Antioxidative Characteristics of Anisomeles indica Extract and Inhibitory Effect of Ovatodiolide on Melanogenesis
Abstract
:1. Introduction
2. Results and Discussion
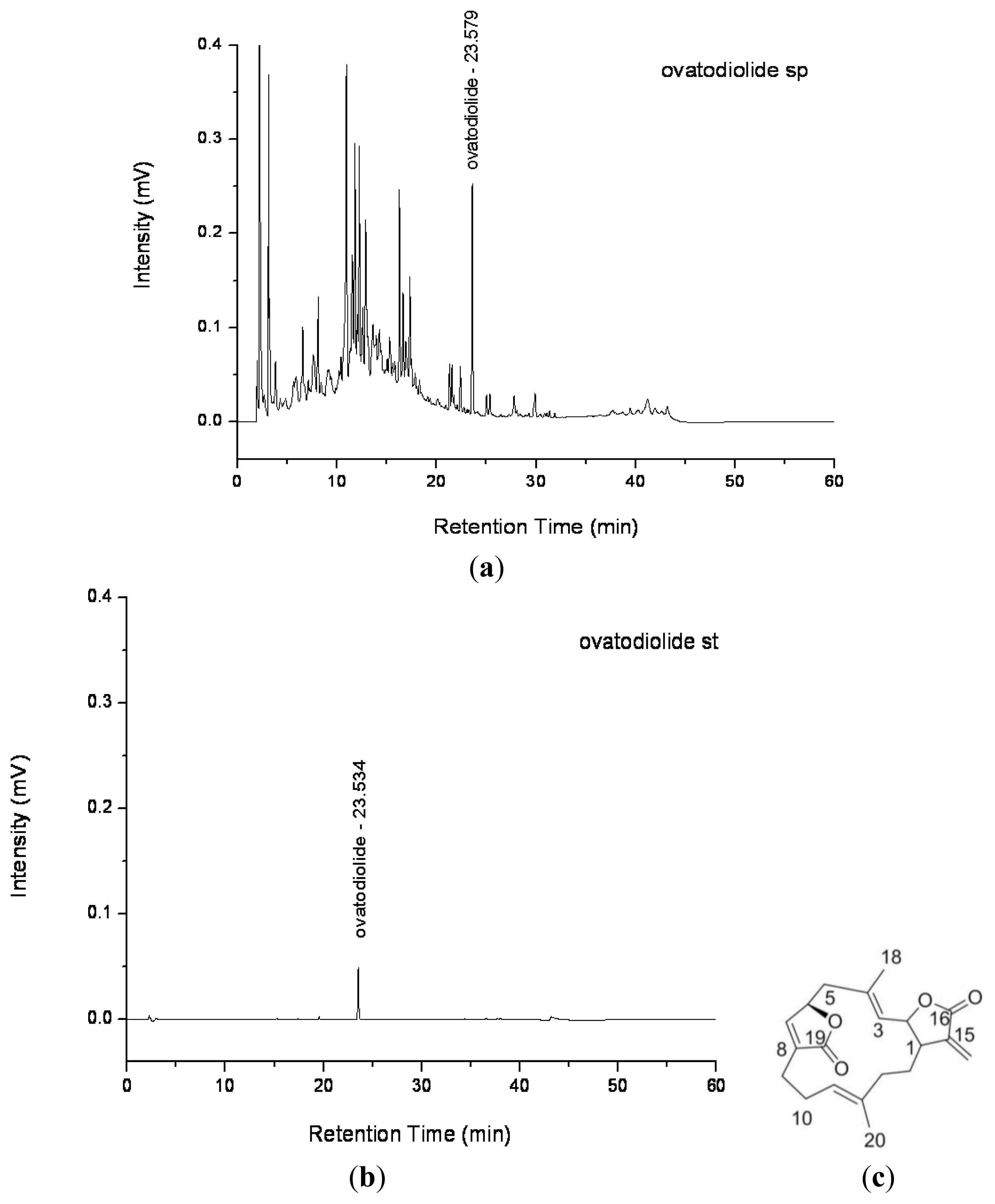
2.1. Purification of Ovatodiolide from A. indica Methanol Extract
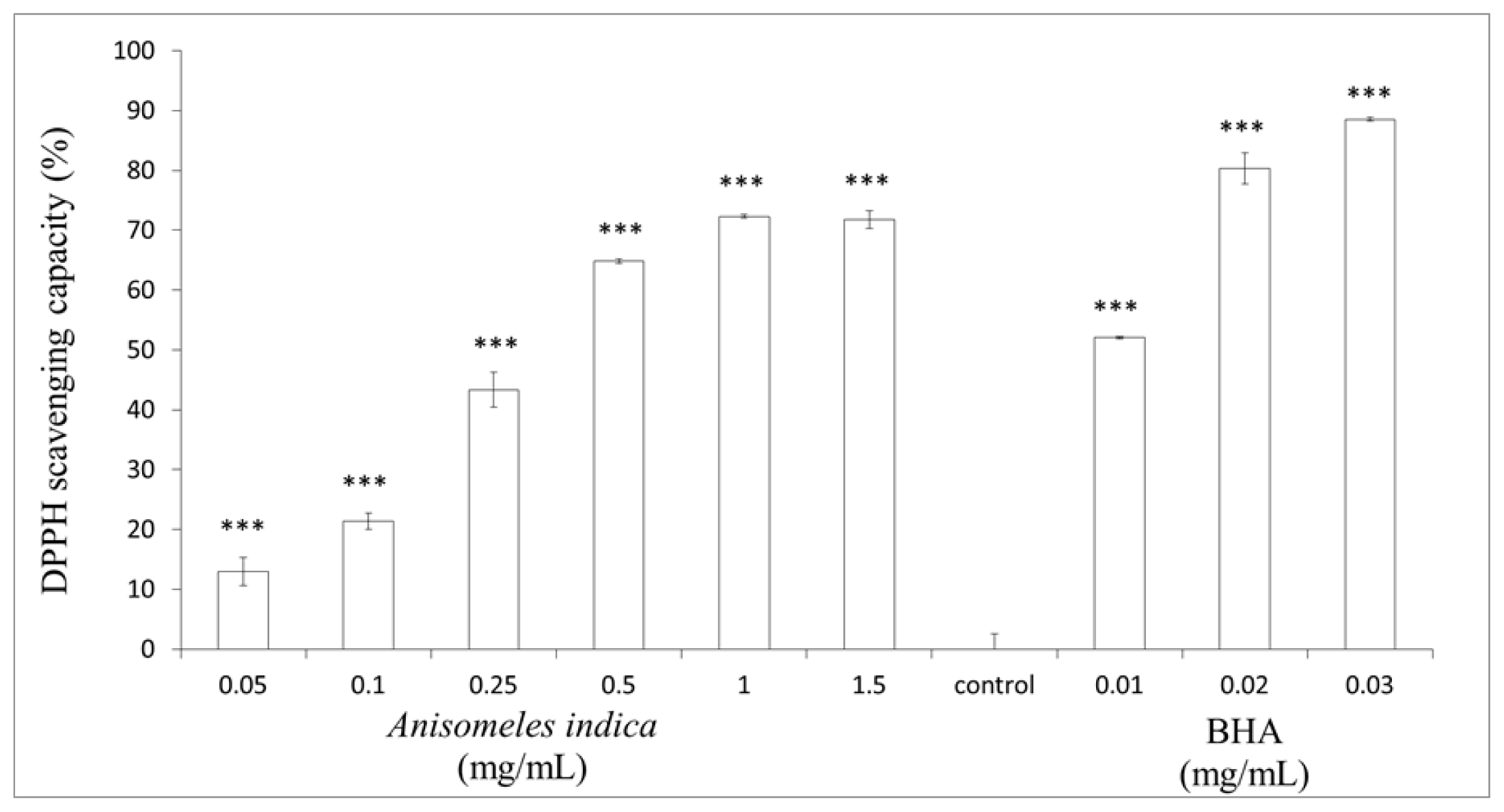
2.2. Determination of DPPH Scavenging Activity
2.3. Determination of ABTS+ Radical Scavenging Capacity
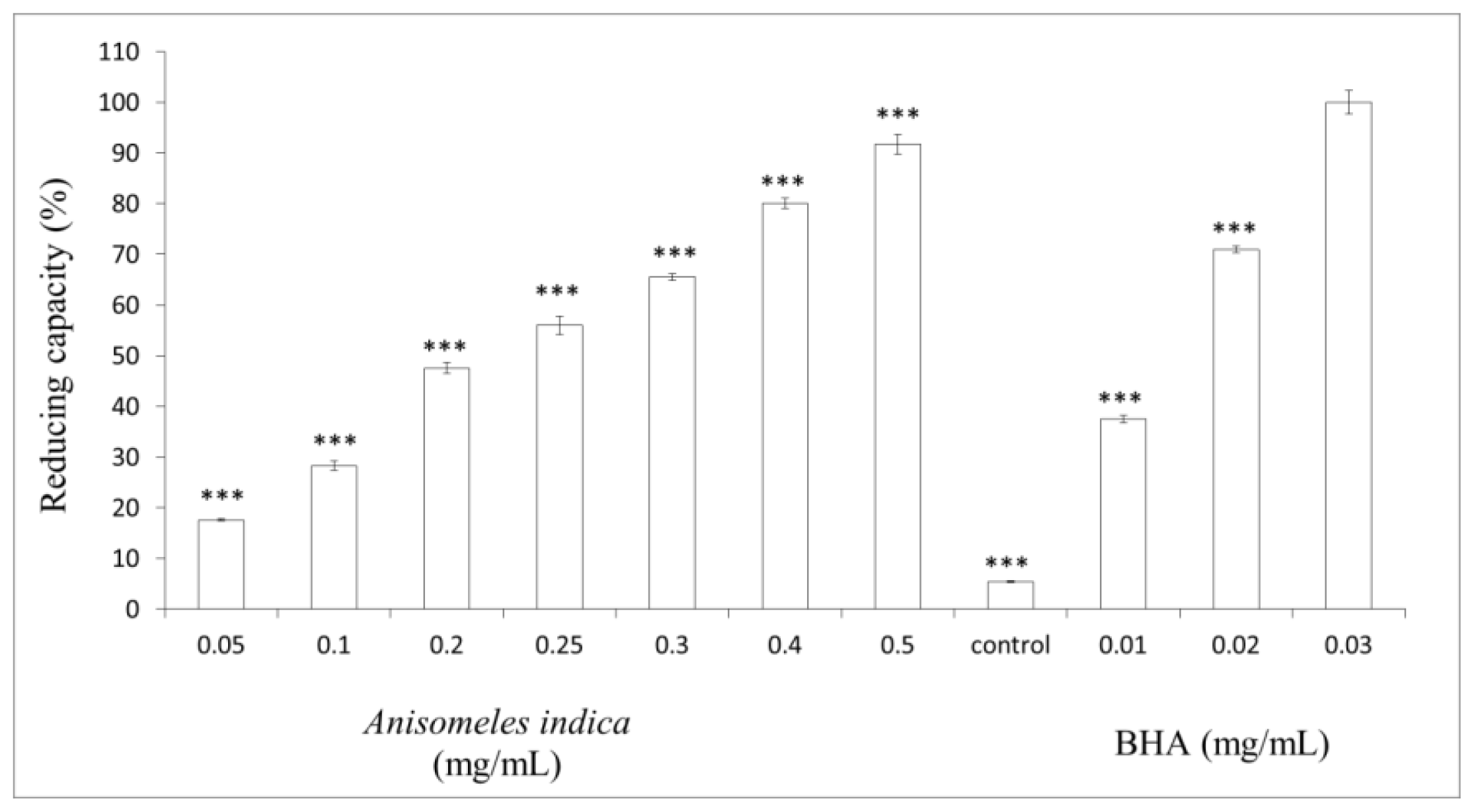
2.4. Measurement of Reducing Capacity
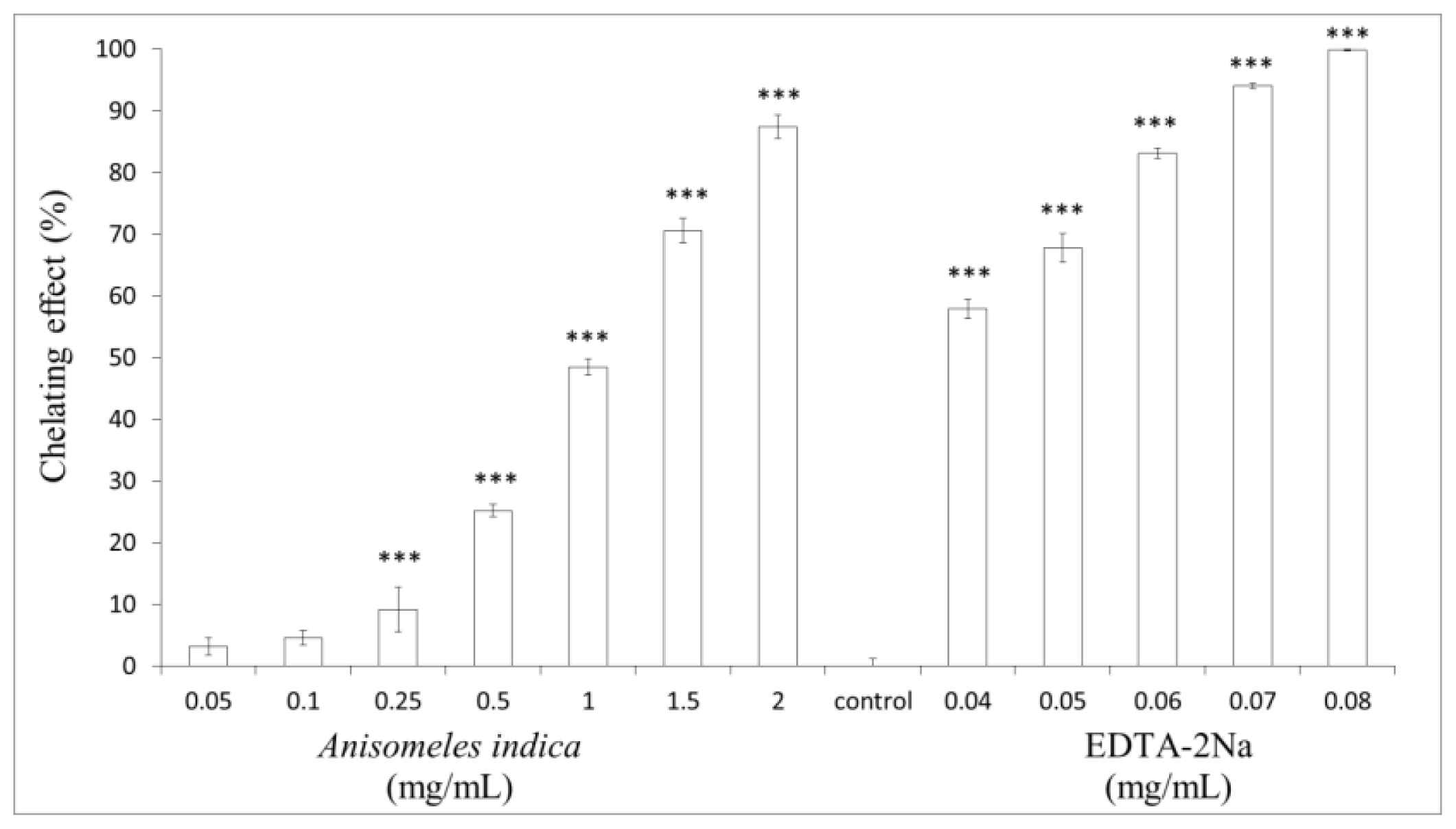
2.5. Measurement of Metal Ion-Chelating Capacity
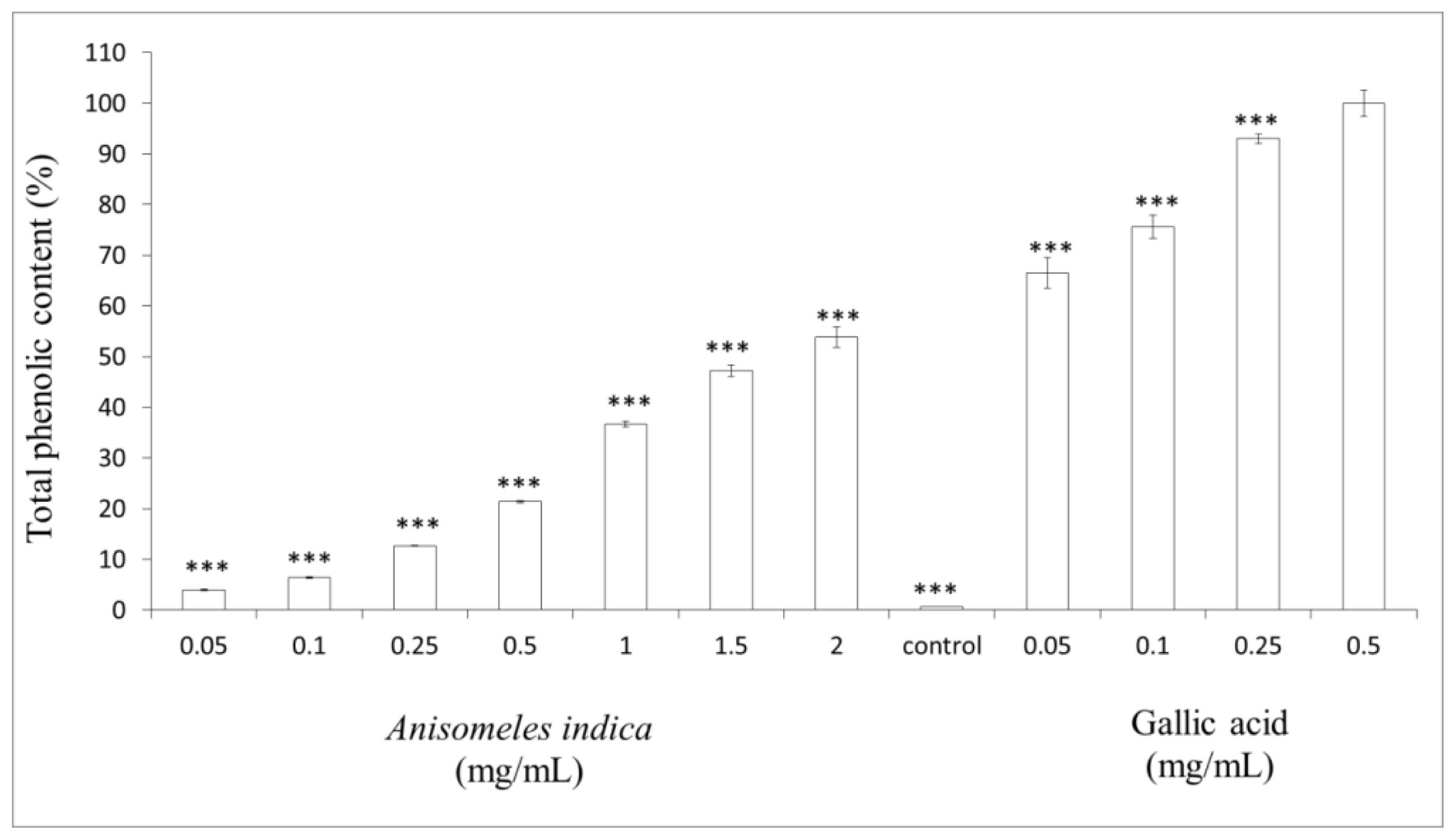
2.6. Measurement of Total Phenol Content
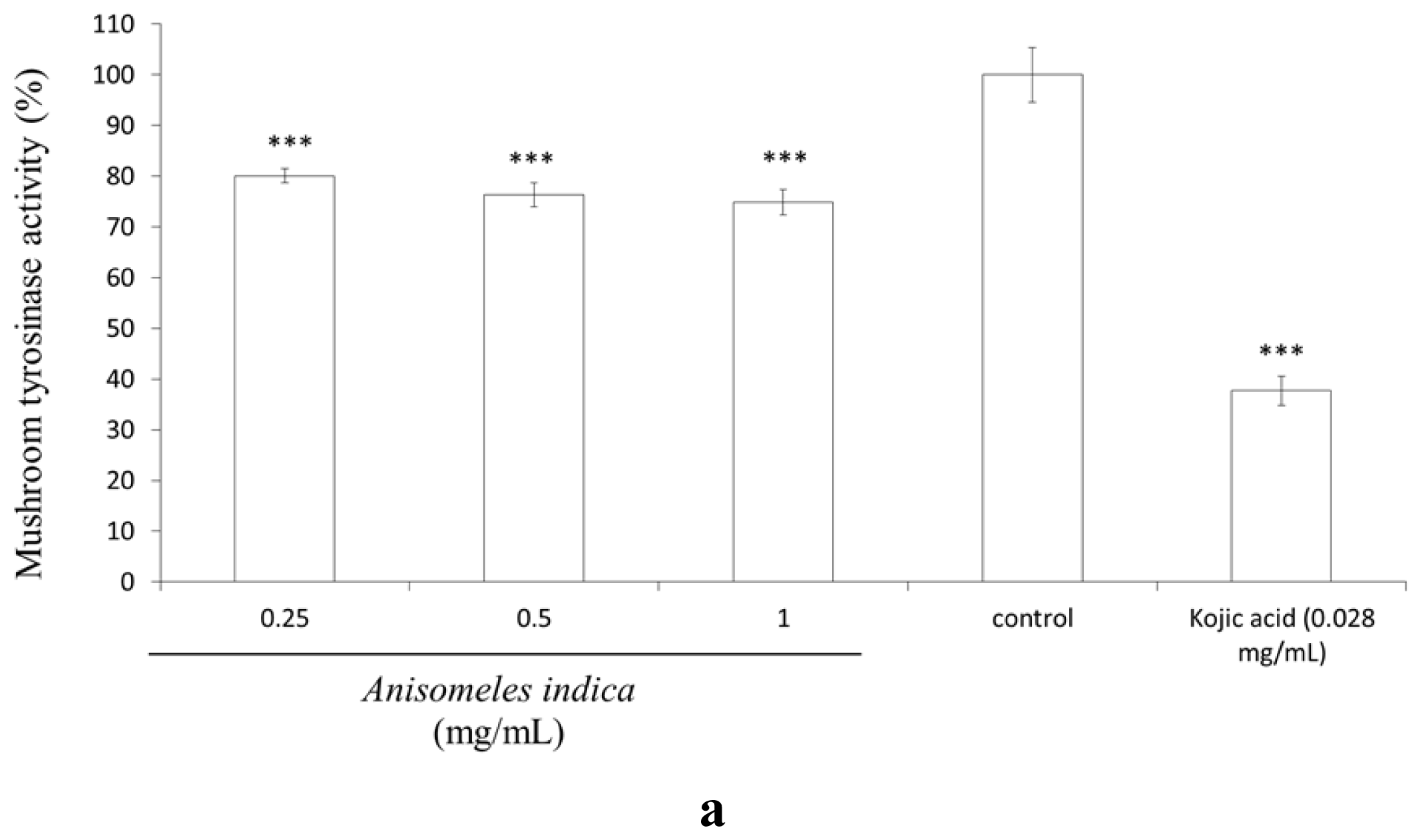
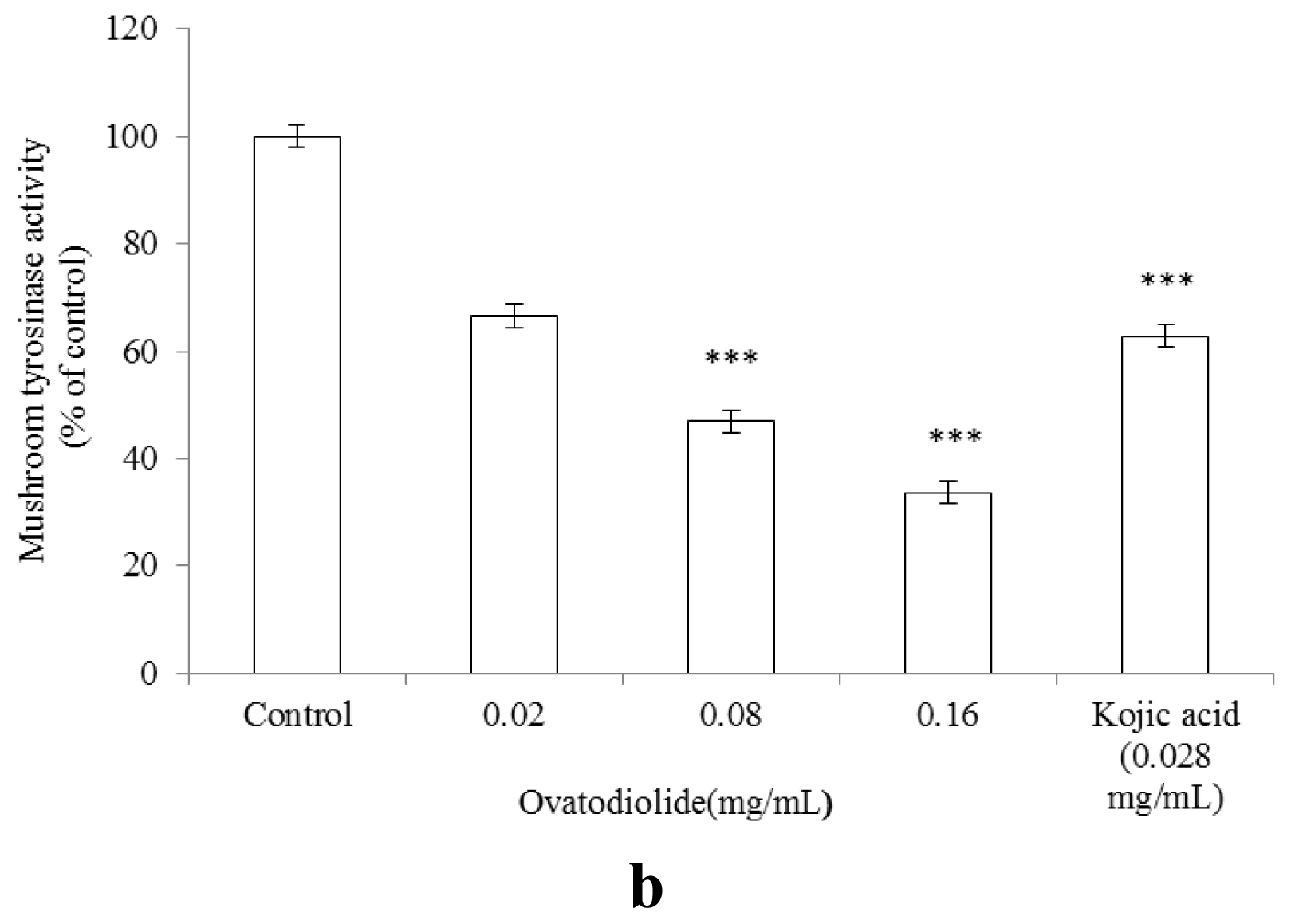
2.7. Inhibitory Effect of A. indica Methanol Extract and Ovatodiolide on Mushroom Tyrosinase Activity
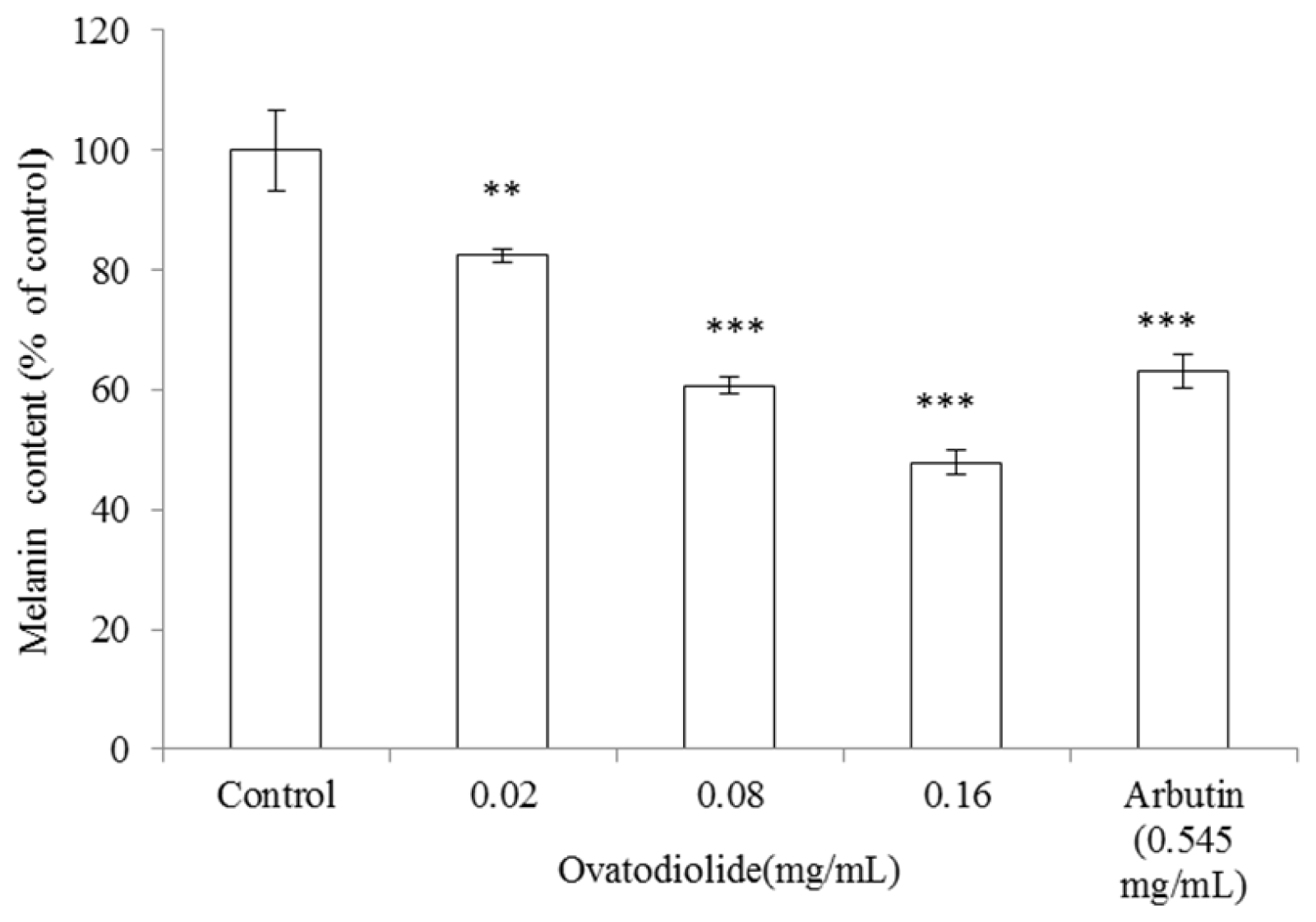
2.8. Inhibitory Effect of Ovatodiolide on Melanin Production
2.9. Inhibitory Effect of Ovatodiolide on B16F10 Tyrosinase Activity
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Plant Material, Extraction and Isolation of Pure Compound
3.3. Cell Culture
3.4. DPPH Scavenging Activity Assay
3.5. ABTS+ Scavenging Capacity Assay
3.6. Determination of Reducing Capacity
3.7. Measurement of Metal-Ion Chelating Capacity
3.8. Determination of Total Phenolic Content
3.9. Assay of Mushroom Tyrosinase Activity
3.10. Measurement of Melanin Content
3.11. Assay of B16F10 Intracellular Tyrosinase Activity
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Bergendi, L.; Benes, L.; Durackova, Z.; Ferencik, M. Chemistry, physiology and pathology of free radicals. Life Sci 1999, 65, 1865–1874. [Google Scholar]
- Darr, D.; Fridovich, I. Free radicals in cutaneous biology. J. Invest. Dermatol 1994, 102, 671–675. [Google Scholar]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol 2001, 54, 176–186. [Google Scholar]
- Sies, H.; Stahl, W. Nutritional protection against skin damage from sunlight. Annu. Rev. Nutr 2004, 24, 173–200. [Google Scholar]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev 2004, 84, 1155–1228. [Google Scholar]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res 2003, 16, 101–110. [Google Scholar]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem 2003, 51, 2837–2853. [Google Scholar]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res 2012, 25, 14–27. [Google Scholar]
- Hearing, V.J.; Jimenez, M. Mammalian tyrosinase—The critical regulatory control point in melanocyte pigmentation. Int. J. Biochem 1987, 19, 1141–1147. [Google Scholar]
- Jimenez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; Garcia-Borron, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J. Biol. Chem 1994, 269, 17993–18000. [Google Scholar]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J 1992, 11, 519–526. [Google Scholar]
- Funasaka, Y.; Komoto, M.; Ichihashi, M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res 2000, 13, 170–174. [Google Scholar]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res 2003, 16, 629–638. [Google Scholar]
- Imokawa, G. Analysis of initial melanogenesis including tyrosinase transfer and melanosome differentiation through interrupted melanization by glutathione. J. Invest. Dermatol 1989, 93, 100–107. [Google Scholar]
- Kumano, Y.; Sakamoto, T.; Egawa, M.; Iwai, I.; Tanaka, M.; Yamamoto, I. In vitro and in vivo prolonged biological activities of novel vitamin C derivative, 2-O-alpha-d-glucopyranosyl-l-ascorbic acid (AA-2G), in cosmetic fields. J. Nutr. Sci. Vitaminol 1998, 44, 345–359. [Google Scholar]
- Huang, T.C.; Hsieh, C.F.; Boufford, D.E.; Kuoh, C.S.; Ohashi, H.; Peng, C.I.; Tsai, J.L.; Yang, K.C.; Hsiao, A.; Tsai, J.M. Flora of Taiwan; National Science Council of the Republic of China: Taiwan, 2003. [Google Scholar]
- Chen, Y.L.; Lan, Y.H.; Hsieh, P.W.; Wu, C.C.; Chen, S.L.; Yen, C.T.; Chang, F.R.; Hung, W.C.; Wu, Y.C. Bioactive cembrane diterpenoids of Anisomeles indica. J. Nat. Prod 2008, 71, 1207–1212. [Google Scholar]
- Hsieh, S.C.; Fang, S.H.; Rao, Y.K.; Tzeng, Y.M. Inhibition of pro-inflammatory mediators and tumor cell proliferation by Anisomeles indica extracts. J. Ethnopharmacol 2008, 118, 65–70. [Google Scholar]
- Rao, Y.K.; Fang, S.H.; Hsieh, S.C.; Yeh, T.H.; Tzeng, Y.M. The constituents of Anisomeles indica and their anti-inflammatory activities. J. Ethnopharmacol 2009, 121, 292–296. [Google Scholar]
- Dharmasiri, M.G.; Ratnasooriya, W.D.; Thabrew, M.I. Anti-inflammatory activity of decoctions of leaves and stems of Anisomeles indica at preflowering and flowering stages. Pharm. Biol 2002, 40, 433–439. [Google Scholar]
- Dharmasiri, M.G.; Ratnasooriya, W.D.; Thabrew, M.I. Water extract of leaves and stems of preflowering but not flowering plants of Anisomeles indica possesses analgesic and antihyperalgesic activities in rats. Pharm. Biol 2003, 41, 37–44. [Google Scholar]
- Wang, Y.C.; Huang, T.L. Screening of anti-Helicobacter pylori herbs deriving from Taiwanese folk medicinal plants. FEMS Immunol. Med. Microbiol 2005, 43, 295–300. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem 2005, 53, 1841–1856. [Google Scholar]
- Chen, X.H.; Xia, L.X.; Zhou, H.B.; Qiu, G.Z. Chemical composition and antioxidant activities of Russula griseocarnosa sp. nov. J. Agric. Food Chem 2010, 58, 6966–6971. [Google Scholar]
- Shahidul Alam, M.; Quader, M.A.; Rashid, M.A. HIV-inhibitory diterpenoid from Anisomeles indica. Fitoterapia 2000, 71, 574–576. [Google Scholar]
- Lin, K.L.; Tsai, P.C.; Hsieh, C.Y.; Chang, L.S.; Lin, S.R. Antimetastatic effect and mechanism of ovatodiolide in MDA-MB-231 human breast cancer cells. Chem. Biol. Interact 2011, 194, 148–158. [Google Scholar]
- Hou, Y.Y.; Wu, M.L.; Hwang, Y.C.; Chang, F.R.; Wu, Y.C.; Wu, C.C. The natural diterpenoid ovatodiolide induces cell cycle arrest and apoptosis in human oral squamous cell carcinoma Ca9-22 cells. Life Sci 2009, 85, 26–32. [Google Scholar]
- Rao, Y.K.; Lien, H.-M.; Lin, Y.-H.; Hsu, Y.-M.; Yeh, C.-T.; Chen, C.-C.; Lai, C.-H.; Tzeng, Y.-M. Antibacterial activities of Anisomeles indica constituents and their inhibition effect on Helicobacter pylori-induced inflammation in human gastric epithelial cells. Food Chem 2012, 132, 780–787. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol 1995, 28, 25–30. [Google Scholar]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric 1998, 76, 270–276. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med 1999, 26, 1231–1237. [Google Scholar]
- Oyaizu, M. Studies on products of browing reaction: Antioxidative activity of product of browing reaction preapared from glucosamine. Jpn. J. Nutr 1986, 44, 307–315. [Google Scholar]
- Dinis, T.C.P.; Maderia, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys 1994, 315, 161–169. [Google Scholar]
- Lister, E.; Wilson, P. Measurement of Total Phenolics and ABTS Assay for Antioxidant Activity in Lincoln; Crop Research Institute: Lincoln, New Zealand, 2001. [Google Scholar]
- Bilodeau, M.L.; Greulich, J.D.; Hullinger, R.L.; Bertolotto, C.; Ballotti, R.; Andrisani, O.M. BMP-2 stimulates tyrosinase gene expression and melanogenesis in differentiated melanocytes. Pigment Cell Res. 2001, 14, 328–336. [Google Scholar]
- Tsuboi, T.; Kondoh, H.; Hiratsuka, J.; Mishima, Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res 1998, 11, 275–282. [Google Scholar]
- Yang, J.Y.; Koo, J.H.; Song, Y.G.; Kwon, K.B.; Lee, J.H.; Sohn, H.S.; Park, B.H.; Jhee, E.C.; Park, J.W. Stimulation of melanogenesis by scoparone in B16 melanoma cells. Acta Pharmacol. Sin 2006, 27, 1467–1473. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, H.-C.; Lien, H.-M.; Ke, H.-J.; Chang, L.-L.; Chen, C.-C.; Chang, T.-M. Antioxidative Characteristics of Anisomeles indica Extract and Inhibitory Effect of Ovatodiolide on Melanogenesis. Int. J. Mol. Sci. 2012, 13, 6220-6235. https://doi.org/10.3390/ijms13056220
Huang H-C, Lien H-M, Ke H-J, Chang L-L, Chen C-C, Chang T-M. Antioxidative Characteristics of Anisomeles indica Extract and Inhibitory Effect of Ovatodiolide on Melanogenesis. International Journal of Molecular Sciences. 2012; 13(5):6220-6235. https://doi.org/10.3390/ijms13056220
Chicago/Turabian StyleHuang, Huey-Chun, Hsiu-Man Lien, Hui-Ju Ke, Li-Ling Chang, Chia-Chang Chen, and Tsong-Min Chang. 2012. "Antioxidative Characteristics of Anisomeles indica Extract and Inhibitory Effect of Ovatodiolide on Melanogenesis" International Journal of Molecular Sciences 13, no. 5: 6220-6235. https://doi.org/10.3390/ijms13056220
APA StyleHuang, H.-C., Lien, H.-M., Ke, H.-J., Chang, L.-L., Chen, C.-C., & Chang, T.-M. (2012). Antioxidative Characteristics of Anisomeles indica Extract and Inhibitory Effect of Ovatodiolide on Melanogenesis. International Journal of Molecular Sciences, 13(5), 6220-6235. https://doi.org/10.3390/ijms13056220




