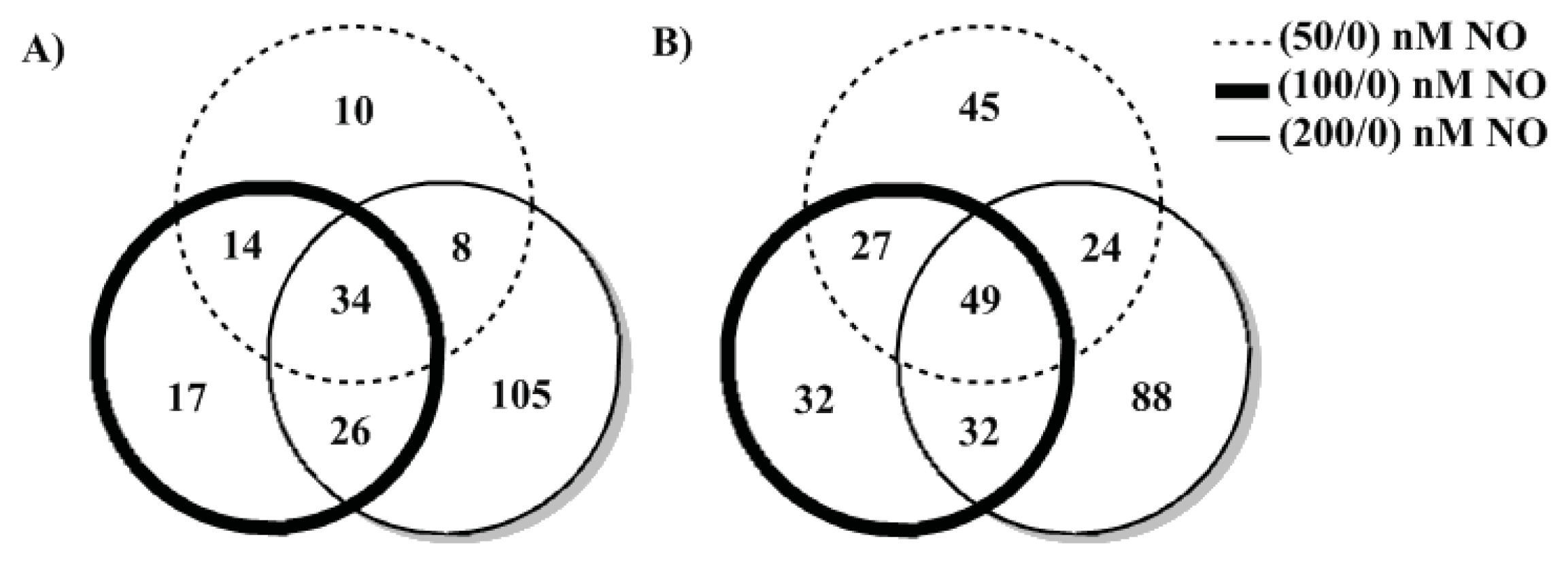
2. Results and Discussion
3. Experimental Section
3.1. Bacterial Strains and Growth Conditions
3.2. Biofilm Imaging by Confocal Microscopy
3.3. Crystal Violet Staining for Biofilm Quantification
3.4. iTRAQ™ Analysis of V. harveyi
4. Conclusions
Supplementary Information
ijms-14-16473-s001.pdfAcknowledgments
Conflict of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol 2004, 2, 95–108. [Google Scholar]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev 2002, 15, 167–193. [Google Scholar]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol 2001, 55, 165–199. [Google Scholar]
- Daniels, R.; Vanderleyden, J.; Michiels, J. Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev 2004, 28, 261–289. [Google Scholar]
- Bjarnsholt, T.; Jensen, P.Ø.; Jakobsen, T.H.; Phipps, R.; Nielsen, A.K.; Rybtke, M.T.; Tolker-Nielsen, T.; Givskov, M.; Høiby, N.; Ciofu, O.; et al. Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS One 2010, 5, e10115. [Google Scholar]
- Henke, J.M.; Bassler, B.L. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol 2004, 186, 3794–3805. [Google Scholar]
- Nealson, K.H.; Hastings, J.W. Bacterial bioluminescence: Its control and ecological significance. Microbiol. Rev 1979, 43, 496–518. [Google Scholar]
- Henares, B.M.; Higgins, K.E.; Boon, E.M. Discovery of a nitric oxide responsive quorum sensing circuit in Vibrio harveyi. ACS Chem. Biol 2012, 7, 1331–1336. [Google Scholar]
- Freeman, J.A.; Bassler, B.L. Sequence and function of LuxU: A two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol 1999, 181, 899–906. [Google Scholar]
- Freeman, J.A.; Bassler, B.L. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol 1999, 31, 665–677. [Google Scholar]
- McCarter, L.L. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J. Bacteriol 1998, 180, 3166–3173. [Google Scholar]
- Enos-Berlage, J.L.; McCarter, L.L. Relation of capsular polysaccharide production and colonial cell organization to colony morphology in Vibrio parahaemolyticus. J. Bacteriol 2000, 182, 5513–5520. [Google Scholar]
- Fidopiastis, P.M.; Miyamoto, C.M.; Jobling, M.G.; Meighen, E.A.; Ruby, E.G. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol 2002, 45, 131–143. [Google Scholar]
- Yildiz, F.H.; Liu, X.S.; Heydorn, A.; Schoolnik, G.K. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol 2004, 53, 497–515. [Google Scholar]
- Zhu, J.; Mekalanos, J.J. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 2003, 5, 647–656. [Google Scholar]
- Waters, C.M.; Lu, W.; Rabinowitz, J.D.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol 2008, 190, 2527–2536. [Google Scholar]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol 2000, 54, 49–79. [Google Scholar]
- Waters, C.M.; Bassler, B.L. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev 2006, 20, 2754–2767. [Google Scholar]
- Yildiz, F.H.; Visick, K.L. Vibrio biofilms: So much the same yet so different. Trends Microbiol 2009, 17, 109–118. [Google Scholar]
- Liu, N.; Xu, Y.; Hossain, S.; Huang, N.; Coursolle, D.; Gralnick, J.A.; Boon, E.M. Nitric oxide regulation of cyclic di-GMP synthesis and hydrolysis in Shewanella woodyi. Biochemistry 2012, 51, 2087–2099. [Google Scholar]
- Plate, L.; Marletta, M.A. Nitric oxide modulates bacterial biofilm formation through a multicomponent cyclic-di-GMP signaling network. Mol. Cell 2012, 46, 449–460. [Google Scholar]
- Carlson, H.K.; Vance, R.E.; Marletta, M.A. H-NOX regulation of c-di-GMP metabolism and biofilm formation in Legionella pneumophila. Mol. Microbiol 2010, 77, 930–942. [Google Scholar]
- Römling, U.; Amikam, D. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol 2006, 9, 218–228. [Google Scholar]
- Jenal, U. Cyclic di-guanosine-monophosphate comes of age: A novel secondary messenger involved in modulating cell surface structures in bacteria? Curr. Opin. Microbiol 2004, 7, 185–191. [Google Scholar]
- Barraud, N.; Hassett, D.J.; Hwang, S.-H.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol 2006, 188, 7344–7353. [Google Scholar]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol 2005, 21, 319–346. [Google Scholar]
- Schmidt, I.; Steenbakkers, P.J.M.; op den Camp, H.J.M.; Schmidt, K.; Jetten, M.S.M. Physiologic and proteomic evidence for a role of nitric oxide in biofilm formation by Nitrosomonas europaea and other ammonia oxidizers. J. Bacteriol 2004, 186, 2781–2788. [Google Scholar]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol 1998, 30, 295–304. [Google Scholar]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol 1998, 30, 285–293. [Google Scholar]
- Watnick, P.I.; Kolter, R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol 1999, 34, 586–595. [Google Scholar]
- Barak, R.; Eisenbach, M. Correlation between phosphorylation of the chemotaxis protein CheY and its activity at the flagellar motor. Biochemistry 1992, 31, 1821–1826. [Google Scholar]
- Clegg, D.O.; Koshland, D.E. The role of a signaling protein in bacterial sensing: Behavioral effects of increased gene expression. Proc. Natl. Acad. Sci. USA 1984, 81, 5056–5060. [Google Scholar]
- Ravid, S.; Matsumura, P.; Eisenbach, M. Restoration of flagellar clockwise rotation in bacterial envelopes by insertion of the chemotaxis protein CheY. Proc. Natl. Acad. Sci. USA 1986, 83, 7157–7161. [Google Scholar]
- Wolfe, A.J.; Conley, M.P.; Kramer, T.J.; Berg, H.C. Reconstitution of signaling in bacterial chemotaxis. J. Bacteriol 1987, 169, 1878–1885. [Google Scholar]
- Lupp, C.; Ruby, E.G. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol 2005, 187, 3620–3629. [Google Scholar]
- Bassler, B.L.; Wright, M.; Showalter, R.E.; Silverman, M.R. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol. Microbiol 1993, 9, 773–786. [Google Scholar]
- Boyd, C.D.; O’Toole, G.A. Second messenger regulation of biofilm formation: Breakthroughs in understanding c-di-GMP effector systems. Annu. Rev. Cell Dev. Biol 2012, 28, 439–462. [Google Scholar]
- Nadell, C.D.; Xavier, J.B.; Levin, S.A.; Foster, K.R. The evolution of quorum sensing in bacterial biofilms. PLoS Biol 2008, 6, e14. [Google Scholar]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol 2005, 13, 27–33. [Google Scholar]
- Coggan, K.A.; Wolfgang, M.C. Global regulatory pathways and cross-talk control Pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr. Issues Mol. Biol 2012, 14, 47–70. [Google Scholar]
- Williams, P.; Camara, M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol 2009, 12, 182–191. [Google Scholar]

| Gene ID | Protein ID | 50 nM NO fold change a | 100 nM NO fold change a | 200 nM NO fold change a | Annotation |
|---|---|---|---|---|---|
| Vibhar_01300 | A7MT73 | 0.364 | 0.625 | 0.813 | Flagellin |
| Vibhar_01301 | A7MT74 | 0.421 | 0.637 | 1.012 | Flagellin |
| Vibhar_03171 | A7MS06 | 0.443 | 0.673 | 0.955 | Flagellin |
| Vibhar_03173 | A7MS08 | 0.425 | 0.705 | 1.000 | Flagellin |
| Vibhar_03174 | A7MS09 | 0.553 | 0.856 | 0.925 | Flagellin |
| Vibhar_03143 | A7MS30 | 1.052 | 0.953 | 0.455 | CheY |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Henares, B.M.; Xu, Y.; Boon, E.M. A Nitric Oxide-Responsive Quorum Sensing Circuit in Vibrio harveyi Regulates Flagella Production and Biofilm Formation. Int. J. Mol. Sci. 2013, 14, 16473-16484. https://doi.org/10.3390/ijms140816473
Henares BM, Xu Y, Boon EM. A Nitric Oxide-Responsive Quorum Sensing Circuit in Vibrio harveyi Regulates Flagella Production and Biofilm Formation. International Journal of Molecular Sciences. 2013; 14(8):16473-16484. https://doi.org/10.3390/ijms140816473
Chicago/Turabian StyleHenares, Bernadette M., Yueming Xu, and Elizabeth M. Boon. 2013. "A Nitric Oxide-Responsive Quorum Sensing Circuit in Vibrio harveyi Regulates Flagella Production and Biofilm Formation" International Journal of Molecular Sciences 14, no. 8: 16473-16484. https://doi.org/10.3390/ijms140816473




