Significant Decline in Galactomannan Signal during Storage of Clinical Serum Samples
Abstract
:1. Introduction
2. Results and Discussion
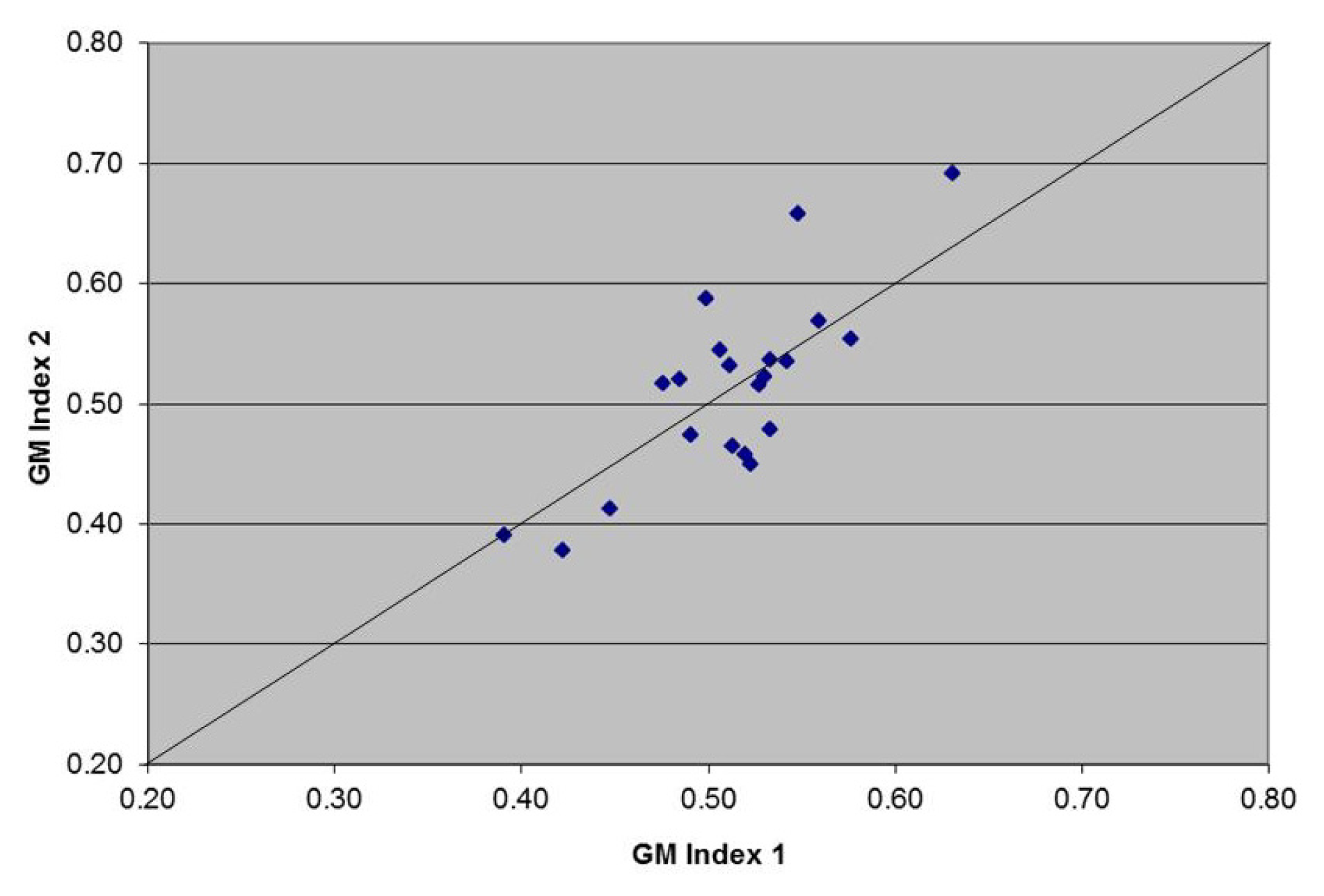
2.1. Sample Results (Scatter Plot)
2.1.1. Hypothesis Testing
2.1.2. Assay Repeatability
2.2. Discussion
3. Experimental Section
3.1. Clinical Samples
3.2. Analysis of Clinical Data
3.3. Assay Repeatability
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Kuderer, N.M.; Dale, D.C.; Crawford, J.; Cosler, L.E.; Lyman, G.H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006, 106, 2258–2266. [Google Scholar]
- Wheat, L.J.; Walsh, T.J. Diagnosis of invasive aspergillosis by galactomannan antigenemia detection using an enzyme immunoassay. Eur. J. Clin. Microbiol. Infect. Dis 2008, 27, 245–251. [Google Scholar]
- Pereira, C.N.; del Nero, G.; Lacaz, C.S.; Machado, C.M. The contribution of galactomannan detection in the diagnosis of invasive aspergillosis in bone marrow transplant recipients. Mycopathologia 2005, 159, 487–493. [Google Scholar]
- Upton, A.; Gugel, A.; Leisenring, W.; Limaye, A.; Alexander, Barbara; Hayden, R.; Marr, K.A. Reproducibility of low galactomannan enzyme immunoassay index values tested in multiple laboratories. J. Clin. Microbiol. 2005, 43, 4796–4800. [Google Scholar]
- Oren, I.; Avidor, I.; Sprecher, H. Lack of intra-laboratory reproducibility in using Platelia Aspergillus enzyme immunoassay test for detection of Aspergillus galactomannan antigen. Transplant Infect Dis 2012, 14, 107–109. [Google Scholar]
- Bizzini, A.; Marchetti, O.; Meylan, P. Response to: Lack of intra-laboratory reproducibility in using Platelia Aspergillus enzyme immunoassay test for detection of Aspergillus galactomannan antigen. Transplant Infect. Dis 2012, 14, 218–219. [Google Scholar]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the european organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (eortc/msg) consensus group. Clin. Infect. Dis 2008, 46, 1813–1821. [Google Scholar]
- Tsitsikas, D.A.; Morin, A.; Araf, S.; Murtagh, B.; Johnson, G.; Vinnicombe, S.; Ellis, S.; Suaris, T.; Wilks, M.; Doffman, S.; et al. Impact of the revised. EORTC/MSG definitions for invasive fungal disease on the rates of diagnosis of invasive aspergillosis. Med. Mycol. [CrossRef]
- Furfaro, E.; Mikulska, M.; Miletich, F.; Viscoli, C. Galactomannan: Testing the same sample twice? Transpl. Infect. Dis 2012, 14, E38–E39. [Google Scholar]
- Jones, R.; Payne, B. Clinical investigation and statistics in laboratory medicine. Am. Assoc. Clin. Chem 1997, 90, 41–42. [Google Scholar]
- Agrawal, S.; Hope, W.; Sinkó, J.; Kibbler, C. Optimizing management of invasive mould diseases. J. Antimicrob. Chemother 2011, 66, i45–i53. [Google Scholar]
- Johnson, G.L.; Bibby, D.F.; Wong, S.; Agrawal, S.G.; Bustin, S.A. A MIQE-compliant real-time PCR assay for Aspergillus detection. PLoS One 2012, 7, e40022. [Google Scholar]

| Sample size | Samples with GM reduction | Median GM1 index | Median GM2 index | p-value | |
|---|---|---|---|---|---|
| Short-Term Serum | 65 | 44 | 0.65 | 0.19 | <0.001 |
| Long-Term Serum | 35 | 31 | 0.56 | 0.10 | <0.001 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Johnson, G.L.; Sarker, S.-J.; Hill, K.; Tsitsikas, D.A.; Morin, A.; Bustin, S.A.; Agrawal, S.G. Significant Decline in Galactomannan Signal during Storage of Clinical Serum Samples. Int. J. Mol. Sci. 2013, 14, 12970-12977. https://doi.org/10.3390/ijms140712970
Johnson GL, Sarker S-J, Hill K, Tsitsikas DA, Morin A, Bustin SA, Agrawal SG. Significant Decline in Galactomannan Signal during Storage of Clinical Serum Samples. International Journal of Molecular Sciences. 2013; 14(7):12970-12977. https://doi.org/10.3390/ijms140712970
Chicago/Turabian StyleJohnson, Gemma L., Shah-Jalal Sarker, Kate Hill, Dimitris A. Tsitsikas, Amelie Morin, Stephen A. Bustin, and Samir G. Agrawal. 2013. "Significant Decline in Galactomannan Signal during Storage of Clinical Serum Samples" International Journal of Molecular Sciences 14, no. 7: 12970-12977. https://doi.org/10.3390/ijms140712970
APA StyleJohnson, G. L., Sarker, S.-J., Hill, K., Tsitsikas, D. A., Morin, A., Bustin, S. A., & Agrawal, S. G. (2013). Significant Decline in Galactomannan Signal during Storage of Clinical Serum Samples. International Journal of Molecular Sciences, 14(7), 12970-12977. https://doi.org/10.3390/ijms140712970





