Hydrogenation of the Exocyclic Olefinic Bond at C-16/C-17 Position of ent-Kaurane Diterpene Glycosides of Stevia rebaudiana Using Various Catalysts
Abstract
:1. Introduction
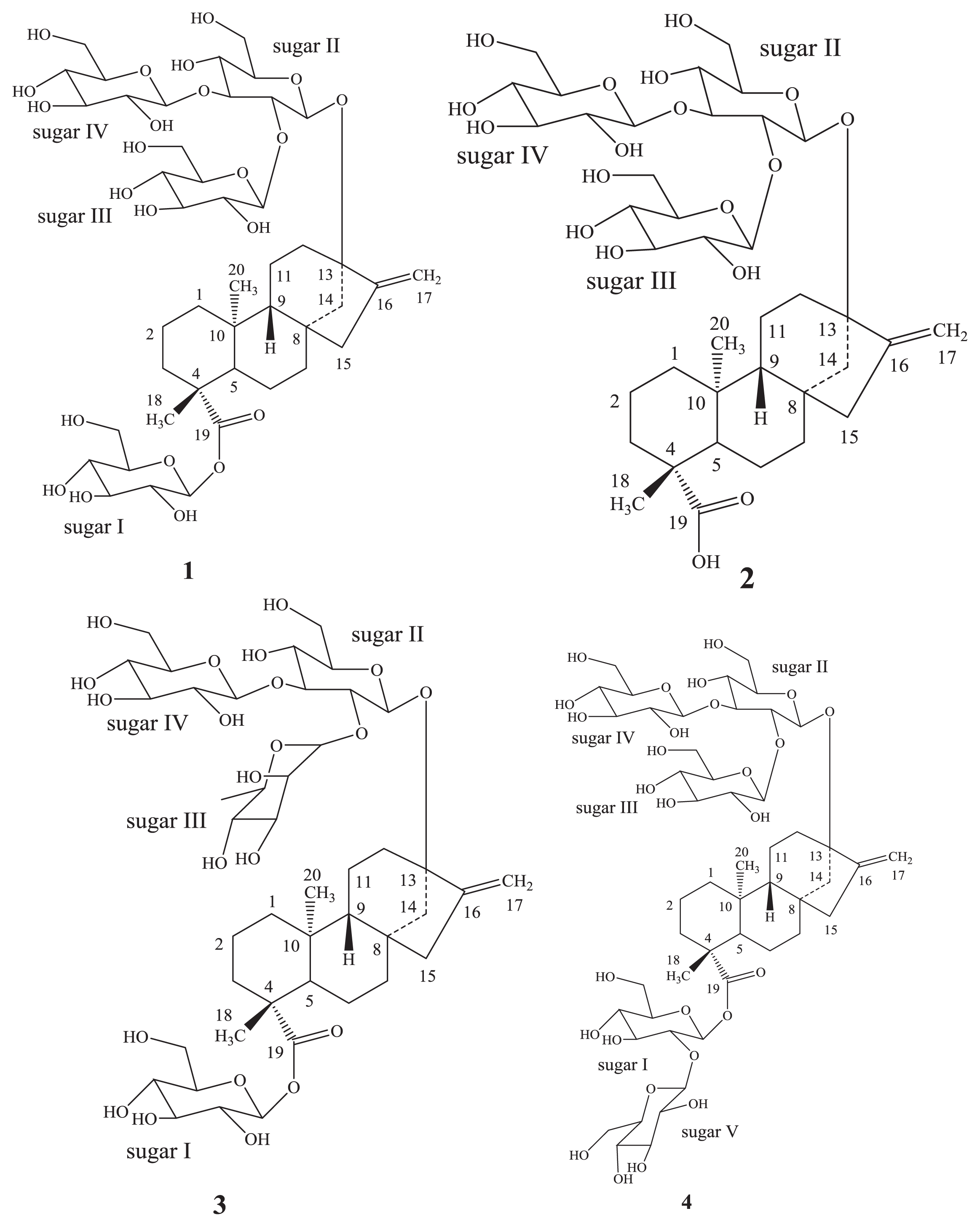
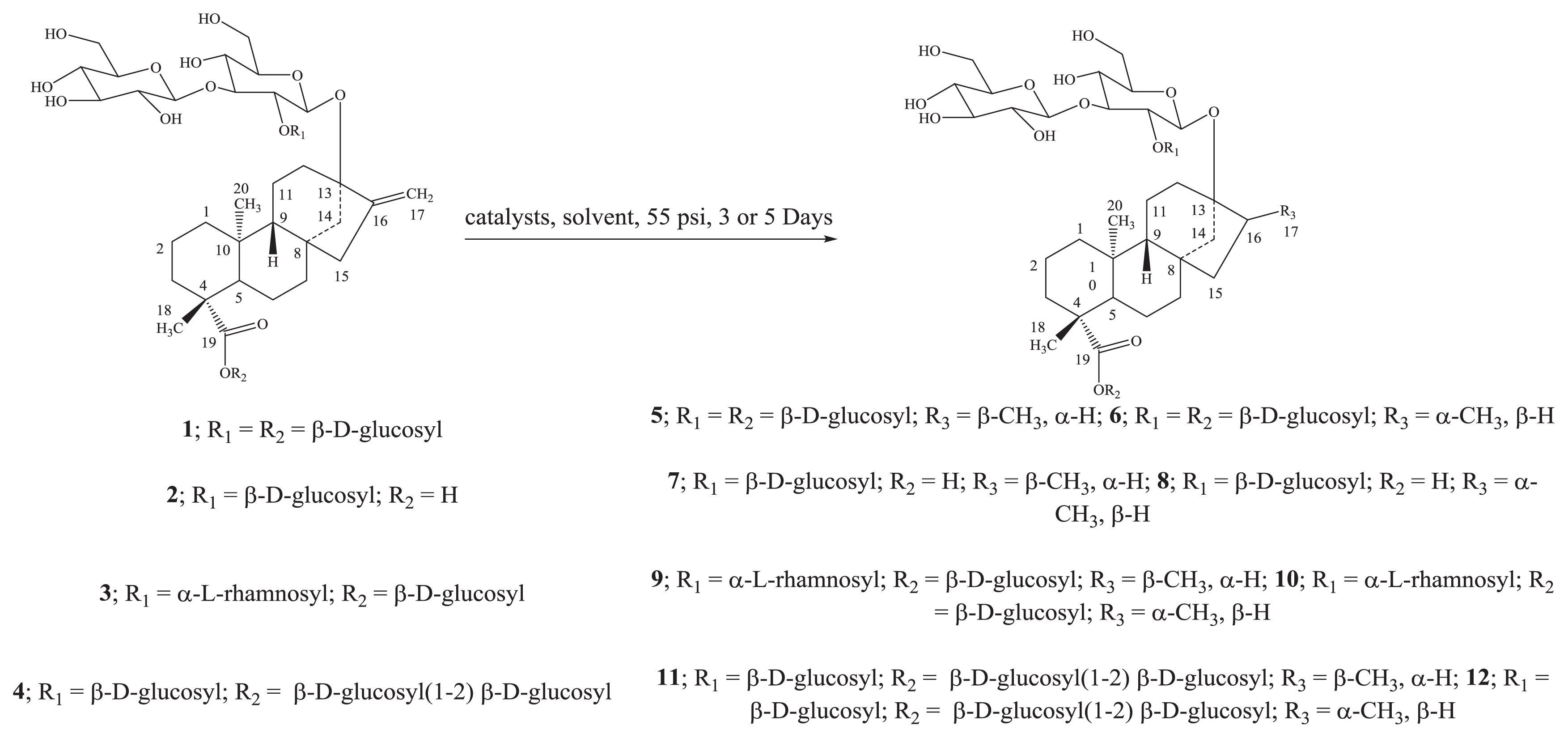
2. Results and Discussion
2.1. Chemistry
2.2. Spectroscopy
3. Experimental Section
3.1. General
3.2. Isolation of Reduced Steviol Glycosides 5–12
3.2.1. General Procedure for the Catalytic Hydrogenation of Steviol Glycosides 1–4 with Pd(OH)2
3.2.2. General Procedure for the Catalytic Hydrogenation of Steviol Glycosides 1–4 with Pt/Charcoal
3.2.3. General Procedure for the Catalytic Hydrogenation of Steviol Glycosides 1–4 with Rh/Charcoal
3.2.4. General Procedure for the Catalytic Hydrogenation of Steviol Glycosides 1–4 with Raney Ni
3.2.5. General Procedure for the Catalytic Hydrogenation of Steviol Glycosides 1–4 with 5% Pd/BaCO3
3.2.6. General Procedure for the Catalytic Hydrogenation of Steviol Glycosides 1–4 with PtO2
3.2.7. Dihydrorebaudiososide A1/Dihydrorebaudiososide A2 (5/6)
3.2.8. Dihydrorebaudioside B1/Dihydrorebaudioside B2 (7/8)
3.2.9. Dihydrorebaudioside C1/Dihydrorebaudioside C2 (9/10)
3.2.10. Dihydrorebaudioside D1/Dihydrorebaudioside D2 (11/12)
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Brandle, J.E.; Starrratt, A.N.; Gijen, M. Stevia rebaudiana: Its agricultural, biological and chemical properties. Can. J. Plant Sci 1998, 78, 527–536. [Google Scholar]
- Chaturvedula, V.S.P.; Rhea, J.; Milanowski, D.; Mocek, U.; Prakash, I. Two minor diterpene glycosides from the leaves of Stevia rebaudiana. Nat. Prod. Commun 2011, 6, 175–178. [Google Scholar]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Diterpene glycosides from Stevia rebaudiana. Molecules 2011, 16, 3552–3562. [Google Scholar]
- Chaturvedula, V.S.P.; Prakash, I. A new diterpenoid glycoside from Stevia rebaudiana. Molecules 2011, 16, 2937–2943. [Google Scholar]
- Chaturvedula, V.S.P.; Clos, J.F.; Rhea, J.; Milanowski, D.; Mocek, U.; DuBois, G.E.; Prakash, I. Minor diterpenoid glycosides from the leaves of Stevia rebaudiana. Phytochem. Lett 2011, 4, 209–212. [Google Scholar]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Structures of the novel α-glucosyl linked diterpene glycosides from Stevia rebaudiana. Carbohydr. Res 2011, 346, 2034–2038. [Google Scholar]
- Chaturvedula, V.S.P.; Klucik, J.; Mani, U.; Prakash, I. Synthesis of ent-kaurane diterpene glycosides. Molecules 2011, 16, 8402–8409. [Google Scholar]
- Prakash, I.; Clos, J.F.; Chaturvedula, V.S.P. Stability of rebaudioside A under acidic conditions and its degradation products. Food Res. Intl 2012, 48, 65–75. [Google Scholar]
- Chaturvedula, V.S.P.; Campbell, M.; Miguel, R.I.S.; Prakash, I. Synthesis and sensory evaluation of ent-kaurane diterpene glycosides. Molecules 2012, 17, 8908–8916. [Google Scholar]
- Chaturvedula, V.S.P.; Campbell, M.; Prakash, I. Catalytic hydrogenation of the sweet principles of Stevia rebaudiana, rebaudioside B, rebaudioside C, and rebaudioside D and sensory evaluation of their reduced derivatives. Int. J. Mol. Sci 2012, 13, 15126–15136. [Google Scholar]
- Kasai, R.; Kaneda, N.; Tanaka, O.; Yamasaki, K.; Sakamoto, I.; Morimoto, K.; Okada, S.; Kitahata, S.; Furukawa, H. Sweet diterpene glycosides of leaves of Stevia rebaudiana Bertoni: Synthesis and structure-sweetness relation of rebaudiosides A, D, and E and their related glycosides. Nippon Kagaku Kaishi 1981, 5, 726–735. [Google Scholar]
- Nanayakkara, N.P.D.; Klocke, J.A.; Compadre, C.M.; Hussain, R.A.; Pezzuto, J.M.; Kinghorn, A.D. Characteriztaion and feeding deterrent effects on the aphid, Schizaphis graminum, of some derivatives of the sweet compounds, stevioside and rebaudioside A. J. Nat. Prod 1987, 50, 434–441. [Google Scholar]
- Pezzuto, J.M.; Compadre, C.M.; Swanson, S.M.; Nanayakkara, N.P.D.; Kinghorn, A.D. Metabolically activated steviol, the aglycone of stevioside, is mutagenic. Proc. Natl. Acad. Sci. USA 1985, 82, 2478–2482. [Google Scholar]
| S. No. | Solvent | Conditions | Duration | Yield |
|---|---|---|---|---|
| Method 1 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 72 h | 72.2%–77.5% |
| Method 2 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.5% |
| Method 3 | H2O | Temp.: 25 °C; Pressure: 55 psi | 72 h | 78.0%–83.0% |
| Method 4 | H2O | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.8% |
| Method 5 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 72 h | 69.0%–72.5% |
| Method 6 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.2%–99.5% |
| S. No. | Solvent | Conditions | Duration | Yield |
|---|---|---|---|---|
| Method 1 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 72 h | 64.0%–69.5% |
| Method 2 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.5% |
| Method 3 | H2O | Temp.: 25 °C; Pressure: 55 psi | 72 h | 72.2%–78.0% |
| Method 4 | H2O | Temp.: 25 °C; Pressure: 55 psi | 120 h | 98.8%–99.5% |
| Method 5 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 72 h | 66.2%–70.5% |
| Method 6 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.5% |
| S. No. | Solvent | Conditions | Duration | Yield |
|---|---|---|---|---|
| Method 1 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 72 h | 62.0%–67.5% |
| Method 2 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.5% |
| Method 3 | H2O | Temp.: 25 °C; Pressure: 55 psi | 72 h | 78.2%–81.0% |
| Method 4 | H2O | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.5%–99.8% |
| Method 5 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 72 h | 68.4%–73.2% |
| Method 6 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 120 h | 96.5%–97.2% |
| S. No. | Solvent | Conditions | Duration | Yield |
|---|---|---|---|---|
| Method 1 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 72 h | 57.0%–61.2% |
| Method 2 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.5% |
| Method 3 | H2O | Temp.: 25 °C; Pressure: 55 psi | 72 h | 76.5%–81.2% |
| Method 4 | H2O | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.5%–99.8% |
| Method 5 | EtOH-H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 72 h | 68.5%–73.5% |
| Method 6 | EtOH-H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 120 h | 97.5%–98.4% |
| S. No. | Solvent | Conditions | Duration | Yield (17β/17α) |
|---|---|---|---|---|
| Method 1 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 72 h | 55.2%–59.5% |
| Method 2 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.7% |
| Method 3 | H2O | Temp.: 25 °C; Pressure: 55 psi | 72 h | 78.3%–81.5% |
| Method 4 | H2O | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.8% |
| Method 5 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 72 h | 67.5%–73.0% |
| Method 6 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.3%–99.7% |
| S. No. | Solvent | Conditions | Duration | Yield (17β/17α) |
|---|---|---|---|---|
| Method 1 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 72 h | 62.0%–67.5% |
| Method 2 | MeOH | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.0%–99.5% |
| Method 3 | H2O | Temp.: 25 °C; Pressure: 55 psi | 72 h | 81.2%–84.4% |
| Method 4 | H2O | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.3%–99.8% |
| Method 5 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 72 h | 65.4%–73.5% |
| Method 6 | EtOH/H2O (8:2) | Temp.: 25 °C; Pressure: 55 psi | 120 h | 99.4%–99.9% |
| Position | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|
| 17 | 1.10 (d, 6.4, 1H) | 1.16 (d, 6.4, 1H) | 1.15 (d, 6.6, 1H) | 1.32 (d, 6.4, 1H) | 1.18 (d, 6.5, 1H) | 1.37 (d, 6.4, 1H) | 1.14 (d, 6.6, 1H) | 1.16 (d, 6.4, 1H) |
| 18 | 1.26 (s, 3H) | 1.25 (s, 3H) | 1.15 (s, 3H) | 1.17 (s, 3H) | 1.24 (s, 3H) | 1.27 (s, 3H) | 1.14 (s, 3H) | 1.15 (s, 3H) |
| 20 | 1.31 (s, 3H) | 1.32 (s, 3H) | 1.17 (s, 3H) | 1.34 (s, 3H) | 1.29 (s, 3H) | 1.28 (s, 3H) | 1.41 (s, 3H) | 1.42 (s, 3H) |
| Sugar I-1′ | 6.16 (d, 6.8, 1H) | 6.16 (d, 6.6, 1H) | 6.13 (d, 6.8, 1H) | 6.14 (d, 6.5, 1H) | 6.86 (d, 6.4, 1H) | 6.84 (d, 6.5, 1H) | ||
| Sugar II-1″ | 5.02 (d, 6.7, 1H) | 5.01 (d, 6.7, 1H) | 5.04 (d, 6.6, 1H) | 5.01 (d, 6.4, 1H) | 5.10 (d, 6.7, 1H) | 5.07 (d, 6.4, 1H) | 5.50 (d, 6.6, 1H) | 5.53 (d, 6.4, 1H) |
| Sugar III-1‴ | 5.36 (d, 6.7, 1H) | 5.34 (d, 6.4, 1H) | 5.33 (d, 6.4, 1H) | 5.34 (d, 6.3, 1H) | 5.92 (d, 6.4, 1H) | 5.75 (d, 6.8, 1H) | 5.52 (d, 6.6, 1H) | 5.56 (d, 6.6, 1H) |
| Sugar IV-1‴′ | 5.51 (d, 6.4, 1H) | 5.42 (d, 6.8, 1H) | 5.47 (d, 6.1, 1H) | 5.51 (d, 6.4, 1H) | 6.51 (d, 1.8, 1H) | 6.84 (d, 1.6, 1H) | 5.36 (d, 6.4, 1H) | 5.41 (d, 6.6, 1H) |
| Sugar V-1‴″ | 1.64 (d, 6.1, 3H) | 1.72 (d, 6.4, 3H) | 6.31 (d, 6.4, 1H) | 6.33 (d, 6.2, 1H) |
| Position | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|
| 1 | 41.1 | 41.3 | 40.3 | 40.2 | 41.4 | 41.3 | 41.2 | 41.2 |
| 2 | 20.2 | 20.2 | 20.3 | 20.5 | 20.3 | 20.2 | 20.4 | 20.3 |
| 3 | 38.8 | 38.7 | 38.7 | 38.7 | 38.8 | 38.8 | 38.7 | 38.6 |
| 4 | 44.5 | 43.1 | 44.3 | 43.2 | 44.4 | 43.4 | 44.4 | 42.9 |
| 5 | 57.7 | 57.7 | 57.6 | 55.8 | 58.0 | 58.1 | 58.1 | 58.0 |
| 6 | 22.9 | 23.0 | 23.1 | 23.4 | 22.8 | 23.1 | 23.0 | 23.0 |
| 7 | 41.6 | 40.3 | 41.6 | 40.4 | 41.6 | 40.2 | 41.4 | 40.2 |
| 8 | 44.5 | 43.2 | 44.3 | 43.2 | 44.4 | 43.1 | 43.5 | 42.5 |
| 9 | 55.7 | 54.8 | 55.8 | 50.6 | 56.4 | 54.6 | 55.4 | 55.1 |
| 10 | 40.2 | 40.3 | 40.2 | 40.3 | 40.3 | 40.3 | 40.1 | 40.3 |
| 11 | 20.3 | 20.7 | 20.5 | 20.8 | 20.4 | 20.7 | 20.6 | 20.7 |
| 12 | 35.4 | 44.4 | 35.5 | 44.0 | 35.4 | 44.2 | 35.9 | 44.0 |
| 13 | 88.3 | 88.2 | 88.6 | 88.2 | 86.2 | 86.2 | 88.3 | 88.1 |
| 14 | 47.3 | 50.5 | 47.6 | 50.6 | 47.1 | 50.3 | 47.5 | 51.1 |
| 15 | 47.2 | 44.6 | 47.7 | 44.2 | 47.1 | 44.9 | 47.6 | 44.8 |
| 16 | 41.2 | 38.9 | 41.5 | 38.7 | 41.2 | 39.0 | 41.1 | 38.9 |
| 17 | 14.5 | 19.7 | 16.5 | 16.4 | 14.2 | 19.7 | 14.4 | 17.2 |
| 18 | 28.6 | 28.6 | 29.7 | 29.8 | 28.6 | 28.6 | 29.4 | 29.8 |
| 19 | 177.4 | 177.7 | 180.5 | 180.3 | 177.8 | 177.6 | 176.5 | 176.3 |
| 20 | 15.6 | 15.8 | 15.8 | 16.2 | 15.7 | 16.1 | 15.6 | 16.0 |
| Sugar I | ||||||||
| 1′ | 96.3 | 96.2 | 96.2 | 95.9 | 96.2 | 96.3 | ||
| 2′ | 75.8 | 75.4 | 75.4 | 75.3 | 81.6 | 81.2 | ||
| 3′ | 79.6 | 79.8 | 79.0 | 79.3 | 78.9 | 78.7 | ||
| 4′ | 71.6 | 71.4 | 71.2 | 71.4 | 71.6 | 71.4 | ||
| 5′ | 78.6 | 78.8 | 78.5 | 78.6 | 78.5 | 78.6 | ||
| 6′ | 63.1 | 63.2 | 62.5 | 62.5 | 63.1 | 63.2 | ||
| Sugar II | ||||||||
| 1″ | 99.1 | 98.8 | 98.7 | 98.9 | 98.4 | 96.8 | 94.2 | 94.3 |
| 2″ | 78.4 | 78.5 | 78.8 | 78.6 | 78.4 | 78.6 | 79.1 | 79.2 |
| 3″ | 86.5 | 85.6 | 85.6 | 86.6 | 87.3 | 86.2 | 86.1 | 86.7 |
| 4″ | 71.9 | 72.1 | 72.1 | 72.2 | 70.6 | 70.6 | 71.2 | 71.1 |
| 5″ | 77.3 | 77.0 | 77.0 | 77.0 | 75.6 | 75.4 | 77.2 | 77.1 |
| 6″ | 62.7 | 62.9 | 62.9 | 62.8 | 62.7 | 62.7 | 62.8 | 62.9 |
| Sugar III | ||||||||
| 1‴ | 105.2 | 105.5 | 105.5 | 105.0 | 103.2 | 102.2 | 105.2 | 104.9 |
| 2‴ | 74.6 | 74.7 | 74.6 | 74.6 | 71.9 | 71.6 | 75.9 | 75.9 |
| 3‴ | 77.5 | 77.9 | 77.8 | 77.8 | 72.9 | 72.9 | 78.7 | 78.6 |
| 4‴ | 72.1 | 72.0 | 72.1 | 72.3 | 73.0 | 73.2 | 72.4 | 72.2 |
| 5‴ | 78.9 | 79.1 | 79.0 | 79.3 | 70.3 | 70.2 | 79.0 | 79.3 |
| 6‴ | 62.6 | 62.5 | 62.9 | 62.9 | 19.6 | 19.3 | 62.6 | 62.8 |
| Sugar IV | ||||||||
| 1‴′ | 105.4 | 105.9 | 105.6 | 105.5 | 105.2 | 104.8 | 105.2 | 105.7 |
| 2‴′ | 74.3 | 74.6 | 75.6 | 75.7 | 74.8 | 74.7 | 74.3 | 74.4 |
| 3‴′ | 79.9 | 79.9 | 81.8 | 81.2 | 79.8 | 79.9 | 79.8 | 79.7 |
| 4‴′ | 72.2 | 72.3 | 72.0 | 72.1 | 72.4 | 72.1 | 72.2 | 72.3 |
| 5‴′ | 79.8 | 79.7 | 79.1 | 79.2 | 79.0 | 78.7 | 79.6 | 79.8 |
| 6‴′ | 63.2 | 63.0 | 63.3 | 63.3 | 63.4 | 63.1 | 63.2 | 63.3 |
| Sugar V | ||||||||
| 1‴″ | 106.1 | 105.6 | ||||||
| 2‴″ | 76.8 | 76.8 | ||||||
| 3‴″ | 78.9 | 78.9 | ||||||
| 4‴″ | 72.3 | 72.2 | ||||||
| 5‴″ | 81.8 | 81.3 | ||||||
| 6‴″ | 63.5 | 63.5 | ||||||
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chaturvedula, V.S.P.; Prakash, I. Hydrogenation of the Exocyclic Olefinic Bond at C-16/C-17 Position of ent-Kaurane Diterpene Glycosides of Stevia rebaudiana Using Various Catalysts. Int. J. Mol. Sci. 2013, 14, 15669-15680. https://doi.org/10.3390/ijms140815669
Chaturvedula VSP, Prakash I. Hydrogenation of the Exocyclic Olefinic Bond at C-16/C-17 Position of ent-Kaurane Diterpene Glycosides of Stevia rebaudiana Using Various Catalysts. International Journal of Molecular Sciences. 2013; 14(8):15669-15680. https://doi.org/10.3390/ijms140815669
Chicago/Turabian StyleChaturvedula, Venkata Sai Prakash, and Indra Prakash. 2013. "Hydrogenation of the Exocyclic Olefinic Bond at C-16/C-17 Position of ent-Kaurane Diterpene Glycosides of Stevia rebaudiana Using Various Catalysts" International Journal of Molecular Sciences 14, no. 8: 15669-15680. https://doi.org/10.3390/ijms140815669
APA StyleChaturvedula, V. S. P., & Prakash, I. (2013). Hydrogenation of the Exocyclic Olefinic Bond at C-16/C-17 Position of ent-Kaurane Diterpene Glycosides of Stevia rebaudiana Using Various Catalysts. International Journal of Molecular Sciences, 14(8), 15669-15680. https://doi.org/10.3390/ijms140815669




