A Comparative Analysis of the Photo-Protective Effects of Soy Isoflavones in Their Aglycone and Glucoside Forms
Abstract
:1. Introduction
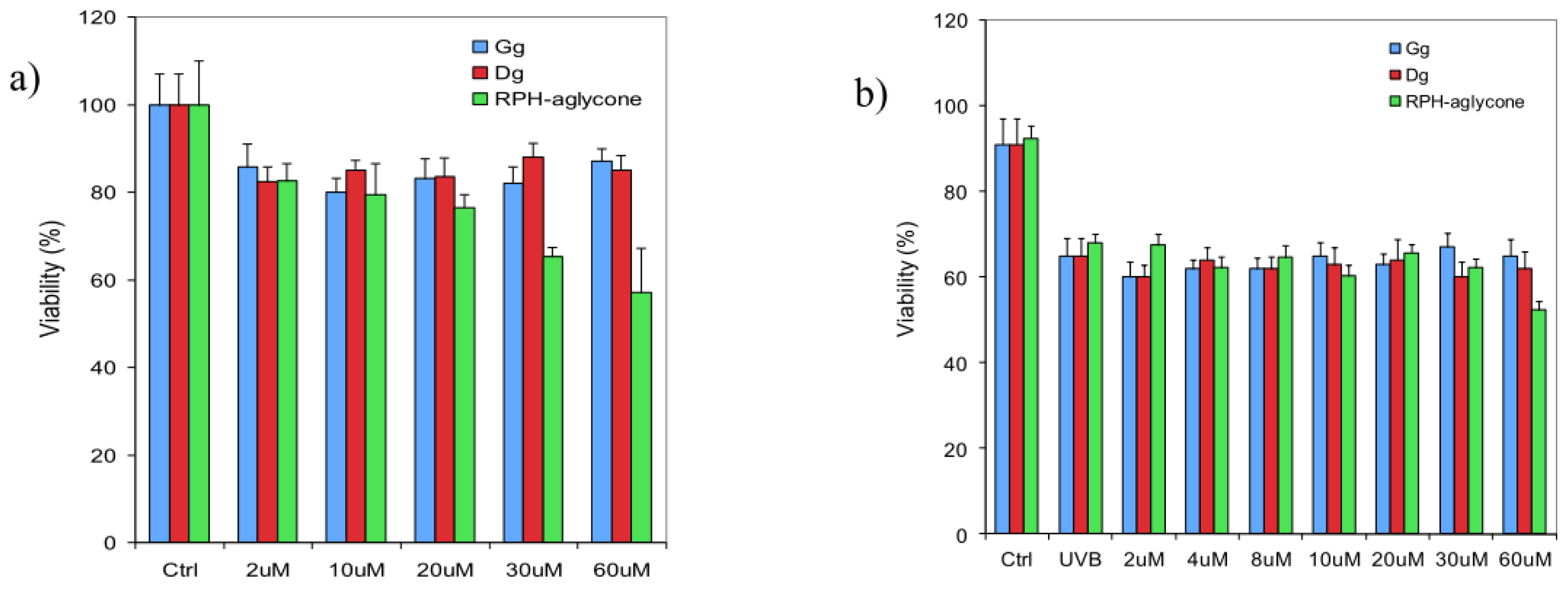
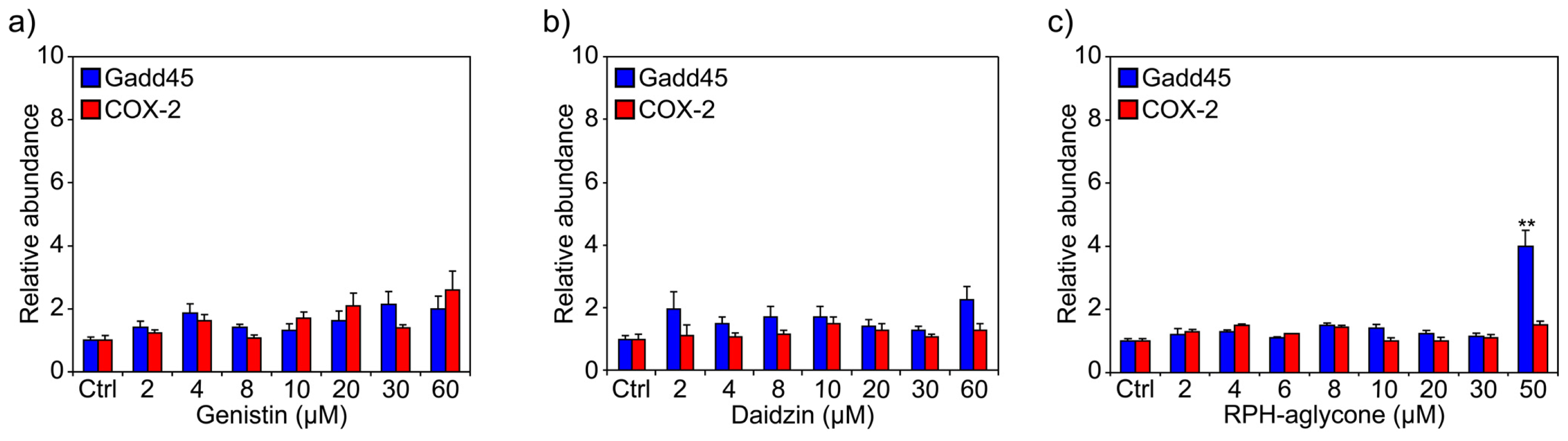
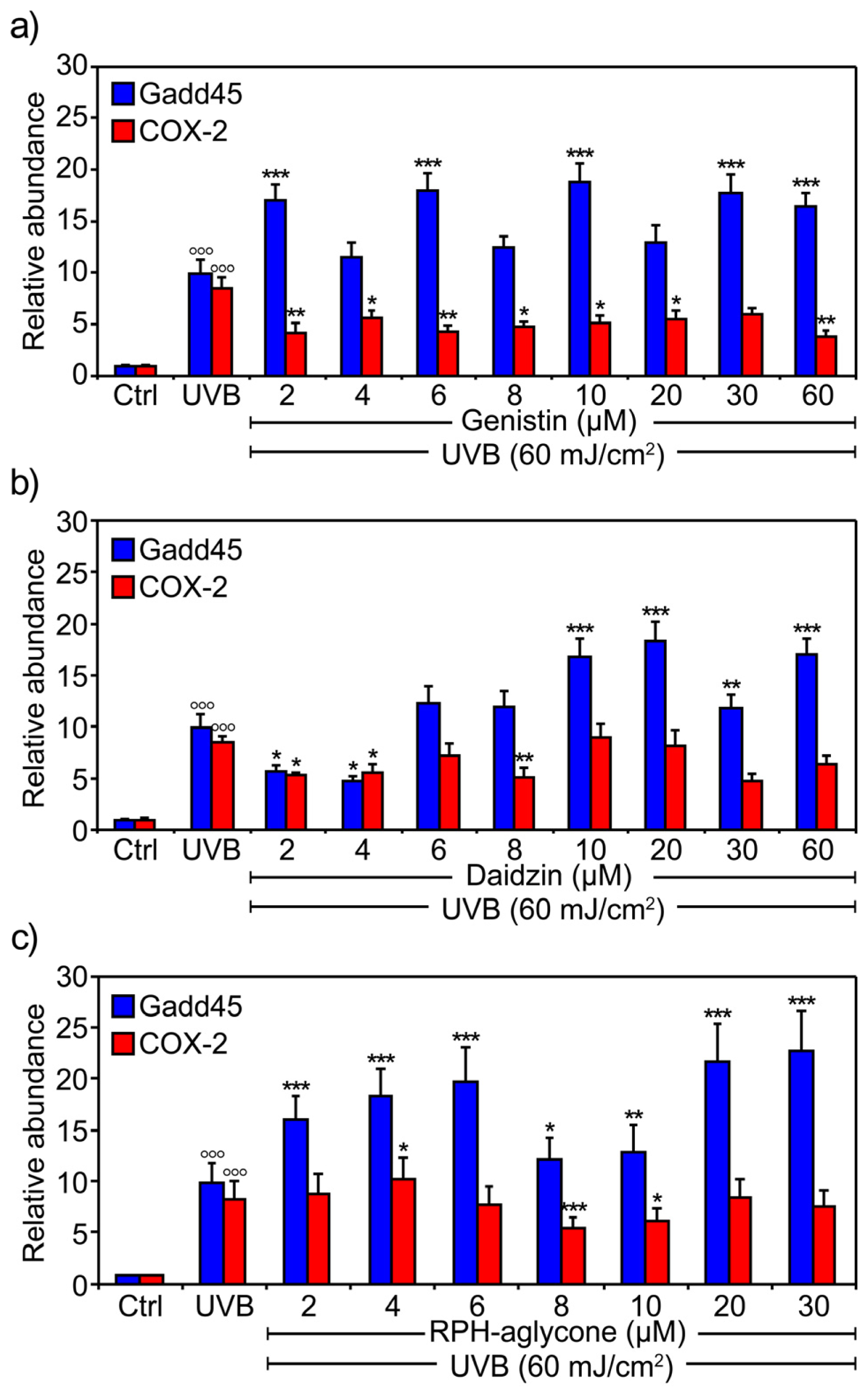
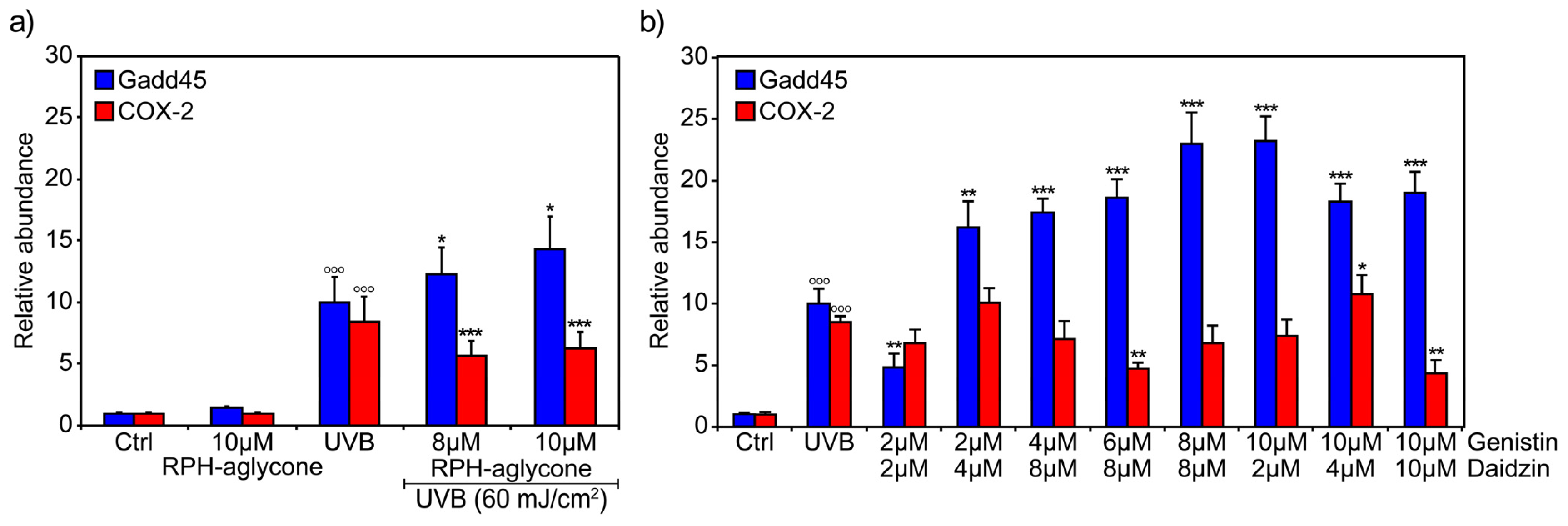
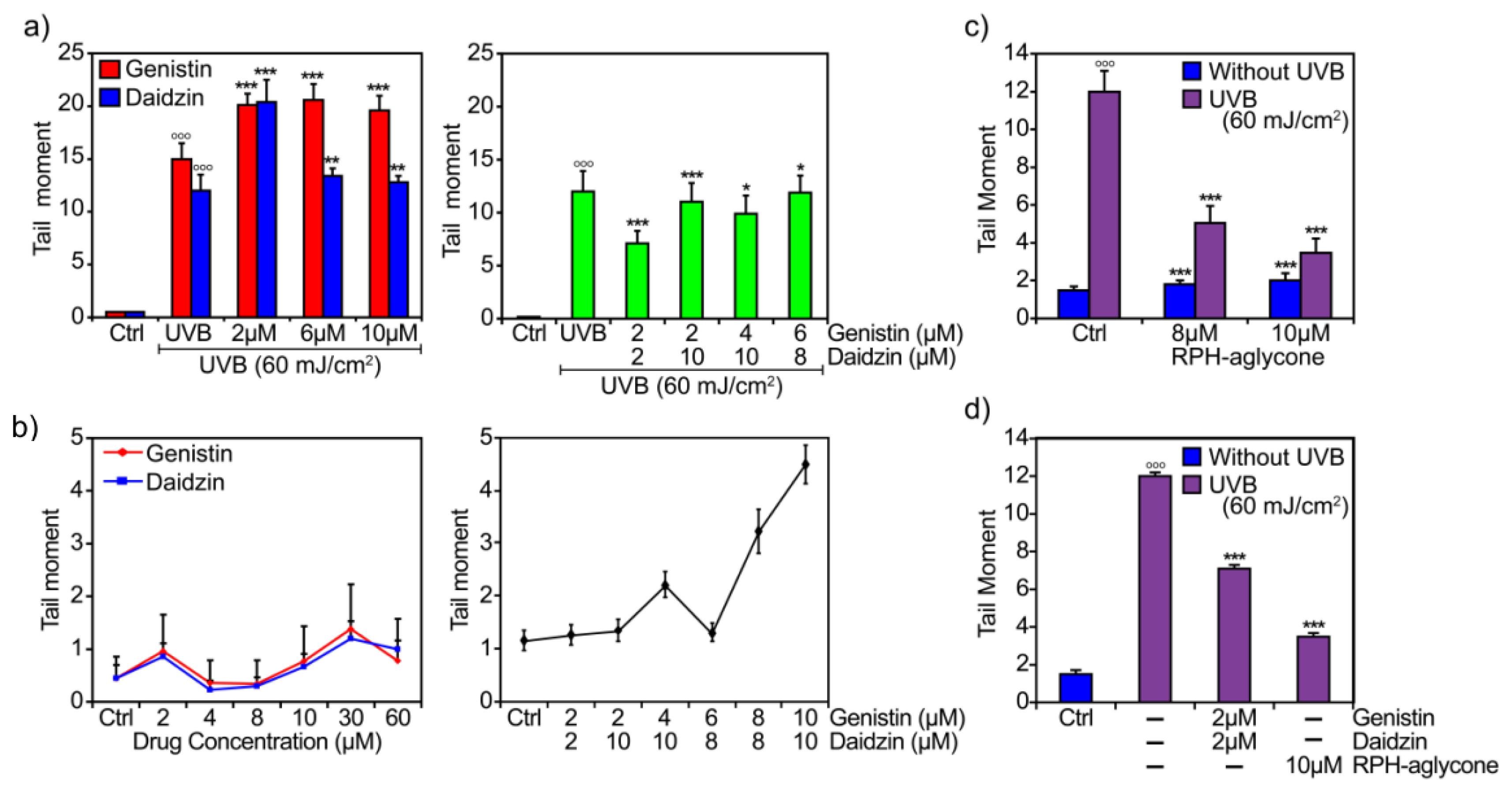
2. Results
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Chemicals
4.3. Treatment with Genistin and/or Daidzin or with RPH-aglycone and UVB Irradiation
4.4. Determination of Cell Viability
4.5. RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
- Gadd45 forward: 5′-AGACCCCGGACCTGCACT-3′
- Gadd45 reverse: 5′-CCGGCAAAAACAAATAAGTTGACT-3′
- COX-2 forward: 5′-CCTGGCGCTCAGCCATAC-3′
- COX-2 reverse: 5′-GGTACAATCGCACTTATACTGGTCAA-3′
- actin forward: 5′-CCTCACCCTGAAGTACCCCA-3′
- actin reverse: 5′-TCGTCCCAGTTGGTGACGAT-3′
4.6. Single-Cell Gel Electrophoresis (Comet Assay)
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
- Conflict of InterestThese authors disclose the following: Gasparri is an advisor for Rottapharm-Madaus and Giannini is an Dermo-Cosmetic R & D Specialist for Rottapharm-Madaus. The remaining authors disclose no conflicts.
References
- Messina, M. A brief historical overview of the past two decades of soy and isoflavone research. J. Nutr 2010, 140, 1350S–1354S. [Google Scholar]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr 2000, 130, 1695–1699. [Google Scholar]
- Zubik, L.; Meydani, M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am. J. Clin. Nutr 2003, 77, 1459–1465. [Google Scholar]
- Setchell, K.D.R.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans and supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar]
- Theil, C.; Briese, V.; Gerber, B.; Richter, D.U. The effects of different lignans and isoflavones, tested as aglycones and glycosides, on hormone receptor-positive and -negative breast carcinoma cells in vitro. Arch. Gynecol. Obstet 2011, 284, 459–465. [Google Scholar]
- Nielsen, I.L.; Williamson, G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr. Cancer 2007, 57, 1–10. [Google Scholar]
- Widyarini, S.; Domanski, D.; Painter, N.; Reeve, V.E. Photoimmune protective effect of the phytoestrogenic isoflavonoid equol is partially due to its antioxidant activities. Photochem. Photobiol. Sci 2012, 11, 1186–1192. [Google Scholar]
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Monfrecola, G.; Bevilacqua, M.A. Synergic effect of genistein and daidzein on UVB-induced DNA damage: An effective photoprotective combination. J. Biomed. Biotechnol 2011, 43, 1664–1667. [Google Scholar]
- Andlauer, W.; Kolb, J.; Furst, P. Isoflavones from tofu are absorbed and metabolized in the isolated rat small intestine. J. Nutr 2000, 130, 3021–3027. [Google Scholar]
- Russo, A.; Cardile, V.; Lombardo, L.; Vanella, L.; Acquaviva, R. Genistin inhibits UV light-induced plasmid DNA damage and cell growth in human melanoma cells. J. Nutr. Biochem. 2006, 17, 103–108. [Google Scholar]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr 2003, 133, 3811S–3819S. [Google Scholar]
- Wen, K.C.; Lin, S.P.; Yu, C.P.; Chiang, H.M. Comparison of puerariae radix and its hydrolysate on stimulation of hyaluronic acid production in NHEK cells. Am. J. Chin. Med 2010, 38, 143–155. [Google Scholar]
- Español, A.J.; Goren, N.; Ribeiro, M.L.; Sales, M.E. Nitric oxide synthase 1 and cyclooxygenase-2 enzymes are targets of muscarinic activation in normal and inflamed NIH3T3 cells. Inflamm. Res 2010, 59, 227–238. [Google Scholar]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem 2000, 69, 145–182. [Google Scholar]
- Seo, M.; Juhnn, Y.S. Gq protein mediates UVB-induced cyclooxygenase-2 expression by stimulating HB-EGF secretion from HaCaT human keratinocytes. Biochem. Biophys. Res. Commun 2010, 393, 190–195. [Google Scholar]
- Takano-Ishikawa, Y.; Goto, M.; Yamaki, K. Structure-activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytomedicine 2006, 13, 310–317. [Google Scholar]
- Isoherranen, K.; Punnonen, K.; Jansen, C.; Uotila, P. Ultraviolet irradiation induces cyclooxygenase-2 expression in keratinocytes. Br. J. Dermatol 1999, 140, 1017–1022. [Google Scholar]
- Cretu, A.; Sha, X.; Tront, J.; Hoffman, B.; Liebermann, D. Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther 2009, 7, 268–276. [Google Scholar]
- Gao, M.; Guo, N.; Huang, C.; Song, L. Diverse roles of GADD45alpha in stress signaling. Curr. Protein Pept. Sci 2009, 10, 388–394. [Google Scholar]
- Minghetti, P.; Cilurzo, F.; Casiraghi, A.; Montanari, L. Evaluation of ex vivo human skin permeation of genistein and daidzein. Drug Delivery 2006, 13, 411–415. [Google Scholar]
- Vänttinen, K.; Moravcova, J. Transdermal absorption of phytoestrogens. Pharmazie 2001, 56, 711–717. [Google Scholar]
- Lin, J.Y.; Tournas, J.A.; Burch, J.A.; Monteiro-Riviere, N.A.; Zielinski, J. Topical isoflavones provide effective photoprotection to skin. Photodermatol. Photoimmunol. Photomed 2008, 24, 61–66. [Google Scholar]
- Huang, Z.R.; Hung, C.F.; Lin, Y.K.; Fang, J.Y. In vitro and in vivo evaluation of topical delivery and potential dermal use of soy isoflavones genistein and daidzein. Int. J. Pharm 2008, 364, 36–44. [Google Scholar]
- Chadha, G.; Sathigari, S.; Parsons, D.L.; Jayachandra, B.R. In vitro percutaneous absorption of genistein from topical gels through human skin. Drug Dev. Ind. Pharm 2011, 37, 498–505. [Google Scholar]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. Methods Mol. Biol 2011, 740, 7–12. [Google Scholar]
- Iovine, B.; Nino, M.; Irace, C.; Bevilacqua, M.A.; Monfrecola, G. Ultraviolet B and A irradiation induces fibromodulin expression in human fibroblasts in vitro. Biochimie 2009, 91, 364–372. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res 1988, 175, 184–189. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iovine, B.; Iannella, M.L.; Gasparri, F.; Giannini, V.; Monfrecola, G.; Bevilacqua, M.A. A Comparative Analysis of the Photo-Protective Effects of Soy Isoflavones in Their Aglycone and Glucoside Forms. Int. J. Mol. Sci. 2012, 13, 16444-16456. https://doi.org/10.3390/ijms131216444
Iovine B, Iannella ML, Gasparri F, Giannini V, Monfrecola G, Bevilacqua MA. A Comparative Analysis of the Photo-Protective Effects of Soy Isoflavones in Their Aglycone and Glucoside Forms. International Journal of Molecular Sciences. 2012; 13(12):16444-16456. https://doi.org/10.3390/ijms131216444
Chicago/Turabian StyleIovine, Barbara, Maria Luigia Iannella, Franco Gasparri, Valentina Giannini, Giuseppe Monfrecola, and Maria Assunta Bevilacqua. 2012. "A Comparative Analysis of the Photo-Protective Effects of Soy Isoflavones in Their Aglycone and Glucoside Forms" International Journal of Molecular Sciences 13, no. 12: 16444-16456. https://doi.org/10.3390/ijms131216444
APA StyleIovine, B., Iannella, M. L., Gasparri, F., Giannini, V., Monfrecola, G., & Bevilacqua, M. A. (2012). A Comparative Analysis of the Photo-Protective Effects of Soy Isoflavones in Their Aglycone and Glucoside Forms. International Journal of Molecular Sciences, 13(12), 16444-16456. https://doi.org/10.3390/ijms131216444



