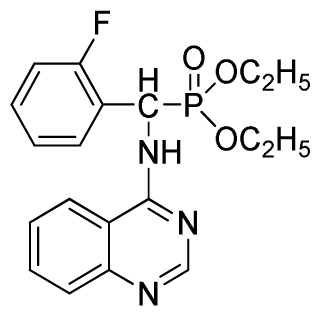
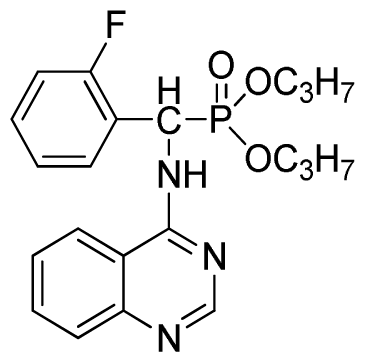
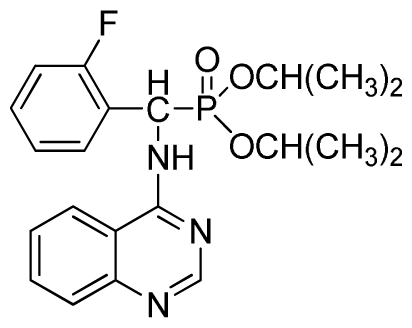
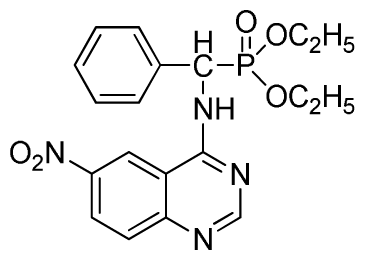
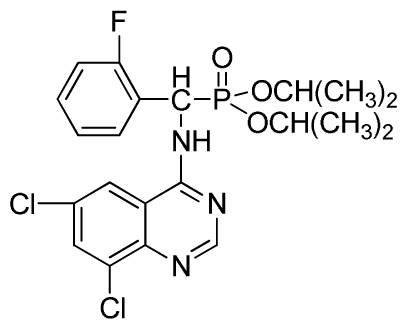
Abstract
This study describes the simple synthesis of new (quinazolin-4-ylamino) methylphosphonates via microwave irradiation. Substituted-2-aminobenzonitrile reacted with 1,1-dimethoxy-N,N-dimethylmethanamine at a reflux condition to obtain N′-(substituted-2-cyanophenyl)-N,N-dimethylformamidine (1). The subsequent reaction of this intermediate product with α-aminophosphonate (2) in a solution containing glacial acetic acid in 2-propanol through microwave irradiation resulted in the formation of (quinazolin-4-ylamino)methyl-phosphonate derivatives 3a to 3x, which were unequivocally characterized by the spectral data and elemental analysis. The influence of the reaction conditions on the yield of 3a was investigated to optimize the synthetic conditions. The relative optimal conditions for the synthesis of 3a include a 1:1 molar ratio of N′-(2-cyanophenyl)-N,N-dimethylformamidine to diethyl amino(phenyl)methylphosphonate and a 4:1 volume ratio of isopropanol to HOAc in the solvent mixture, at a reaction temperature of 150 °C, with a microwave power of 100 W and a corresponding pressure of 150 psi for 20 min in the microwave synthesizer. The yield of 3a was approximately 79%, whereas those of 3b to 3x were approximately 77% to 86%. Some of the synthesized compounds displayed weak to good anti-Tobacco mosaic virus (TMV) activity.
1. Introduction
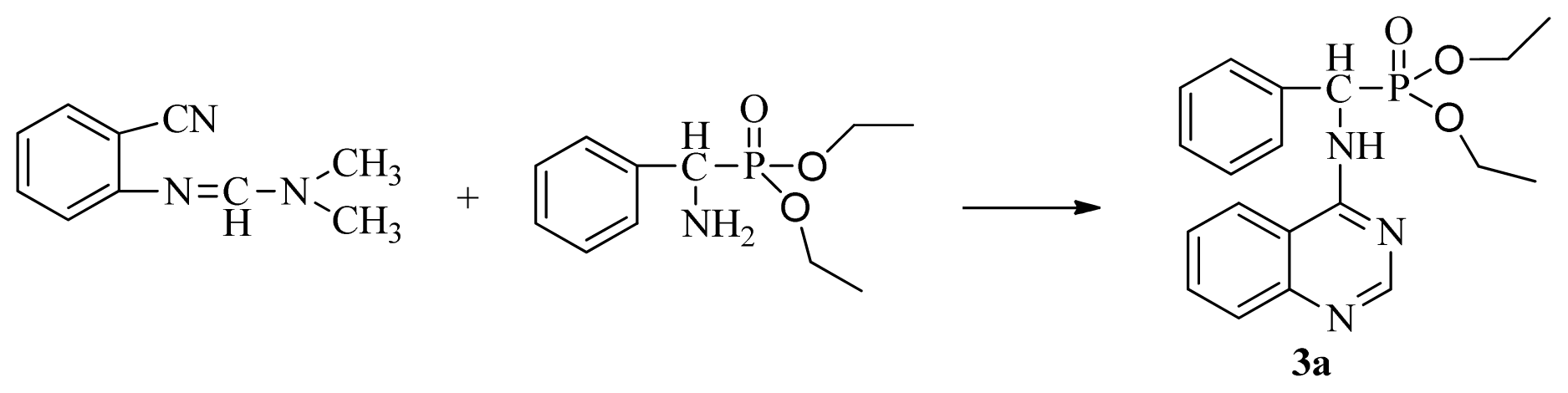
4-Aminoquinazoline derivatives, an important class of heterocyclic compounds [1], have received wide attention due to their anticancer [2–4], antimalarial [5], antimicrobial [6], and antiviral activities [7,8]. In addition, these derivatives can act as selective inhibitors of tyrosine kinase [9,10]. Suitably substituted derivatives of α-aminophosphonates exhibit a wide range of bioactivities [11–15], thus incorporating this pharmacaphore into the parent quinazoline unit may elicit synergistic effects in the design of lead structures with potent antiviral activity for plants. Based on this concept, we planned to simultaneously synthesize novel compounds containing α-aminophosphonates and chloroquinazolines (3). A classic, well-known method for synthesis of 3 is through a conventional nucleophilic reaction involving α-aminophosphonates and chloroquinazolines (Scheme I). This nucleophilic reaction offers the advantage of cheap and available materials with mild reaction conditions. However, it requires long reaction times, poor yield, and the sensitive nature of 4-chloroquinazoline, accompanied by a complicated route to access the desired product through the classic mode, has motivated us to investigate alternative methodologies [16].
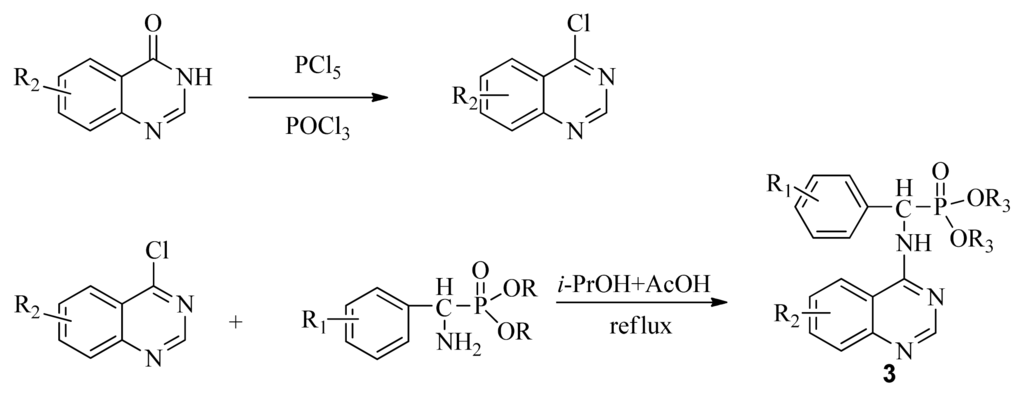
Scheme I.
The traditional method of producing the title compound 3.
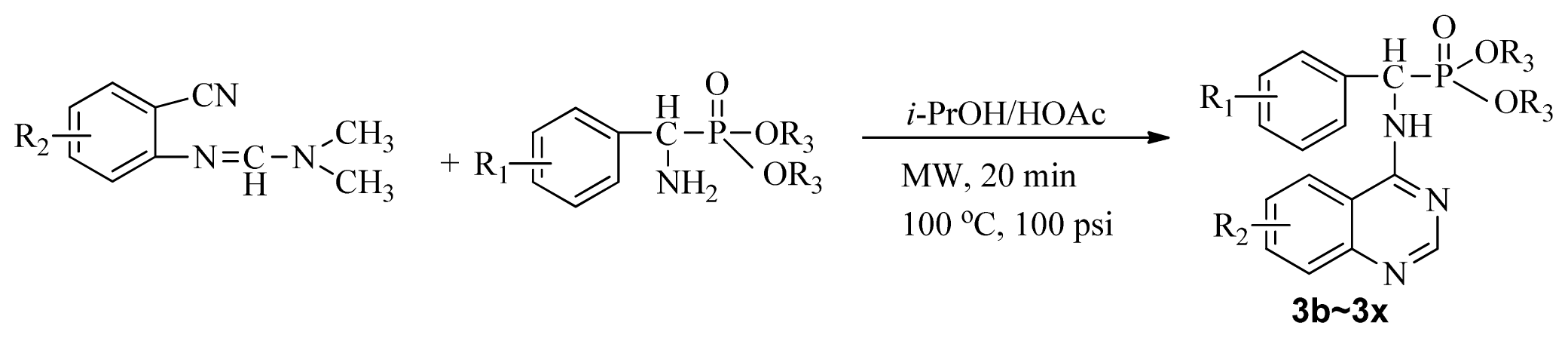
Microwave heating has been developed as a possible alternative to conventional heating with the potential to emerge as the preferred heating mode in organic synthesis [17–20]. In this perspective, microwave heating has an edge over the traditional method both in medicinal and high-throughput chemistry applications because it can offer an expanded reaction range and diminished reaction times from several hours or days down to minutes. Microwave heating is the result of the molecular rotation of materials that contain polar molecules with an electrical dipole moment. In the presence of an oscillating electromagnetic field, rotating molecules continuously collide with one another, thereby distributing the energy to adjacent molecules and causing an increase in temperature [21–23]. This kind of dipole rotation, normally referred to as dielectric heating, is usually observed in liquid water and other polar solvents placed in the microwave oven, but much less on fats, sugars, and frozen water [24–26]. Microwave irradiation has attracted the attention of the scientific community as a powerful tool for rapid, green, and efficient synthesis of a variety of quinazoline compounds [27–29]. In view of the advantages and disadvantages of the classical synthetic methods, we followed a cyclic method under microwave irradiation as illustrated in Scheme II. Title compounds (3a–3x) can easily be synthesized in a solvent of isopropanol and acetic acid (v/v, 4:1) using N′-(substituted-2-cyanophenyl)-N,N-dimethyl-formamidine derivatives and dialkyl amino (phenyl) methylphosphonate as material under microwave irradiation (100 °C, 100 psi) for 20 min. The new method requires short reaction times; it is very easy, mild, and environmentally friendly. To the best of our knowledge, this is the first report on the synthesis of new quinazoline compounds containing α-aminophosphonate moieties using microwave irradiation. This study aims to optimize the reaction conditions of each step and supply useful data for a one-pot reaction. Preliminary antiviral activity tests showed that some compounds exhibited in vivo antiviral activities against tobacco mosaic virus (TMV) at 500 mg/L. Among them, compounds 3p, 3v exhibited slightly similar activities compared to the commercial agent Ningnanmycin.
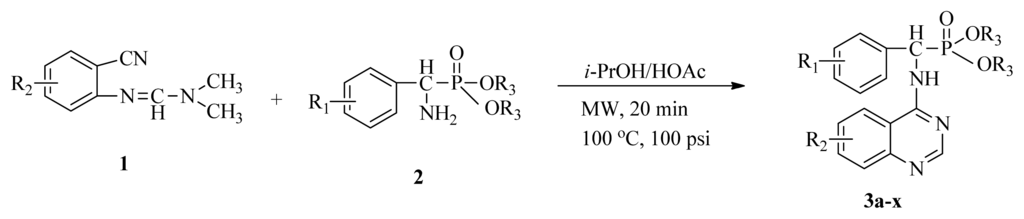
Scheme II.
Synthesis of the title compounds 3a to 3x.
2. Results
2.1. Chemistry
Microwave technology was applied to the synthetic reaction to shorten the reaction time and increase the yields of (quinazolin-4-ylamino)methylphosphonate derivatives (3). As expected, when the mixture of formamidine (obtained from 2-amino-benzonitrile) (1) and α-aminophosphonate (2) in ethanol was irradiated with microwaves, the reaction was completed in 25 min with higher yields compared with the conventional mode of heating. We optimized the reaction parameters for the synthesis of compound 3a as a model under different conditions. The influence of the reaction times and reaction temperatures with or without microwave irradiation and the role of microwave power were investigated. Table 1 indicates that microwave irradiation can accelerate the reaction (Table 1, entry 1 to 16). The reaction was relatively slow and only 34.0% yield was obtained within 6 h when microwave irradiation was not applied (Table 1, entry 12). The effect of three different mixture solvents was studied. Table 1 also shows that the reaction can yield 59.0% of the product in the mixture solvent containing isopropanol/acetic acid (v/v, 4:1), whereas in the other solvents, the yield of 3a was significantly lower, particularly in the mixture solvent containing acetonitrile/acetic acid (v/v, 4:1), which only produced 40.1% yield. This result indicated the superior efficiency of isopropanol/acetic acid compared with other mixture solvents (Table 1, entries 1 to 3). The yield of 3a increased from 45.1% and 59.0% to 62.5% (Table 1, entries 1, 5, and 6) when the reaction time was prolonged from 10 and 20 min to 30 min at 100 °C and 100 psi. When the reaction time was further prolonged to 30 min, the yield of 3a (62.5%, entry 6) was slightly increased compared with that produced after 20 min. A decrease in the yield was observed at lower temperatures (Table 1, entries 1 and 7) compared with that at 150 °C (Table 1, entry 5), and a slight improvement was noted when the reaction system was heated to 170 °C (Table 1, entry 11). In addition, the effect of microwave power and pressure on the reaction was also studied. Compound 3a was obtained with 43.0%, 70.0%, and 79.0% yields, when the microwave power was increased from 60 W to 80 and 100 W with the corresponding pressure of 100, 100, and 150 psi, respectively (Table 1, entries 10 and 11). Hence, the reaction should be carried out at 100 W and 150 psi to yield better results than at a different power output or pressure.

Table 1.
Synthesis of 3a under different reaction conditions.
The optimized reaction conditions were exploited with starting materials containing different substituents and resulted in excellent yields. The results are listed in Table 2. This indicates the versatility of the reported method. The proposed mechanism shown in Scheme III can account for the role of acetic acid in the reaction. Protonation of nitrogen by acetic acid initially activates the electrophilic carbon of the nitrile group toward a nucleophilic attack by the phosphonate amine. This reaction is followed by an increase in the electrophilic reactivity of the imine carbon on the formamidine group due to the possible protonation of nitrogen and the subsequent expulsion of a secondary amine. The intramolecular attack by the nitrogen at the electrophilic center leads to the formation of the desired cyclic product.

Table 2.
Synthesis of (quinazolin-4-ylamino)methylphosphonate derivatives via microwave irradiation.
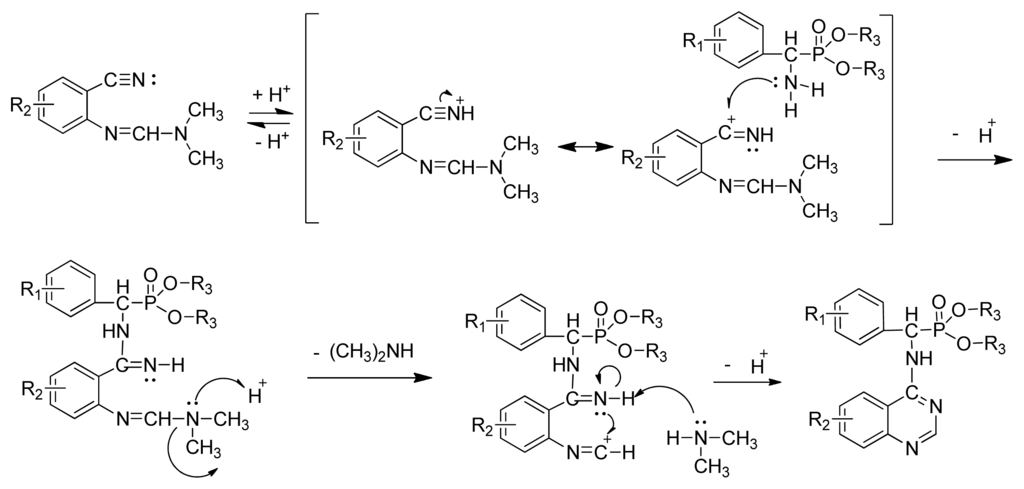
Scheme III.
Possible mechanism for the formation of the title compounds.
3. Experimental Section
3.1. General
Unless otherwise stated, all the reagents and reactants were purchased from commercial suppliers; the melting points were uncorrected and were determined on a XT-4 binocular microscope (Beijing Tech Instrument Co., China). 1H NMR and 13C NMR spectra were obtained using a JEOL ECX 500 NMR spectrometer (Tokyo, Japan) at room temperature, operating at 500 MHz for 1H NMR, 125 MHz for 13C NMR, and 470 MHz for 19F NMR, using CDCl3 as solvents and tetramethylsilane as an internal standard. Infrared spectra were obtained using KBr on a Bruker VECTOR 22 spectrometer (Ettlingen, Germany). Microwave irradiations were carried out using Discovery™ LabMate microwave apparatus. Elemental analysis was performed on the Elemental Vario-III CHN analyzer (Hanau, Germany). The course of the reactions was monitored through TLC; analytical TLC was performed on silica gel GF254; column chromatographic purification was carried out using silica gel. The intermediate products, including diethyl amino(phenyl)methylphosphonate (2a), di-n-propyl amino(phenyl)methylphosphonate (2b), diisopropyl amino(phenyl)methylphosphonate (2c), diethyl amino(2-fluorophenyl)methylphosphonate (2d), di-n-propyl amino(2-fluorophenyl)methylphosphonate (2e), and diisopropyl amino (2-fluorophenyl)methylphosphonate (2f) were prepared according to a previously described procedure [31].
3.2. Preparation of the Intermediates N′-(2-cyanophenyl)-N,N-Dimethylformamidine (1a to 1d)
The substituted-2-aminobenzonitrile (10 mmol) and 1,1-dimethoxy-N,N-dimethylmethanamine (23.8 g, 20 mmol) were placed in a dried, round-bottom flask containing a magnetic stir bar. The reaction mixture was refluxed for 3 h, and then cooled to room temperature. Water (20 mL) was then added and the resulting mixture was stirred for 10 min. The mixture was subsequently filtered and recrystallized from methanol to obtain pure N′-(substituted-2-cyanophenyl)-N,N-dimethylformamidine 1.
N′-(2-cyanophenyl)-N,N-dimethylformamidine (1a): White crystal, yield 90.8%; mp 101 °C to 103 °C; 1H NMR (CDCl3, 500 MHz): δ 8.66 (s, 1H, CH), 7.49 to 7.66 (m, 4H, Ar-3,4,5,6-H), 3.32 (s, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 156.53, 141.06, 135.16, 134.15, 128.02, 125.43, 116.92, 107.69, 40.86, 40.19; IR (KBr): ν 2949.16, 2709.99, 2227.78, 1701.22, 1597.06, 1338.60, 1134.14, 1058.92, 769.60, 532.35 cm−1; Anal. Calcd for C10H11N3: C 69.34%, H 6.40%, N 24.26%; Found. C 69.51%, H 6.23%, N 24.41%.
N′-(2-cyano-4-nitrophenyl)-N,N-dimethylformamidine (1b): White crystal, yield 91.4%; mp 137 °C to 139 °C; 1H NMR (CDCl3, 500 MHz): δ 8.42 (s, 1H, Ar-3-H), 8.23 to 8.25 (m, 1H, Ar-5-H), 7.79 (s, 1H, CH), 7.00 (d, 1H, J = 10.0 Hz, Ar-6-H), 3.19 (s, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 156.54, 135.16, 134.46, 128.15, 125.59, 116.89, 107.71, 43.72, 40.01, 38.09; IR (KBr): ν 2223.92, 1625.99, 1595.13, 1330.88, 1109.07, 829.39, 501.49 cm−1; Anal. Calcd for C10H10N4O2: C 55.04%, H 4.62%, N 25.68%; Found. C 55.11%, H 4.73%, N 26.51%.
N′-(2-cyano-4-fluorophenyl)-N,N-dimethylformamidine (1c): White crystal, yield 92.2%; mp 83 °C to 85 °C; 1H NMR (CDCl3, 500 MHz): δ 8.43 (s, 1H, Ar-3-H), 8.24 to 8.26 (m, 1H, Ar-5-H), 7.77 (s, 1H, CH), 7.02 (d, 1H, J = 5.0 Hz, Ar-6-H), 3.18 (s, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.29 (d, 1JCF = 246.4 Hz), 154.41, 141.39, 129.61 (d, 2JCF = 23.8 Hz), 128.69 (d, 2JCF = 22.6 Hz), 118.81(d, 3JCF = 7.8 Hz), 107.28 (d, 3JCF = 7.4 Hz), 77.16, 40.98, 35.19; IR (KBr): ν 2223.92, 1627.92, 1502.55, 1332.81, 1172.72, 829.39, 520.78 cm−1; Anal. Calcd for C10H10FN3: C 62.82%, H 5.27%, N 21.98%; Found. C 62.56%, H 5.08%, N 21.95%.
N′-(2,4-dichloro-6-cyanophenyl)-N,N-dimethylformamidine (1d): White crystal, yield 89.7%; mp 153 °C to 155 °C; 1H NMR (CDCl3, 500 MHz): δ 8.42 (s, 1H, Ar-3-H), 8.24 to 8.26 (m, 1H, Ar-5-H), 7.78 (s, 1H, CH), 3.19 (s, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.29, 154.41, 141.39, 129.61, 128.69, 118.81, 107.28, 77.16, 40.98, 35.19; IR (KBr): ν 2358.94, 1627.92, 1502.55, 1311.59, 1109.57, 889.18, 520.79 cm−1; Anal. Calcd for C10H9Cl2N3: C 49.61%, H 3.75%, N 17.36%; Found. C 49.46%, H 3.83%, N 17.05%.
3.3. Preparation of Title Compounds 3a–3x
The intermediates N′-(2-cyanophenyl)-N,N-dimethylformamidine 1 (0.5 mmol) and diethyl amino(phenyl)methylphosphonate (0.5 mmol) were added to a 10 mL microwave reaction vial equipped with a magnetic stir bar. Glacial acetic acid [1 mL; 20% (v/v)] solution in 2-propanol (4 mL) was also added. The mixture was capped and irradiated in the microwave for 20 min at 150 °C then cooled to ambient temperature and purified in parallel using the following procedure. The contents of the vial were poured into single-mouth bottles (50 mL). The reaction vessel was washed with 2-propanol (3 mL × 5 mL), and then 2-propanol was poured into the single-mouth bottles. The solvent was concentrated under reduced pressure, the solid was obtained, and the crude product was purified then preparative TLC with a mixture of petroleum ether and ethyl acetate (v/v = 1:1) as the developing solvent to yield the title compounds 3a to 3x.
Diethyl phenyl(quinazolin-4-ylamino)methylphosphonate (3a): White solid, yield 78.5%; mp 201 °C to 203 °C; 1H NMR: (CDCl3, 500 MHz): δ 8.65 (s, 1H, quinazoline-2-H), 7.95 (d, J = 8.0 Hz, 1H, quinazoline-8-H), 7.86 (d, J = 8.6 Hz, 1H, quinazoline-5-H), 7.76 to 7.78 (m, 1H, Ar-4-H), 7.51 to 7.61 (m, 2H, quinazoline-6,7-H), 7.26 to 7.37 (m, 4H, Ar-2,3,5,6-H), 6.84 (s, 1H, N-H), 6.12 (d, J = 10.0 Hz, 1H, CH), 3.97 to 4.15 (m, 4H, 2CH2), 1.11 to 1.25 (m, 6H, 2CH3 ); 13C NMR (CDCl3, 125 MHz): δ 155.05, 133.04, 128.78, 128.65, 128.35, 128.28, 128.23, 126.46, 120.90, 77.36, 77.11, 76.86, 63.77, 63.71, 63.29, 52.01, 50.79, 16.48,16.28; 31P NMR(CDCl3, 500 MHz): δ 22.7; IR: ν 3296.76 (NH), 3070.14 (ArH), 1779.78 (CN), 1577.65 (Ar), 1206.79 (P=O), 987.02 (P-O-C) cm−1; Anal. Calcd for C19H22N3O3P: C 61.45%, H 5.97%, N 11.31%; Found. C 61.05%, H 5.93%, N 10.99%.
Di-n-propyl phenyl(quinazolin-4-ylamino)methylphosphonate (3b): White solid, yield 78.0%; mp 173 °C to 175 °C; 1H NMR (CDCl3, 500 MHz): δ 8.65 (s, 1H, quinazoline-2-H), 7.98 d, J = 8.0 Hz, 1H, quinazoline-8-H), 7.84 (d, J = 8.1 Hz, 1H, quinazoline-5-H), 7.74 to 7.77 (m, 1H, Ar-4-H), 7.61 to 7.63 (m, 2H, quinazoline-6,7-H), 7.26 to 7.34 (m, 4H, Ar-2,3,5,6-H), 6.79 (s, 1H, N-H), 6.18 (d, J = 20.0 Hz, 1H, CH), 3.63 to 4.07 (m, 4H, 2OCH2), 1.47 to 1.61 (m, 4H, 2CH2), 0.78 to 0.83 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.66, 158.59, 155.02, 149.69, 135.46, 132.84, 128.65, 128.61, 128.27, 128.22, 126.24, 120.95, 68.93, 68.60, 51.88, 50.65, 23.70, 23.66, 9.90, 9.82; 31P NMR(CDCl3, 500 MHz): δ 22.6; IR: ν 3263.64 (NH), 2968.56 (ArH), 1714.78 (CN), 1579.72 (Ar), 1238.36 (P=O), 1012.24 (P-O-C) cm−1; Anal. Calcd for C21H26N3O3P: C 63.15%, H 6.56%, N 10.52%; Found. C 63.19%, H 6.22%, N 10.63%.
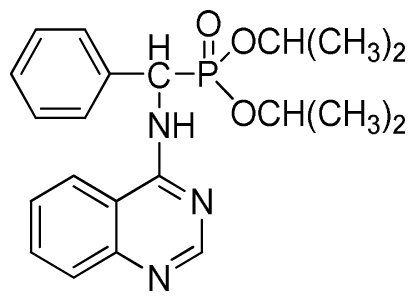
Diisopropyl phenyl(quinazolin-4-ylamino)methylphosphonate (3c): White solid, yield 78.1%; mp 188 °C to 190 °C; 1H NMR (CDCl3, 500 MHz): δ 8.65 (s, 1H, quinazoline-2-H), 8.00 (d, J = 8.6 Hz, 1H, quinazoline-8-H), 7.85 (d, J = 8.0 Hz, 1H, quinazoline-5-H), 7.73 to 7.77 (m, 1H, Ar-4-H), 7.47 to 7.50 (m, 2H, quinazoline-6,7-H), 7.26 to 7.48 (m, 5H, Ar-2,3,5,6-H), 7.02 (s, 1H, N-H), 6.10 (d, J = 9.2 Hz, 1H, CH), 4.47 to 4.79 (m, 2H, 2CH), 0.89 to 1.31 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 158.77, 158.70, 155.10, 149.68, 132.78, 128.57, 128.52, 128.46, 128.41, 128.05, 126.21, 120.95, 71.99, 58.34, 52.48, 51.24, 24.28, 24.26, 24.16, 24.13; 31P NMR(CDCl3, 200 MHz): δ 20.9; IR: ν 3275.15 (NH), 2980.07 (ArH), 1620.24 (CN), 1577.85 (Ar), 1238.36 (P=O), 987.68 (P-O-C) cm−1; Anal. Calcd for C21H26N3O3P: C 63.15%, H 6.56%, N 10.52%; Found. C 63.04%, H 6.36%, N 10.62%.
Diethyl(2-fluorophenyl)(quinazolin-4-ylamino)methylphosphonate (3d): White solid, yield 77.8%; mp 163 °C to 165 °C; 1H NMR (CDCl3, 500 MHz): δ 8.65 (s, 1H, quinazoline-2-H), 7.95 (d, J = 10.0 Hz, 1H, Ar-3-H), 7.85 (d, J = 15.0 Hz, 1H, quinazoline-8-H), 7.76 to 7.79 (m, 1H, quinazoline-5-H), 7.51 to 7.61 (m, 2H, quinazoline-6,7-H), 7.26 to 7.38 (m, 4H, Ar-4, 5, 6-H), 6.84 (s, 1H, NH), 6.16 (d, J = 10.0 Hz, 1H, CH), 4.15 to 4.17 (m, 2H, CH2), 3.98 to 4.14 (m, 2H, CH2), 1.11 to 1.27 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 161.92, 159.94, 158.67 (d, 1JCF = 246.5 Hz), 155.15, 149.78, 133.05, 129.57 (d, 2JCF = 23.8 Hz), 128.75 (d, 3JCF = 7.7 Hz), 126.46 (d, 3JCF = 7.6 Hz), 124.53, 121.15, 115.92 (d, 2JCF = 24.2 Hz), 115.13, 63.84, 63.77, 46.49, 45.14, 16.43, 16.26; 31P NMR (CDCl3, 200 MHz): δ 21.7; IR: ν 3304.06 (NH), 2987.74 (CH3), 1577.74, 1525.69, 1409.96 (C=C), 1361.74 (CH3), 1234.44 (P=O), 1228.2 (P-O-C), 804.32, 769.60 (=C–H) cm−1; Anal. Calcd for C19H21FN3O3P: C 58.61%; H, 5.44%, N 10.79%; Found. C 58.42%, H 5.33%, N 10.58%.
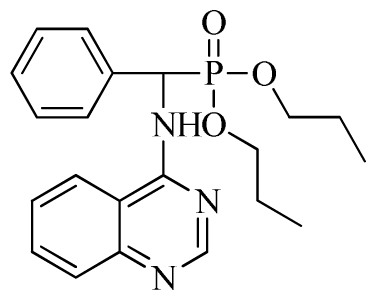
Di-n-propyl(2-fluorophenyl)(quinazolin-4-ylamino)methylphosphonate (3e): White solid, yield 77.5%; mp 111 °C to 113 °C; 1H NMR (DMSO-d6, 500 MHz): δ 9.05 (d, J = 6.0 Hz, 2H, quinazoline-2-H, Ar- 3-H), 7.85 to 8.10 (m, 4H, quinazoline-5,6,7,8-H), 7.35 (s, 1H, NH), 7.31 to 7.33 (m, 3H, Ar-4,5,6-H), 6.85 (d, J = 20.0 Hz, CH), 3.98 to 4.02 (m, 4H, 2-OCH2), 1.49 to 1.51 (m, 4H, 2-CH2), 1.46 to 1.51 (m, 4H, 2CH2), 0.74 to 0.78 (m, 3H, J = 7.5 Hz, CH3), 0.73 to 0.75 (m, H, 2CH3), 0.71 (t, 3H, J = 7.5 Hz, CH3); 13C NMR (DMSO-d6, 125 MHz): δ 161.30, 151.85 (d, 1JCF = 245.8 Hz), 136.64, 131.53, 131.15, 128.92 (d, 2JCF =24.2 Hz), 125.34 (d, 3JCF = 7.5 Hz), 125.15 (d, 3JCF = 7.5 Hz), 121.87, 121.76, 121.12, 115.91 (d, 2JCF =23.6 Hz), 115.57, 113.70, 68.71, 68.65, 45.74, 23.88, 23.84, 10.24, 10.19; 31P NMR(DMSO-d6, 200 MHz): δ 19.5; IR: ν 3294.05 (NH), 2977.74 (CH3), 1571.17, 1520.61, 1412.12 (C=C) 1360.12 (CH3), 1234.44 (P=O), 1228.2 (P-O-C) cm−1; Anal. Calcd for C21H25FN3O3P: C 60.43%, H 6.04%, N 10.07%; Found. C 60.14%, H 6.16%, N 10.42%.
Diisopropyl (2-fluorophenyl)(quinazolin-4-ylamino)methylphosphonate (3f): White solid, yield 77.5%; mp 111 °C to 113 °C; 1H NMR (DMSO-d6, 500 MHz): δ 9.05 (d, J = 6.0 Hz, 2H, quinazoline-2-H, Ar- 3-H), 7.85 to 8.10 (m, 4H, quinazoline-5,6,7,8-H), 7.35 (s, 1H, NH), 7.31 to 7.33 (m, 3H, Ar-4,5,6-H), 6.85 (d, J = 20.0 Hz, CH), 3.98 to 4.02 (m, 4H, 2-OCH2), 1.49 to 1.51 (m, 4H, 2-CH2), 1.46 to 1.51 (m, 4H, 2CH2), 0.74 to 0.78 (m, 3H, J = 7.5 Hz, CH3), 0.73 to 0.75 (m, H, 2CH3), 0.71 (t, 3H, J = 7.5 Hz, CH3); 13C NMR (DMSO-d6, 125 MHz): δ 161.30, 151.85 (d, 1JCF = 246.5 Hz), 136.64, 131.53, 131.15, 128.92 (d, 2JCF = 24.6 Hz), 125.34 (d, 3JCF = 7.5 Hz), 125.15 (d, 3JCF = 7.5 Hz), 121.87, 121.76, 121.12, 115.91 (d, 2JCF = 23.6 Hz), 115.57, 113.70, 68.71, 68.65, 45.74, 23.88, 23.84, 10.24, 10.19; 31P NMR(DMSO-d6, 200 MHz): δ 19.5; IR: ν 3294.05 (NH), 2977.74 (CH3), 1571.17, 1520.61, 1412.12 (C=C) 1360.12 (CH3), 1234.44 (P=O), 1228.2 (P-O-C) cm−1; Anal. Calcd for C21H25FN3O3P: C 60.43%, H 6.04%, N 10.07%; Found. C 60.14%, H 6.16%, N 10.42%.
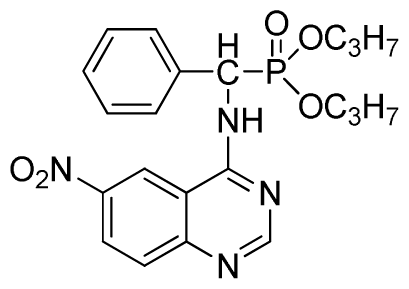
Diethyl (6-nitroquinazolin-4-ylamino)(phenyl)methylphosphonate (3g): White solid, yield 85.9%; mp 132 °C to 134 °C; 1H NMR (CDCl3, 500 MHz): δ 8.62 (s, 1H, quinazoline-2-H), 8.30 (s, 1H, quinazoline-5-H), 8.05 (s, 1H, NH), 7.70 to 7.74 (m, 2H, quinazoline-7,8-H), 7.26~7.69 (m, 5H, Ar-2,3,4,5,6-H), 6.36 (d, J = 10 Hz, CH), 3.85 to 4.23 (m, 4H, 2-CH2), 1.16 to 1.24 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.46, 1H, 155.20, 135.24, 133.60, 131.47, 129.93, 128.66, 128.19, 121.55, 116.20, 119.13, 98.37, 63.72, 52.01, 50.81, 16.52, 16.47; 31P NMR(CDCl3, 200 MHz): δ 22.1; IR: ν 3435.28 (NH), 3134.83 (ArH), 1713.74 (CN), 1571.31 (Ar), 1227.79 (P=O), 1001.53 (P-O-C) cm−1; Anal. Calcd for C19H21N4O5P: C 54.81%, H 5.08%, N 13.46%; Found. C 54.62%, H 5.13%, N 13.66%.
Dipropyl (6-nitroquinazolin-4-ylamino)(phenyl)methylphosphonate (3h): Yellow solid, yield 85.7%; mp 196 °C to 198 °C; 1H NMR (CDCl3, 500 MHz): δ 8.62 (s, 1H, quinazoline-2-H), 8.73 (s, 1H, quinazoline-5-H), 8.13 (s, 1H, NH), 7.64 to 7.81 (m, 2H, quinazoline-7,8-H), 7.27 to 7.62 (m, 5H, Ar-2,3,4,5,6-H), 6.28 (d, 1H, J = 10 Hz, CH), 3.77 to 4.22 (m, 4H, 2-OCH2), 1.79 to 2.18 (m, 4H, 2CH2), 1.13 to 1.26 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.39, 158.33, 155.65, 145.42, 134.87, 133.22, 130.86, 128.68, 128.45, 128.40, 128.30, 120.09, 76.69, 63.81, 63.42, 63.36, 52.23, 50.90, 30.97, 16.44, 16.39; 31P NMR(CDCl3, 200 MHz): δ 21.6; IR: ν 3281.12 (NH), 2989.66 (–CH), 1630.35 (C=N), 1565.56, 1515.33, 1470.85, 1448.21 (Ar), 1240.13 (P=O) cm−1; Anal. Calcd for C21H25N4O5P: C 56.75%, H 5.67%, N 12.61%; Found. C 56.55%, H 5.58%, N 12.55%.
Dipropyl (6-nitroquinazolin-4-ylamino)(phenyl)methylphosphonate (3i): Yellow solid, yield 85.6%; mp 43 °C to 45 °C; 1H NMR (CDCl3, 500 MHz): δ 8.61 (s, 1H, quinazoline-2-H),7.92 (s, 1H, quinazoline-5-H),7.89 (s, 1H, NH), 7.67 to 7.68 (m, 2H, quinazoline-7,8-H), 7.25 to 7.64 (m, 5H, Ar-2,3,4,5,6-H), 6.22 (d, 1H, J = 10 Hz, CH), 4.58 to 4.84 (m, 4H, 2-OCH), 1.15 to 1.31 (m, 6H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 160.94, 158.93, 158.87, 154.56, 146.60, 135.73, 130.82, 128.77, 128.67, 128.49, 127.96, 122.35, 122.14, 106.34, 106.16, 72.52, 52.58, 51.36, 24.25, 24.18; 31P NMR(CDCl3, 200 MHz): δ 20.0; IR: ν 3277.06 (NH), 2980.02 (CH), 1614.42 (C=N), 1573.91, 1527.62, 1490.97, 1456.26(Ar), 1224.80 (P=O) cm−1; Anal. Calcd for C21H25N4O5P: C 56.75%, H 5.67%, N 12.61%; Found. C 56.61%, H 5.82%, N 12.83%.
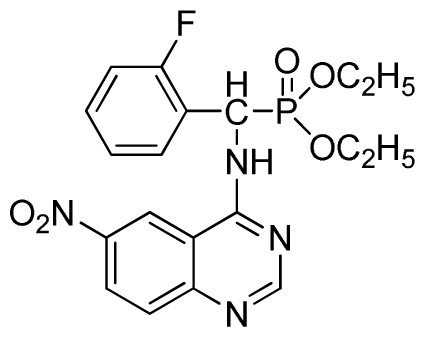
Diethyl (2-fluorophenyl)(6-nitroquinazolin-4-ylamino)methylphosphonate (3j): Yellow solid, yield 85.2%; mp 176 °C to 178 °C; 1H NMR (DMSO-d6, 500 MHz): δ 9.84 (s, 1H, quinazoline-2-H), 9.68 to 9.70 (d, J = 8.0 Hz, 1H, Ar-3-H), 8.67 (s, 1H, quinazoline-5-H), 8.04 to 8.50 (m, 2H, quinazoline-7, 8-H), 7.8 (s, 1H, NH), 7.20 to 7.35 (m, 3H, Ar-4,5,6-H), 6.70 (d, J = 8.0 Hz, 1H, CH), 3.90 to 4.04 (m, 4H, 2CH2), 1.01 to 1.07 (m, 6H, 2CH3); 13C NMR (DMSO-d6, 125 MHz): δ 160.57, 160.51, 158.12 (d, 1JCF = 246.4 Hz), 153.43, 145.03, 131.26, 129.89 (d, 2JCF = 24.8 Hz), 127.36 (d, 3JCF = 7.4 Hz), 125.06 (d, 3JCF = 7.5 Hz), 121.82, 115.78 (d, 2JCF = 23.5 Hz), 114.53, 63.48, 63.42, 44.73, 43.45, 39.98, 16.78, 16.45; 31P NMR(DMSO-d6, 200 MHz): δ 20.8; IR: ν 3259.70 (NH), 1575.84, 1525.69, 1490.97, 1456.26 (Ar), 2989.66 (–CH), 1622.13 (C=N), 1234.44 (P=O) cm−1; Anal. Calcd for C19H20FN4O5P: C 52.54%; H 4.64%; N 12.90%; Found. C 52.41%, H 4.59%, N 12.86%.
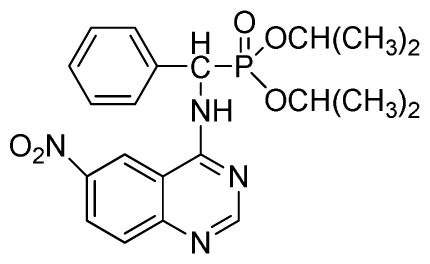
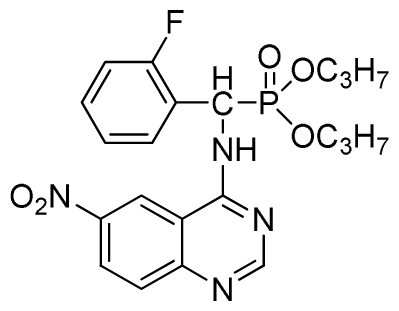
Dipropyl (2-fluorophenyl)(6-nitroquinazolin-4-ylamino)methylphosphonate (3k): Yellow solid, yield 85.0%; mp 168 °C to 170 °C; 1H NMR (CDCl3, 500 MHz): δ 9.30 (s, 1H, quinazoline-2-H), 8.73 to 8.75 (d, J = 10.0 Hz, 1H, Ar-3-H), 8.65 (s, 1H, quinazoline-5-H), 7.89 to 8.47 (m, 2H, quinazoline-7,8- H), 7.80 (s, 1H, NH), 7.09 to 7.27 (m, 3H, Ar-4,5,6-H), 6.81 (d, J = 15.0 Hz, 1H, CH), 4.14 to 4.19 (m, 4H, 2OCH2), 1.54 to 1.62 (m, 4H, 2CH2), 0.82 to 0.85 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 161.53, 160.16, 159.65 (d, 1JCF = 245.8 Hz), 158.03, 153.25, 144.74, 130.27, 129.91 (d, 2JCF = 24.8 Hz), 126.52 (d, 3JCF = 7.8 Hz), 124.56 (d, 3JCF = 7.5 Hz), 122.63, 119.92, 115.58 (d, 2JCF = 22.8 Hz), 114.69, 69.34, 45.37, 44.09, 23.72, 9.93; 31P NMR(CDCl3, 200 MHz): δ 20.2; IR: ν 3230.34 (NH), 1567.34, 1523.22, 1493.17, 1452.56 (Ar), 2990.05 (–CH), 1625.02 (C=N) 1230.48 (P=O) cm−1; Anal. Calcd for C21H24FN4O5P: C 54.55%, H 5.23%, N 12.12%; Found. C 54.43%, H 5.32%, N 12.20%.
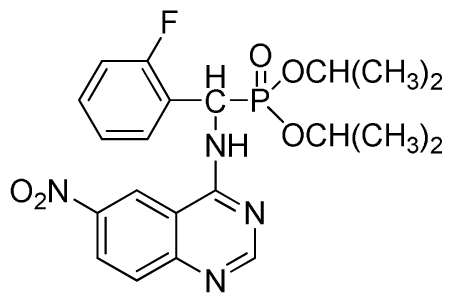
Diisopropyl(2-fluorophenyl)(6-nitroquinazolin-4-ylamino)methylphosphonate (3l): Yellow solid, yield 83.9%; mp 141 °C to 142 °C; 1H NMR (CDCl3, 500 MHz): δ 9.14 (s, 1H, quinazoline-2-H), 8.74 (d, J = 8.5 Hz, 1H, Ar-3-H), 8.50 (s, 1H, quinazoline-5-H), 7.91 to 7.92 (m, 2H, quinazoline-7, 8-H), 7.25 (s, 1H, NH), 7.07 to 7.12 (m, 3H, Ar-4,5,6-H), 6.56 (d, J = 10.0 Hz, 1H, CH), 4.58 to 4.83 (m, 4H, 2OCH), 0.92 to 1.29 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 161.80, 159.80, 158.14 (d, 1JCF = 246.2 Hz), 153.19, 144.87, 130.15, 129.88, 129.73 (d, 2JCF = 24.5 Hz), 126.61 (d, 3JCF = 7.9 Hz), 124.53 (d, 3JCF = 7.6 Hz), 122.96, 119.18, 115.58 (d, 2JCF = 23.5 Hz), 114.36, 73.15, 46.71, 45.43, 24.20, 24.11, 23.91, 23.17; 31P NMR(CDCl3, 200 MHz): δ 18.8; IR: ν 3255.84 (NH), 1577.77, 1527.62, 1490.97, 1456.26 (Ar), 2981.95 (–CH), 1622.13 (C=N), 1236.37 (P=O) cm−1; Anal. Calcd For C21H24FN4O5P: C 54.55%, H 5.23%, N 12.12%; Found. C 54.62%, H 5.18%, N 11.98%.
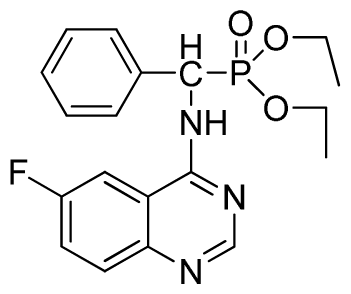
Diethyl (6-fluoroquinazolin-4-ylamino)(phenyl)methylphosphonate (3m): White solid, yield 86.1%; mp >300 °C; 1H NMR (CDCl3, 500 MHz): δ 8.62(s, 1H, quinazoline-2-H), 7.85 (s, 1H, quinazoline-5-H), 7.66 to 7.80 (m, 2H, quinazoline-7,8-H), 7.65 (s, 1H, N-H), 7.24 to 7.51 (m, 5H, Ar-2,3,4,5,6-H), 6.24 (d, J = 10.0 Hz, 1H, C-H), 4.03 to 4.22 (m, 4H, 2CH2), 1.14 to 1.26 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.97 (d, 1JCF = 243.8 Hz), 158.60, 154.41, 146.73, 135.24 (d, 3JCF = 8.0 Hz), 128.60, 128.35, 122.46 (d, 2JCF = 24.5 Hz), 122.27 (d, 3JCF = 7.9 Hz), 106.09 (d, 2JCF = 22.8 Hz), 77.27, 76.76, 63.74, 63.63, 63.31, 63.25, 52.00, 16.43, 16.10; 31P NMR(CDCl3, 200 MHz): δ 22.5; IR: ν 3267.4 (NH), 3068.8 (ArH), 1629.9 (CN), 1232.7 (P=O), 1022.2 (P-O-C) cm−1; Anal. Calcd for C19H21FN3O3P: C 58.61%, H 5.44%, N 10.97%; Found. C 58.77%, H 5.63%, N 10.92%.
Di-n-propyl(6-fluoroquinazolin-4-ylamino)(phenyl)methylphosphonate (3n): White solid, yield 86.1%; mp 186 °C to 188 °C; 1H NMR (CDCl3, 500 MHz): δ 8.62 (s, 1H, quinazoline-2-H), 8.20 (s, 1H, quinazoline-5-H N-H), 7.82 to 8.10 (m, 2H, quinazoline-7,8-H), 7.80 (s, 1H, N-H), 7.23 to 7.72 (m, 5H, Ar-2,3,4,5,6-H), 6.46 (d, J = 8.0 Hz, 1H, C-H), 3.99 to 4.14 (m, 4H, 2OCH2), 1.56 to 1.57 (m, 4H, 2CH2), 0.78~0.85 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.80 (d, 1JCF = 246.2 Hz), 159.05, 158.84, 154.40, 146.79, 135.38, 130.59 (d, 3JCF = 8.1 Hz), 130.52, 128.67, 128.62, 128.45, 127.96, 122.02 (d, 2JCF = 24.8 Hz), 106.96 (d, 3JCF = 7.9 Hz), 106.78 (d, 2JCF = 23.2 Hz), 68.89, 68.83, 51.86, 50.61, 23.86, 23.68; 31P NMR(CDCl3, 200 MHz): δ 22.1; IR: ν 3261.6 (NH), 2968.5 (ArH), 1714.7 (CN), 1236.4 (P=O), 1012.2 (P-O-C) cm−1; Anal. Calcd for C21H25FN3O3P: C 60.43%, H 6.04%, N 10.07%; Found. C 60.33%, H 5.95%, N 10.12%.
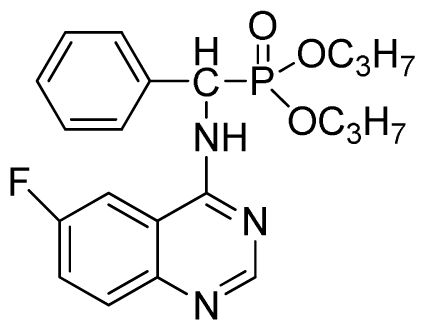
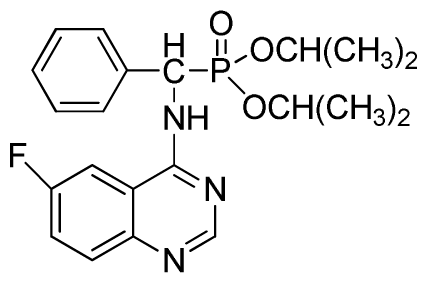
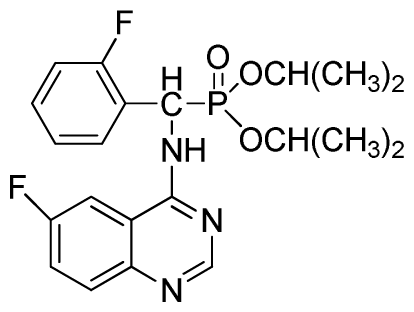
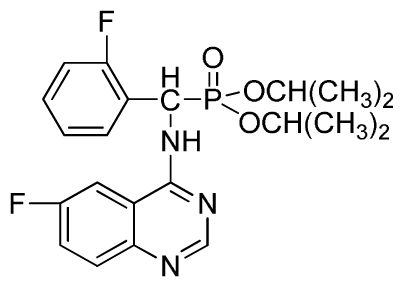
Diisopropyl (6-fluoroquinazolin-4-ylamino)(phenyl)methylphosphonate (3o): White solid, yield 85.3%; mp 204 °C to 206 °C; 1H NMR (CDCl3, 500 MHz): δ 8.63 (s, 1H, quinazoline-2-H), 8.13 (s, 1H, quinazoline-5-H), 7.77 to 8.12 (m, 2H, quinazoline-7,8-H), 7.80 (s, 1H, N-H), 7.27 to 7.75 (m, 5H, Ar-2,3,4,5,6-H), 6.17 (d, J = 10.0 Hz, 1H, C-H), 4.57 to 4.82 (m, 2H, 2CH), 1.16 to 1.31 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 160.92 (d, 1JCF = 245.4 Hz), 158.94, 158.76, 154.48, 146.76, 135.66, 130.8 (d, 3JCF = 8.0 Hz), 128.69, 128.64, 128.43, 127.95, 122.35 (d, 2JCF = 23.8 Hz), 122.15 (d, 3JCF = 8.0 Hz), 106.28, 106.09 (d, 2JCF = 23.4 Hz), 72.48, 52.55, 51.30, 24.20, 24.18, 24.15; 31P NMR(CDCl3, 200 MHz): δ 20.5; IR: ν 3288.6 (NH), 2985.8 (ArH), 1629.9 (CN), 1224.7 (P=O), 987(P-O-C) cm−1; Anal. Calcd for C21H25FN3O3P: C 60.43%, H 6.04%, N 10.07%; Found. C 60.21%, H 5.94%, N 9.85%.
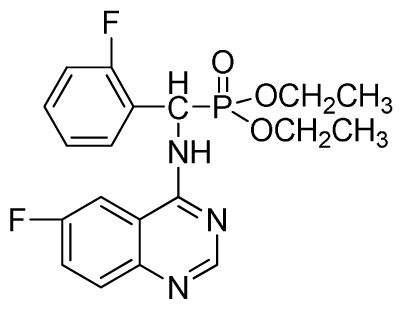
Diethyl (2-fluorophenyl)(6-fluoroquinazolin-4-ylamino)methylphosphonate (3p): White solid, yield 85.3%; mp 142 °C to 144 °C; 1H NMR (CDCl3, 500 MHz): δ 8.61 (s, 1H, quinazoline-2-H), 8.03 (s, 1H, quinazoline-5-H), 7.79 to 7.82 (m, 1H, Ar-3-H), 7.68~7.70 (m, 2H, quinazoline-7,8-H), 7.46 (brs, 1H, N-H), 7.23 to 7.26 (m, 3H, Ar-4,5,6-H), 6.35 (d, J = 10.0 Hz, 1H, C-H), 3.83 to 4.22 (m, 4H, 2CH2), 1.16 to 1.23 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.94 (d, 1JCF = 244.5 Hz), 158.97 (d, 1JCF = 245.2 Hz), 154.48, 146.84, 135.32 (d, 3JCF = 8.0 Hz), 130.61(d, 2JCF = 24.8 Hz), 130.71(d, 2JCF = 24.5 Hz), 128.65 (d, 3JCF = 7.8 Hz), 128.12 (d, 3JCF = 7.6 Hz), 122.39 (d, 3JCF = 7.6 Hz), 106.77 (d, 2JCF = 23.2 Hz), 77.10, 63.47, 63.41, 51.98, 50.74, 16.50, 16.21, 16.17; 31P NMR(CDCl3, 200 MHz): δ 21.8; IR: ν 3265.5 (NH), 2985.8-3068.7 (ArH), 1714.7 (CN), 1244.9 (P=O), 1022.2 (P-O-C) cm−1; Anal. Calcd for C19H20F2N3O3P: C 56.02%, H 4.95%, N 10.32%; Found. C 56.26%, H 4.91%, N 9.89%.
Di-n-propyl (2-fluorophenyl)(6-fluoroquinazolin-4-ylamino)methylphosphonate (3q): White solid, yield 85.3%; mp 213 °C to 214 °C; 1H NMR (CDCl3, 500 MHz): δ 8.72 (s, 1H, quinazoline-2-H), 8.13 (s, 1H, quinazoline-5-H), 7.78 to 7.81 (m, 1H, Ar-3-H), 7.63 to 7.66 (m, 2H, quinazoline-7,8-H), 7.39 (s, 1H, N-H), 7.25 to 7.27 (m, 3H, Ar-4,5,6-H), 6.28 (d, J = 10.0 Hz, 1H, C-H), 3.92 to 4.10 (m, 4H, 2OCH2), 1.49 to 1.81 (m, 4H, 2CH2), 0.78 to 0.81 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.50 (d, 1JCF = 245.2 Hz), 155.71 (d, 1JCF = 244.5 Hz), 145.51, 133.29 (d, 3JCF = 7.8 Hz), 128.75 (d, 2JCF = 24.3 Hz), 128.55 (d, 3JCF = 7.6 Hz), 128.51 (d, 3JCF = 7.8 Hz), 128.34 (d, 2JCF = 23.8 Hz), 120.23 (d, 3JCF = 7.7 Hz), 116.85 (d, 2JCF = 22.5 Hz), 77.11, 69.24, 68.88, 52.26, 51.02, 23.93, 23.69, 9.99, 9.94; 31P NMR(CDCl3, 200 MHz): δ 21.7; IR: ν 3271.33 (NH), 2985.85 to 3070.72 (ArH), 1629.86 (CN), 1246.07 (P=O), 1020.34 (P-O-C) cm−1; Anal. Calcd for C21H24F2N3O3P: C 57.93%, H 5.56%, N 9.65%; Found. C 58.39%, H 5.27%, N 9.80%.
Diisopropyl(2-fluorophenyl)(6-fluoroquinazolin-4-ylamino)methylphosphonate (3r): White solid, yield 83.7%; mp 251 °C to 253 °C; 1H NMR (CDCl3, 500 MHz): δ 8.60 (s, 1H, quinazoline-2-H), 8.03(s, 1H, quinazoline-5-H), 7.80 to 7.82 (m, 1H, Ar-3-H), 7.65 to 7.69 (m, 2H, quinazoline-7,8-H), 7.43 (brs, 1H, N-H), 7.21 to 7.43 (m, 3H, Ar-4,5,6-H), 6.30 (d, J = 10.0 Hz, 1H, C-H), 4.60 to 4.86 (m, 2H, 2OCH), 1.15 to 1.32 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 160.90 (d, 1JCF = 245.6 Hz), 158.94 (d, 1JCF = 244.6 Hz), 154.57, 154.56, 146.84, 135.76 (d, 3JCF = 7.8 Hz), 130.74 (d, 2JCF = 25.8 Hz), 128.91 (d, 2JCF = 24.8 Hz), 128.87 (d, 3JCF = 7.5 Hz), 128.40 (d, 3JCF = 7.6 Hz), 127.95 (d, 2JCF = 23.8 Hz), 122.32 (d, 3JCF = 7.4 Hz), 106.83 (d, 2JCF = 22.5 Hz), 77.12, 72.54, 72.09, 52.55, 51.30, 24.24, 23.84, 23.23; 31P NMR(CDCl3, 200 MHz): δ 21.2; IR: ν 3270.21 (NH), 2981.93 to 3072.64 (ArH), 1629.58 (CN), 1238.43 (P=O), 993.32 (P-O-C) cm−1; Anal. Calcd for C21H24F2N3O3P: C 57.93%, H 5.56%, N 9.65%; Found. C 58.06%, H 5.86%, N9.66%.
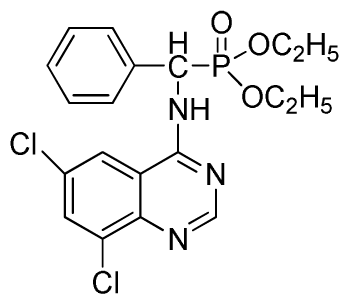
Diethyl(6,8-dichloroquinazolin-4-ylamino)(phenyl)methylphosphonate (3s): White solid, yield 86.0%; mp 178 °C to 180 °C; 1H NMR (CDCl3, 500 MHz): δ 8.73 (s, 1H, quinazoline-2-H), 8.13 (s, 1H, quinazoline-7-H), 7.81 (s, quinazoline-5-H), 7.74 (brs, 1H, N-H), 7.27 to 7.63 (m, 5H, Ar-2,3,4,5,6-H), 6.26 (d, J = 10.0 Hz, 1H, C-H), 3.76 to 4.26 (m, 4H, 2CH2), 1.13 to 1.26 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.39, 155.33, 155.65, 145.43, 134.87, 133.23, 130.86, 128.68, 128.45, 128.40, 128.29, 63.81, 63.42, 63.36, 52.23, 50.90, 30.96, 16.44, 16.40; 31P NMR(CDCl3, 200 MHz): δ 22.9; IR: ν 3275.18 (NH), 3072.64 (ArH), 1604.82 (CN), 1228.75 (P=O), 1022.27 (P-O-C) cm−1; Anal. Calcd for C19H20Cl2N3O3P: C 51.83%, H 4.58%, N 9.54%; Found. C 52.06%, H 4.71%, N 9.67%.
Dipropyl(6,8-dichloroquinazolin-4-ylamino)(phenyl)methylphosphonate (3t): White solid, yield 85.7%; mp 150 °C to 152 °C; 1H NMR (CDCl3, 500 MHz): δ 8.73 (s, 1H, quinazoline-2-H), 8.23 (s, 1H, quinazoline-7-H), 8.13 (brs, 1H, N-H), 7.79 (s, 1H, quinazoline-5-H), 7.25 to 7.68 (m, 5H, Ar-2,3,4,5,6-H), 6.35 (d, J = 10.0 Hz, 1H, C-H), 3.97 to 4.14 (m, 4H, 2OCH2), 1.52 to 1.61 (m, 4H, 2CH2), 0.81 to 0.82 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.55, 155.64, 145.47, 134.98, 133.16, 128.61, 128.57, 128.52, 128.19, 120.41, 116.89, 69.05, 68.91, 52.17, 50.93, 23.86, 23.62, 9.90, 19.88; 31P NMR(CDCl3, 200 MHz): δ 21.7; IR: ν 3275.18 (NH), 3072.66 (ArH), 1714.79 (CN), 1224.81 (P=O), 1031.26 (P-O-C) cm−1; Anal. Calcd for C21H24Cl2N3O3P: C 53.86%, H 5.17%, N 8.97%; Found. C 53.39%, H 5.27%, N 8.80%.
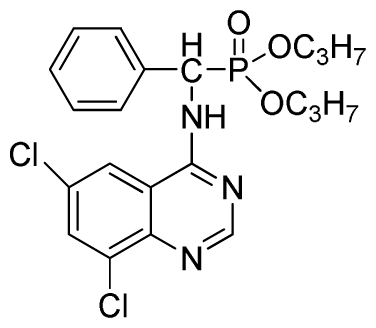
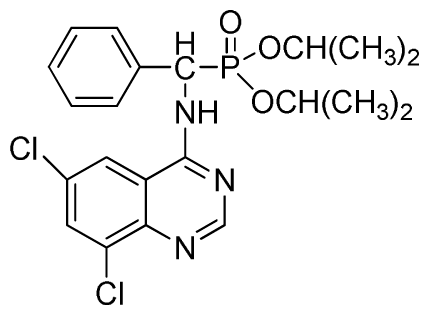
Diisopropyl (6,8-dichloroquinazolin-4-ylamino)(phenyl)methylphosphonate (3u): White solid, yield 85.2%; mp 187 °C to 190 °C; 1H NMR (CDCl3, 500 MHz): δ 8.73 (s, 1H, quinazoline-2-H), 8.05 (s, 1H, quinazoline-7-H), 7.82 (s, 1H, quinazoline-5-H), 7.64 (s, 1H, N-H), 7.27 to 7.43 (m, 5H, Ar-2,3,4,5,6-H), 6.16 (d, J = 10.0 Hz, 1H, C-H), 4.53 to 4.78 (m, 2H, 2OCH), 0.88 to 1.29 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 158.31, 158.24, 155.71, 145.42, 133.25, 128.59, 128.54, 128.21, 119.68, 116.64, 72.86, 60.40, 52.86, 51.61, 24.25, 24.23, 24.12, 23.87, 14.20; 31P NMR(CDCl3, 200 MHz): δ 20.2; IR: ν 3275.18 (NH), 3271.3 (NH), 3076.5 (ArH), 1730.2 (CN), 1238.7 (P=O), 1022.2 (P-O-C) cm−1; Anal. Calcd for C21H24Cl2N3O3P: C 53.86%, H 5.17%, N 8.97%; Found. C 53.92%, H 5.29%, N 8.33%.
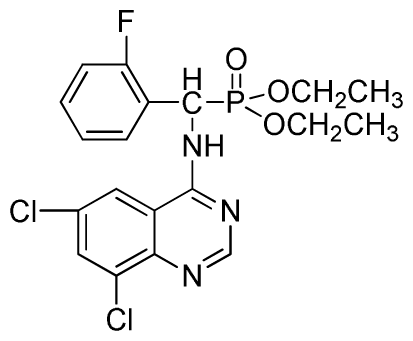
Diethyl(6,8-dichloroquinazolin-4-ylamino)(2-fluorophenyl)methylphosphonate (3v): White solid, yield 84.5%; mp 128 °C to 130 °C; 1H NMR (CDCl3, 500 MHz): δ 8.61 (s, 1H, quinazoline-2-H), 7.96 (s, 1H, N-H), 7.79 to 7.84 (m, 2H, quinazoline-5,7-H), 7.23 to 7.27 (m, 4H, Ar-H), 6.35 (d, J = 10.0 Hz, 1H, C-H), 3.82 to 4.22 (m, 4H, 2CH2), 1.16 to 1.22 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.96 (d, 1JCF = 244.8 Hz), 158.99, 154.48, 146.83, 135.32, 130.82, 130.75(d, 2JCF = 24.8 Hz), 128.61 (d, 3JCF = 7.6 Hz), 128.15 (d, 3JCF = 7.4 Hz), 122.42 (d, 2JCF = 22.8 Hz), 106.69, 77.10, 63.59, 63.46, 51.99, 50.75, 16.50, 16.46, 16.22; 31P NMR(CDCl3, 200 MHz): δ 22.3; IR: ν 3267.45 (NH), 2947.43~3068.76 (ArH), 1629.82 (CN), 1246.19 (P=O), 1022.37 (P-O-C) cm−1; Anal. Calcd for C19H19Cl2FN3O3P: C 49.80%, H 4.18%, N 9.17%; Found. C 50.26%, H 4.61%, N 9.62%.
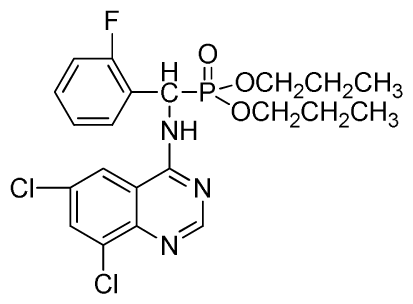
Dipropyl (6,8-dichloroquinazolin-4-ylamino)(2-fluorophenyl)methylphosphonate (3w): White solid, yield 83.4%; mp 178 °C to 180 °C; 1H NMR (CDCl3, 500 MHz): δ 8.72 (s, 1H, quinazoline-2-H), 8.12 (s, 1H, N-H), 7.80 (s, 1H, quinazoline-7-H), 7.63 (s, 1H, quinazoline-5-H), 7.26 to 7.29 (m, 4H, Ar-3,4,5,6-H), 6.27 (d, J = 8.5 Hz, 1H, C-H), 3.64 to 4.10 (m, 4H, 2OCH2), 1.48~1.80 (m, 4H, 2CH2), 0.79 to 0.81 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.46 (d, 1JCF = 246.2 Hz), 155.71, 145.50, 133.31, 130.95, 128.75 (d, 2JCF = 23.8 Hz), 128.54 (d, 3JCF = 7.4 Hz), 128.52, 128.49 (d, 3JCF = 7.5 Hz), 120.16 (d, 2JCF = 23.5 Hz), 116.83, 77.10, 69.25, 52.27, 51.03, 23.88, 23.74, 9.99, 9.94; 31P NMR(CDCl3, 200 MHz): δ 21.9; IR: ν 3275.1 (NH), 3072.6 (ArH), 1714.7 (CN), 1224.8 (P=O), 997.2 (P-O-C) cm−1; Anal. Calcd for C21H23Cl2FN3O3P: C 51.87%, H 4.77%, N 8.64%; Found. C 51.39%, H 5.21%, N 8.80%.
Diisopropyl (6,8-dichloroquinazolin-4-ylamino)(2-fluorophenyl)methylphosphonate (3x): White solid, yield 82.6%; mp 202 °C to 204 °C; 1H NMR (CDCl3, 500 MHz): δ 8.60 (s, 1H, quinazoline-2-H), 8.07 (s, 1H, N-H), 7.81 (s, 1H, N-H), 7.60 (s, 1H, quinazoline-7-H), 7.51 (s, 1H, quinazoline-5-H), 7.21 to 7.25 (m, 4H, Ar-3,4,5,6-H), 6.27 (d, J = 25.0Hz, 1H, CH), 4.60 to 4.86 (m, 2H, 2CH), 1.14 to 1.31 (m, 12H, 4CH3 ); 13C NMR (CDCl3, 125 MHz): δ 160.91, 159.06 (d, 1JCF = 246.2 Hz), 158.94, 154.56, 146.84, 135.75, 128.90 (d, 2JCF = 24.5 Hz), 128.45 (d, 3JCF = 7.4 Hz), 127.95(d, 3JCF = 7.6 Hz), 122.33, 122.14 (d, 2JCF = 23.6Hz), 116.02, 77.12, 72.50, 52.55, 24.24, 23.84, 23.27; 31P NMR(CDCl3, 200 MHz): δ 20.4; IR: ν 3269.36 (NH), 2981.91 (ArH), 1712.8 (CN), 1238.38 (P=O), 1014.66 (P-O-C) cm−1; Anal. Calcd for C21H23Cl2FN3O3P: C 51.87%, H 4.77%, N 8.64%; Found. C 51.46%, H 4.86%, N 8.66%.
4. Conclusions
In summary, an efficient and simple method for the synthesis of quinazolin-4-ylamino)methylphosphonate derivatives was developed through the reaction of N′-(substituted-2-cyanophenyl)-N,N-dimethylformamidine derivatives with dialkyl amino (phenyl)methylphosphonate under microwave irradiation. This novel method has several advantages over the other methods, such as high yield, fewer reaction steps, faster reaction rate, and easy work up without producing any significant by-product. By contrast, the classical method (Scheme 1) of quinazoline derivatives bearing α-aminophosphonate moiety formation involves long reaction times (6 h). All compounds, 3a to 3×, were fully characterized using the spectroscopic methods. Preliminary bioassays indicated that some of these compounds are also associated with good inhibitory activities against TMV at 500 mg/L. Further studies are currently underway to establish a definite structure activity relationship.
Supporting Information
ijms-13-06730-s001.pdfAcknowledgments
We extend our gratitude to the National Basic Research Program of China (No. 2010CB126105) and the National Key Technologies R&D Program of China (No. 2011BAE06B05-6) for their support in our project.
References
- Rocco, S.A.; Barbarini, J.E.; Rittner, R. Synthesis of some 4-anilinoquinazoline derivatives. Synthesis 2004, 3, 429–435. [Google Scholar]
- Yang, S.; Li, Z.; Jin, L.H.; Song, B.A.; Liu, G.; Chen, J.; Chen, Z.; Hu, D.Y.; Xue, W.; Xu, R.Q. Synthesis and bioactivity of 4-alkyl (aryl) thioquinazoline dervatives. Bioorg. Med. Chem. Lett 2007, 17, 2193–2196. [Google Scholar]
- Chandregowda, V.; Kush, A.K.; Reddy, G.C. Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur. J. Med. Chem 2008, 44, 1–10. [Google Scholar]
- Berger, M.; Albrecht, B.; Berces, A.; Ettmayer, P.; Neruda, W.; Woisetschläger, M. S(+)-4-(1-Phenylethylamino)quinazolines as inhibitors of human immunoglobulin E synthesis: Potency is dictated by stereochemistry and atomic point charges at N-1. J. Med. Chem 2001, 44, 3031–3038. [Google Scholar]
- Madapa, S.; Tusi, Z.; Mishra, A.; Srivastava, K.; Pandey, S.K. Synthesis and antimalarial activity of new 6-ureido-4-anilinoquinazolines. Bioorg. Med. Chem 2009, 17, 222–234. [Google Scholar]
- Liu, X.H.; Jing, Z.; Zhou, A.N.; Song, B.A.; Zhu, H.L.; Bai, L.S.; Bhadury, P.S.; Pan, C.X. Synthesis, structure and antibacterial activity of new 2-(1-(2-(substituted-phenyl)-5-methyloxazol- 4-yl)-3-(2-substitued-phenyl)-4,5-dihydro-1H-pyrazol-5-l)-7-substitued-1,2,3,4 tetrahydroisoquinoline derivatives. Bioorg. Med. Chem 2009, 17, 1207–1213. [Google Scholar]
- Zhang, Y.P.; Bai, S.; Song, B.A.; Bhadury, P.S.; Hu, D.Y.; Yang, S.; Zhang, X.Y.; Fan, H.T.; Lu, P. Enantioseparation and plant virucidal bioactivity of new quinazoline derivatives with α-aminophosphonate moiety. J. Chromatogr. B 2010, 878, 1285–1289. [Google Scholar]
- Gao, X.W.; Cai, X.J.; Yan, K.; Song, B.A.; Gao, L.L.; Chen, Z. Synthesis and antiviral bioactivities of 2-phenyl-3-(substituted-benzalamino)-4(3H)-quinazolinone derivatives. Molecules 2007, 12, 2621–2642. [Google Scholar]
- Bridges, A.J. Chemical inhibitors of protein kinases. Chem. Rev 2001, 101, 2541–2572. [Google Scholar]
- Tsou, H.R.; Mamuya, N.; Johnson, B.D.; Reich, M.F.; Gruber, B.C.; Ye, F.; Nilakantan, R.; Shen, R.; Discafani, C.; DeBlanc, R.; et al. 6-substituted-4-(3-bromophenylamino)quinazolines as putative irreversible inhibitors of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER-2) Tyrosine kinase with enhanced antitumor activity. J. Med. Chem 2001, 44, 2719–2734. [Google Scholar]
- Chen, M.H.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Cai, X.J.; Hu, D.Y.; Xue, W.; Zeng, S. Synthesis and antiviral activities of chiral thiourea derivatives containing an α-aminophosphonate moiety. J. Agric. Food Chem 2009, 57, 1383–1388. [Google Scholar]
- Jin, L.H.; Song, B.A.; Zhang, G.P.; Xu, R.Q.; Zhang, S.M.; Gao, X.W.; Hu, D.Y.; Yang, S. Synthesis, X-ray crystallographic analysis and antitumor activity of N-(benzothiazole-2-yl)-1-(fluorophenyl)- O,O-dialkyl- α-aminophosphonates. Bioorg. Med. Chem. Lett. 2006, 16, 1537–1543. [Google Scholar]
- Fan, H.T.; Song, B.A.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Yang, S. Antiviral activity and mechanism of action of novel thiourea containing chiral phosphonate on tobacco mosaic virus. Int. J. Mol. Sci 2011, 12, 4522–4535. [Google Scholar]
- Li, C.H.; Song, B.A.; Yan, K.; Xu, G.F.; Hu, D.Y.; Yang, S.; Jin, L.H.; Xue, W.; Lu, P. One pot synthesis of α-aminophosphonates containing bromo- and 3,4,5-trimethoxybenzyl groups under solvent-free conditions. Molecules 2007, 12, 163–172. [Google Scholar]
- Xu, Y.S.; Yan, K.; Song, B.A.; Xu, G.F.; Yang, S.; Xue, W.; Hu, D.Y.; Lu, P.; Ouyang, G.P.; Jin, L.H.; Chen, Z. Synthesis and antiviral bioactivities of α-aminophosphonates containing alkoxyethyl moieties. Molecules 2006, 11, 666–676. [Google Scholar]
- Luo, H. The Synthesis and biological Activity of the Novel Quinazoline Containing α-amino Phosphate and Fluoride Sulfide. Master Thesis, Guizhou University, Guiyang, China, June 2008. [Google Scholar]
- Mavandadi, F.; Lidstrom, P. Microwave-assisted chemistry in drug discovery. Curr. Top. Med. Chem 2004, 4, 773–792. [Google Scholar]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett 1986, 27, 279–282. [Google Scholar]
- Liu, Y.P.; Yin, D.C.; Chen, H.T.; Sun, B.G. Rapid synthesis of flavor compound 4-ethyloctanoic acid under microwave irradiation. Int. J. Mol. Sci 2010, 11, 4165–4174. [Google Scholar]
- Cleophax, J.; Liagre, M.; Loupy, A.; Petit, A. Application of focused microwaves to the scale-up of solvent-free organic reactions. Org. Process Res. Dev 2000, 4, 498–504. [Google Scholar]
- Perreux, L.; Loupy, A. A tentative realization of microwave effects in organic synthesis according to the reaction medium and mechanistic consideration. Tetrahedron 2001, 57, 9199–9223. [Google Scholar]
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis, a review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar]
- Zhao, H.; Song, Z.Y.; Cowins, J.V.; Olubajo, O. Microwave-assisted esterification of N-Acetyl-l-Phenylalanine using modified mukaiyama’s reagents: A new approach involving ionic liquids. Int. J. Mol. Sci 2008, 9, 33–44. [Google Scholar]
- Lin, Y.J.; Le, G.W.; Wang, J.Y.; Li, Y.X.; Shi, Y.H.; Sun, J. Antioxidative peptides derived from enzyme hydrolysis of bone collagen after microwave assisted acid pre-treatment and nitrogen protection. Int. J. Mol. Sci 2010, 11, 4297–4308. [Google Scholar]
- Santagada, V.; Perissutti, E.; Caliendo, G. The application of microwave irradiation as new convenient synthetic procedure in drug discovery. Curr. Med. Chem 2002, 9, 1251–1283. [Google Scholar]
- Hinou, H.; Saito, N.; Ogawa, M.; Maeda, T.; Nishimura, S.I. Microwave effect for glycosylation promoted by solid super acid in supercritical carbon dioxide. Int. J. Mol. Sci 2009, 10, 5285–5295. [Google Scholar]
- Yoon, D.S.; Han, Y.; Stark, T.M.; Haber, J.C.; Gregg, B.T.; Stankovich, S.B. Efficient synthesis of 4-aminoquinazoline and thieno[3,2-d]pyrimidin-4-ylamine derivatives by microwave irradiation. Org. Lett 2004, 6, 4775–4778. [Google Scholar]
- Zhang, Y.D.; Yao, C.; Houghten, R.A.; Yu, Y.P. Solid-phase synthesis of 4,8-disubstituted-8, 9-dihydropyrazino [2,3-g]quinazolin7(6H)-ones. Pept. Sci 2007, 3, 439–443. [Google Scholar]
- Liu, G.; Yang, S.; Song, B.A.; Xue, W.; Hu, D.Y.; Jin, L.H.; Lu, P. Microwave assisted synthesis of N-arylheterocyclic substituted-4-aminoquinazoline derivatives. Molecules 2006, 11, 272–278. [Google Scholar]
- Gao, X.; Cai, X.; Yan, K.; Song, B.; Gao, L.; Chen, Z. Synthesis and antiviral bioactivities of 2-aryl- or 2-methyl-3-(substituted-Benzalamino)-4(3H)-quinazolinone derivatives. Molecules 2007, 12, 2621–2642. [Google Scholar]
- Kaboudin, B.; Moradi, K. A simple and convenient procedure for the synthesis of 1-aminophosphonates from aromatic aldehydes. Tetrahedron Lett 2005, 46, 2989–2991. [Google Scholar]
- Gooding, G.V., Jr; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).