Fabrication of an Electrically-Resistive, Varistor-Polymer Composite
Abstract
:1. Introduction
- Those that participate in the formation of the basic microstructure of ZnO varistors in sintering provide for the formation of inter-granular layers; Bi2O3 is one such dopant.
- Those used in ensuring the non-linearity of the varistor ceramic promote the creation of deep charge carrier traps and cause the formation of the surface potential of the grains; Co3O4 and MnO are such dopants.
- Those that stabilize inter-granular layers under electrical loads and external environmental factors (temperature and humidity) and increase the stability of the electrical characteristics and reliability of the varistors; Sb2O3 is one such dopant [29].
2. Results and Discussion
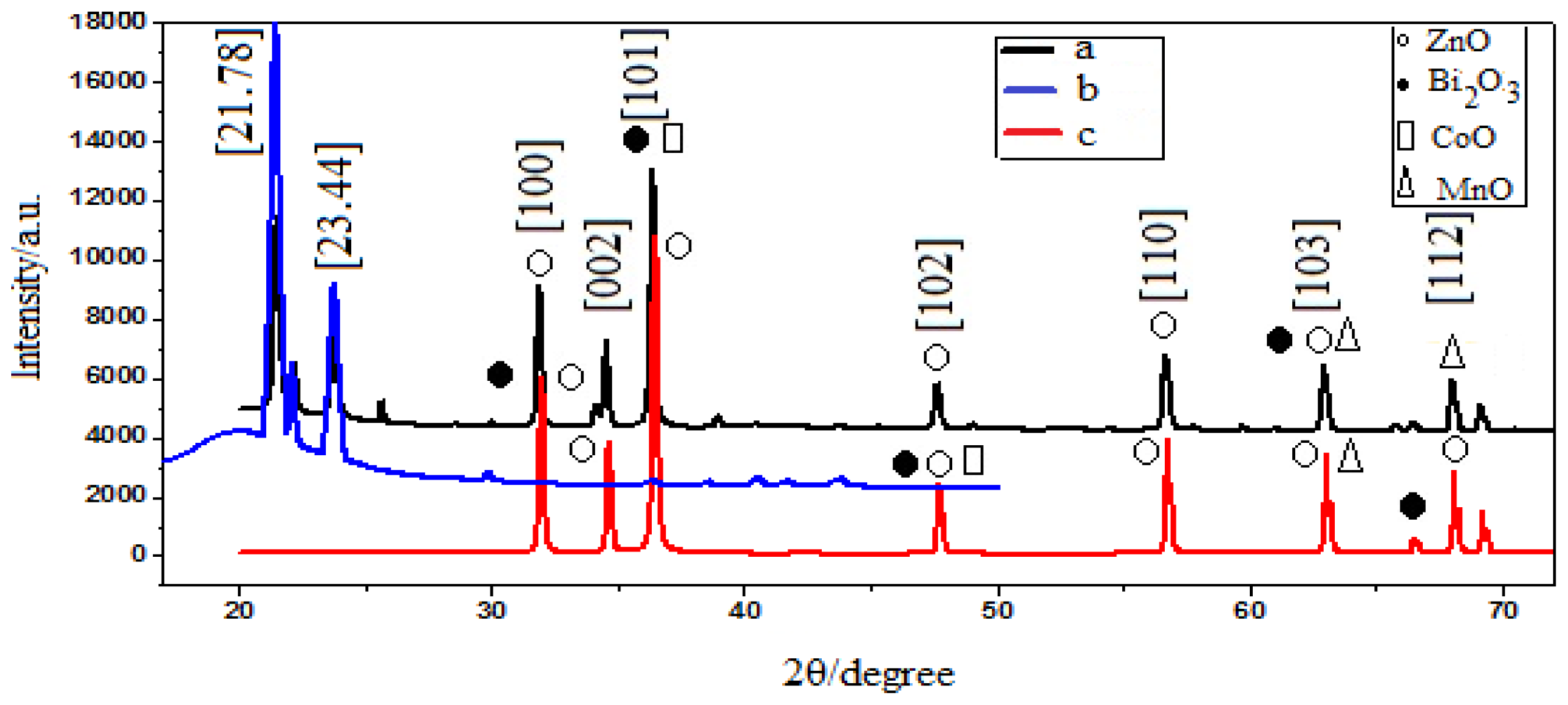
2.1. X-ray Diffraction
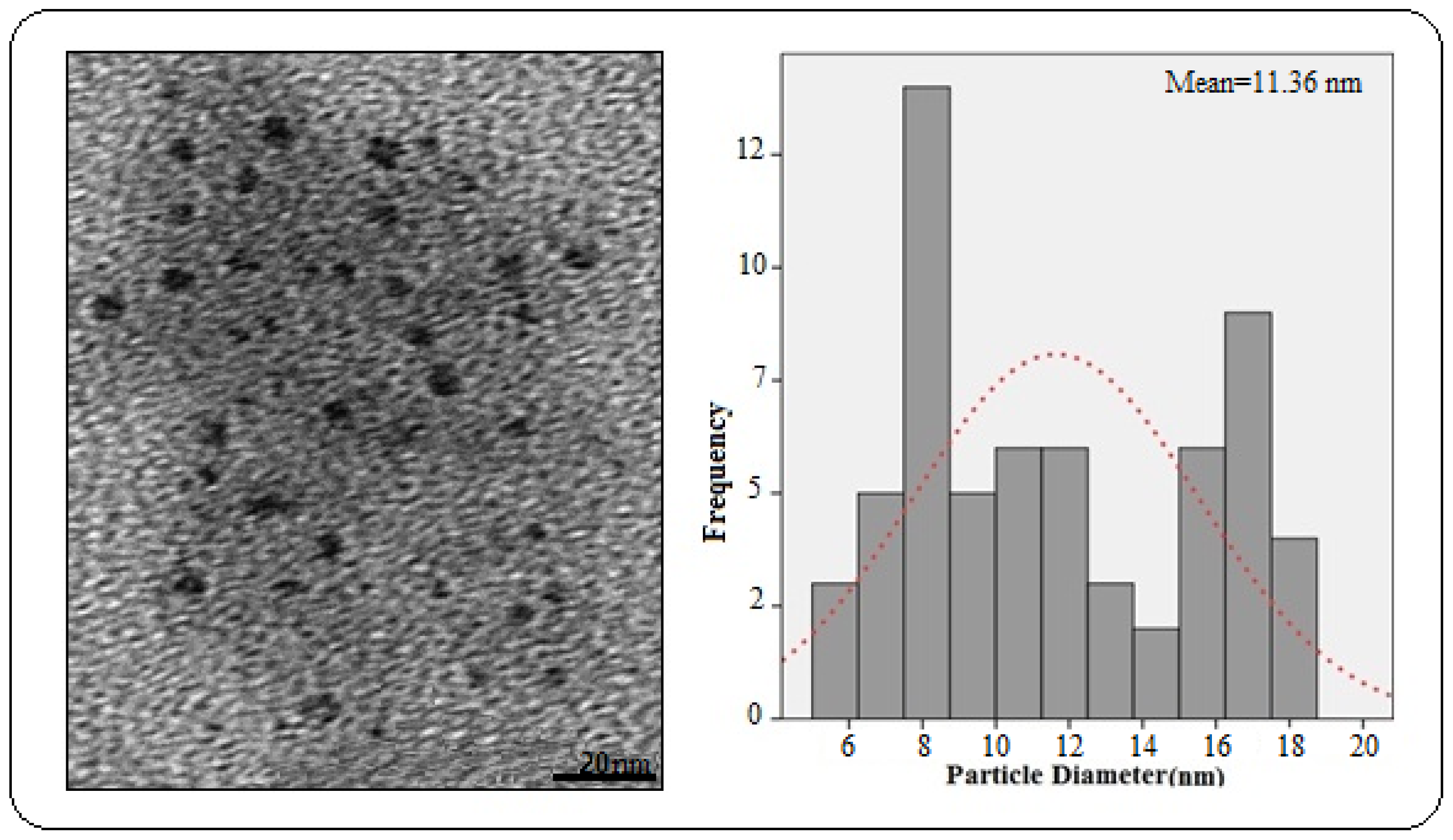
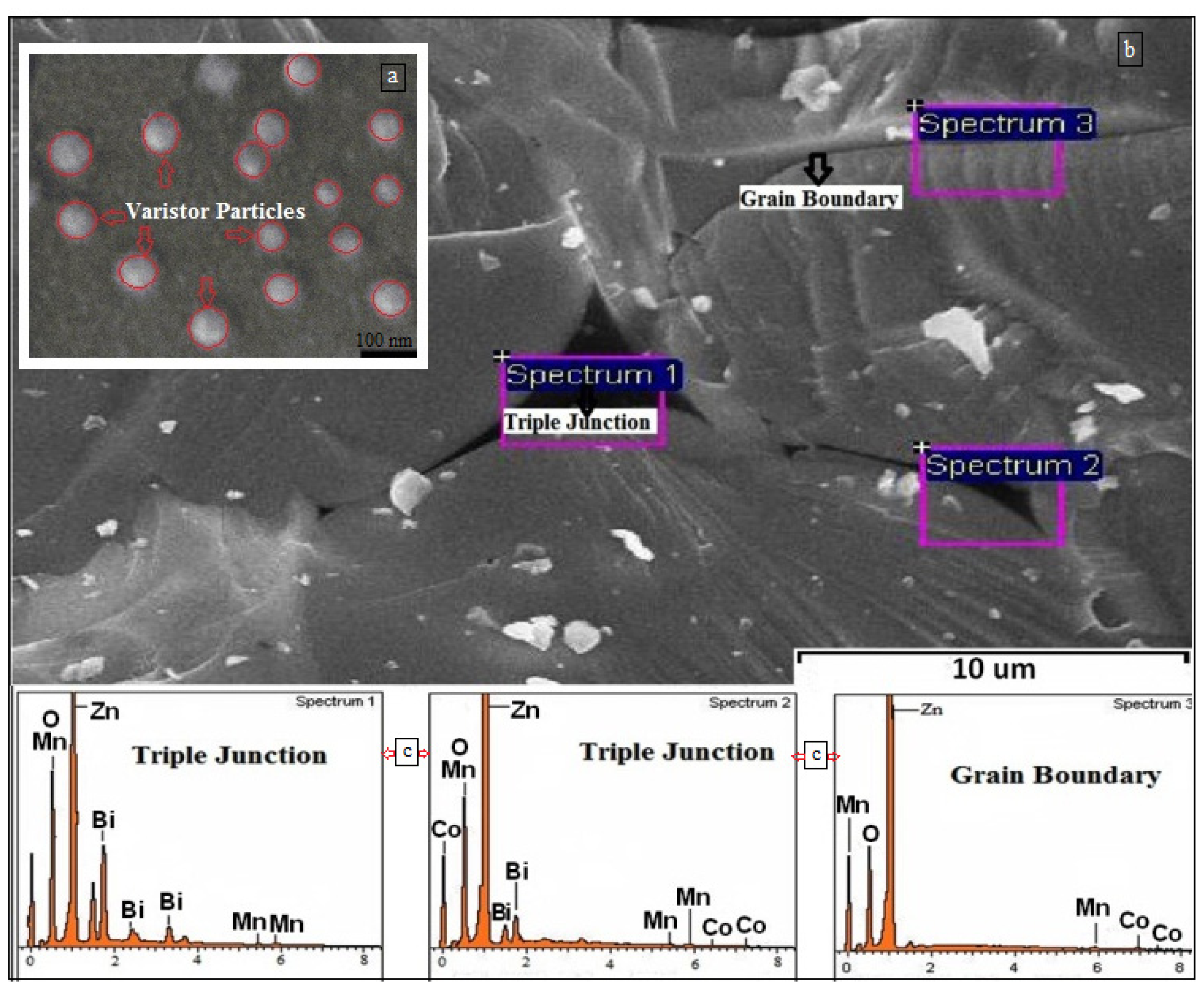
2.2. Electron Microscopic Analysis
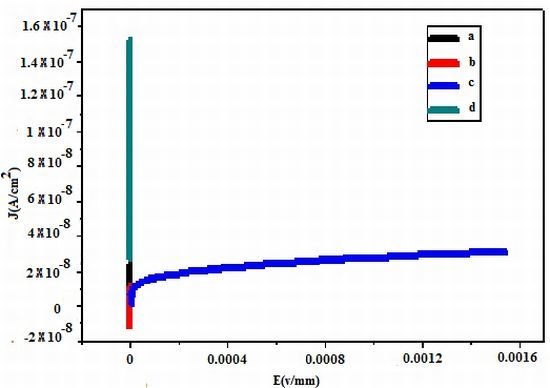
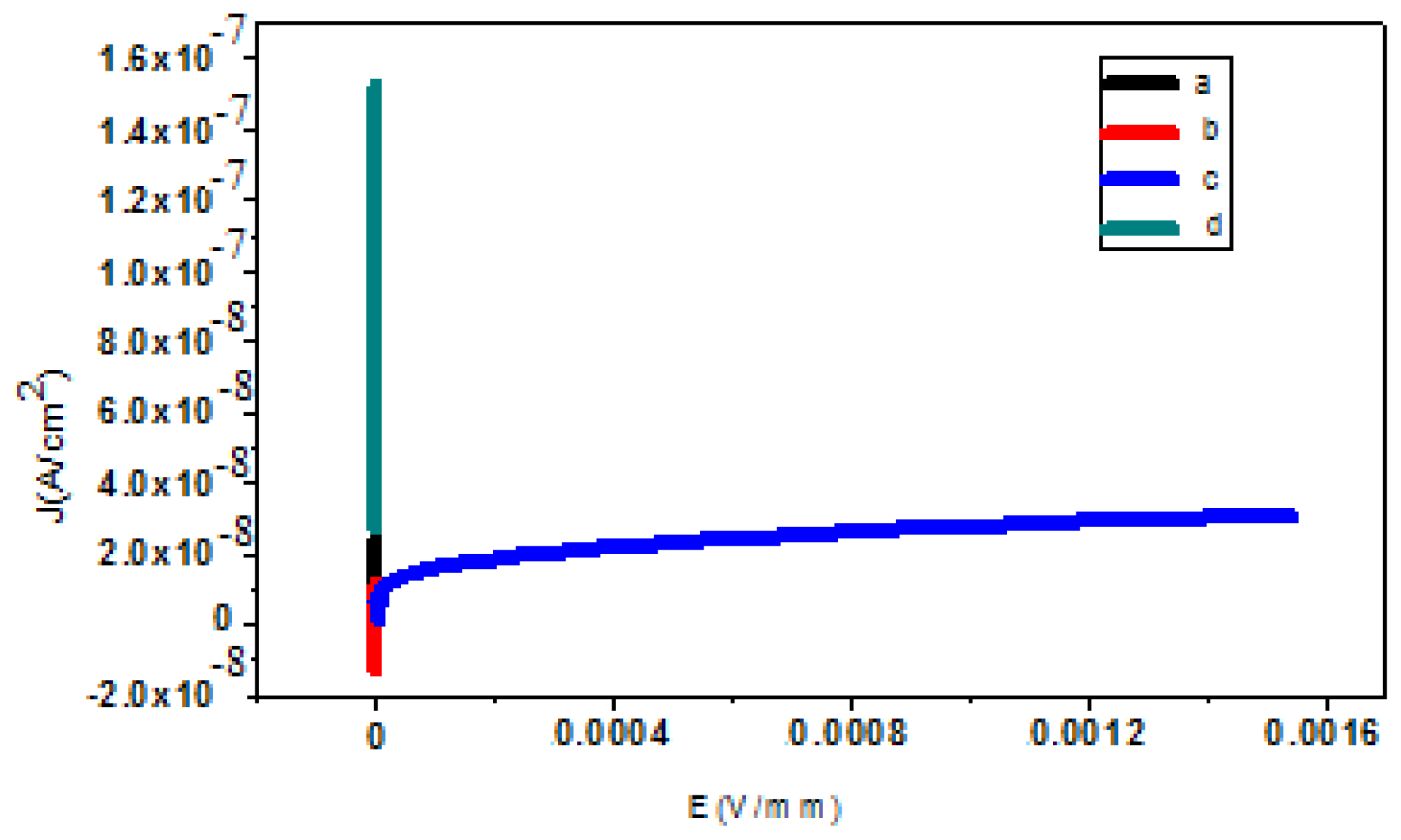
2.3. Electrical Measurement
3. Experimental Section
3.1. Materials
3.2. Preparation ZnO Based Nano-Sized Varistor Powder
3.3. Fabrication of Varistor Polymer Nanocomposite
3.4. Characterization
4. Conclusions
Acknowledgments
References
- Chung, D.D.L. Composite Materials: Functional Materials for Modern Technologies; Springer: New York, NY, USA, 2003; p. 289. [Google Scholar]
- Xanthos, M. Polymer and Polymer Composites. In Functional Fillers for Plastics, 2nd ed; Wiley-VCH Verlag GmbH & Co. KGaA: Piscataway, NJ, USA, 2010; pp. 1–18. [Google Scholar]
- McLachlan, D.S.; Blaszkiewicz, M.; Newnham, R.E. Electrical resistivity of composites. J. Am. Ceram. Soc 1990, 73, 2187–2203. [Google Scholar]
- Xu, H.P.; Wu, Y.H.; Yang, D.D.; Wang, J.R.; Xie, H.Q. Study on theories and influence factors of PTC property in polymer based conductive composites. Rev. Adv. Mater. Sci 2011, 27, 173–183. [Google Scholar]
- Bhattacharya, S.K.; Chaklader, A.C.D. Review on metal-filled plastics. Part1. Electrical conductivity. Polym.-Plast. Technol. Eng 1982, 19, 21–51. [Google Scholar]
- Nano-Bio-Electronic Photonic and Mems Packaging; Wong, C.P.; Moon, K.S.; Li, Y. (Eds.) Springer: New York, NY, USA, 2010; p. 297.
- Strumpler, R.; Glatz-Reichenbach, J. Conducting polymer composites. J. Electroceram 1999, 3, 329–346. [Google Scholar]
- Carmona, F. Conducting filled polymers. Physica A 1989, 157, 461–469. [Google Scholar]
- Bloor, D.; Donnelly, K.; Hands, P.J.; Laughlin, P.; Lussey, D. A metal-polymer composite with unusual properties. J. Phys. D 2005, 38, 2851–2860. [Google Scholar]
- Varlow, B.R.; Rabertson, J.; Donnelly, K.P. Nonlinear fillers in electrical insulating materials. Meas. Sci. Technol 2007, 1, 96–102. [Google Scholar]
- Matrix Active Micro- and Nanocomposites Based on the Polymer, Semiconductive and Ferropiezoceramic Materials. In Nanocomposites and Polymers with Analytical Methods; Cuppoletti, J. (Ed.) InTech: Azerbaijan, 2011; pp. 376–377.
- Souza, F.L.; Gomes, J.W.; Buenoa, P.R.; Cassia-Santos, M.R.; Araujo, A.L.; Liete, E.R.; Longoa, E.; Varela, J.A. Effect of the addition of ZnO seeds on the electrical proprieties of ZnO-based varistors. Mater. Chem. Phys 2003, 80, 512–516. [Google Scholar]
- Greuter, F.; Siegrist, M.; Kluge-Weiss, P.; Kessler, R.; Donzel, L.; Loitzl, R.; Gramespacher, H.J. Microvaristors: Functional fillers for novel electroceramic composites. J. Electroceram 2004, 13, 739–744. [Google Scholar]
- Hingorani, S.; Shah, D.O. Effect of process variables on the grain growth and microstructure of ZnO-Bi2O3 varistors and their nanosize ZnO precursors. J. Mater. Res 1995, 10, 461–467. [Google Scholar]
- Tang, E.; Cheng, G.; Pang, X.; Ma, X.; Xing, F. Synthesis of nano-ZnO/poly(methyl methacrylate) composite microsphere through emulsion polymerization and its UV-shielding property. Colloid. Polym. Sci 2006, 284, 422–428. [Google Scholar]
- Lee, J.; Bhattacharyya, D.; Easteal, A.J.; Metson, J.B. Properties of nano-ZnO/poly(vinyl alcohol)/poly(ethylene oxide) composite thin films. Curr. Appl. Phys 2008, 8, 42–47. [Google Scholar]
- Yang, R.; Christensen, P.A.; Egerton, T.A.; White, J.R. Degradation products formed during UV exposure of polyethylene–ZnO nano-composites. Polym. Degrad. Stabil 2010, 95, 1533–1541. [Google Scholar]
- Naga Raju, B.; Ramji, K.; Prasad, V.S.R.K. Studies on tribological properties of ZnO polymer nanocomposites. ARPN J. Eng. Appl. Sci 2011, 6, 75–82. [Google Scholar]
- Wang, X.; Herth, S.; Hugener, T.; Siegel, R.W.; Nelson, J.K.; Schadler, L.S.; Hilborg, H.; Auletta, T. Nonlinear Electrical Behavior of Treated ZnO-EPDM Nanocomposites. Proceedings of the 2006 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Kansas City, MO, USA, 15–18 October 2006; pp. 421–424.
- Matsuoka, M.; Masuyama, T.; Lida, Y. Voltage nonlinearity of zinc oxide ceramics doped with alkali earth metal oxide. Jpn. J. Appl. Phys 1969, 8, 1275–1276. [Google Scholar]
- Elfwing, M. Nanoscale Characterisation of Barriers to Electron Conduction in ZnO Varistor Materials. In Ph.D. Thesis; Uppsala University: Uppsala, Sweden, 2002. [Google Scholar]
- Clarke, D.R. Varistor ceramics. J. Am. Ceram. Soc 1999, 82, 485–502. [Google Scholar]
- Sato, Y.; Yamamoto, T.; Ikuhara, Y. Atomic structures and electrical properties of ZnO grain boundaries. J. Am. Ceram. Soc 2007, 90, 337–357. [Google Scholar]
- Bueno, P.R.; Leite, E.R.; oliveira, M.M.; Orlandi, M.O.; Longo, E. Role of oxygen at the grain boundary of metal oxide varistors: A potential barrier formation mechanism. Appl. Phys. Lett 2001, 79, 48–50. [Google Scholar]
- Olsson, E. Interfacial Micro Structure in ZnO Varistor Materials. Ph.D. Thesis, Chalmers University of Technology, Goteborg, Sweden, 1988. [Google Scholar]
- Sato, Y.; Buban, J.P.; Mizoguchi, T.; Shibata, N.; Yodogawa, M.; Yamamoto, T.; Ikuhara, Y. Role of Pr segregation in acceptor-state formation at ZnO grain boundaries. Phys. Rev. Lett 2006, 97, 106802. [Google Scholar]
- Pike, G.E.; Kurtz, S.R.; Gourley, P.L.; Philipp, H.R.; Levinson, L.M. Electroluminescence in ZnO varistors: Evidence for hole contributions to the breakdown mechanism. J. Appl. Phys 1985, 57, 5512–5518. [Google Scholar]
- Morris, W.G. Physical properties of the electrical barriers in varistors. Vac. Sci. Technol 1976, 13, 926–931. [Google Scholar]
- Skidan, B.; M’int, M. Effect of metal oxides on the microstructure of zinc ceramic. Glass Ceram 2007, 64, 31–33. [Google Scholar]
- Wang, J.; Cheung, M.K.; Mi, Y. Preparation of cross-linked microparticles of poly(glycidyl methacrylate) by dispersion polymerization of glycidyl methacrylate using a PDMS macromonomer as stabilizer in supercritical carbon dioxide. Polymer 2002, 43, 1357–1364. [Google Scholar]
- Porter, J.R.; Henson, A.; Popat, K.C. Biodegradable poly(e-caprolactone) nanowires for bone tissue engineering applications. Biomaterials 2009, 30, 780–788. [Google Scholar]
- Corden, T.J.; Jones, I.A.; Rudd, C.D.; Christian, P.; Downes, S. Initial development into a novel technique for manufacturing a long fibre thermoplastic bioabsorbable composite: In situ polymerisation of poly-epsilon-caprolactone. Compos. Part A 1999, 30, 737–746. [Google Scholar]
- Corden, T.J.; Jones, I.A.; Rudd, C.D.; Christian, P.; Downes, S.; McDougal, K.E. Physical and biocompatibility properties of poly-epsilon-caprolactone produced using in situ polymerisation: A novel manufacturing technique for long-fibre composite materials. Biomaterials 2000, 21, 713–724. [Google Scholar]
- Koenig, M.F.; Huang, S.J. Biodegradable blends and composites of polycaprolactone and starch derivatives. Polymer 1995, 36, 1877–1882. [Google Scholar]
- Mingotaud, A.F.; Dargelas, F.; Cansell, F. Cationic and anionic ring-opening polymerization in supercritical CO2. Macromol. Symp 2000, 153, 77–86. [Google Scholar]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. PubMed 1991, 12, 292–304. [Google Scholar]
- Kweon, H.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.; Lee, H.-S.; Oh, J.-S.; Akaike, T.; Cho, C.-S. A novel degradable polycaprolactone networks for tissue engineering. J. Biomater 2003, 24, 801–808. [Google Scholar]
- Bhaw-Luximon, A.; Jhurry, D.; Motala-Timol, S.; Lochee, Y. Polymerization of ɛ-caprolactone and its copolymerization with γ-butyrolactone using metal complexes. Macromol. Symp 2005, 231, 60–68. [Google Scholar]
- Kitayama, T.; Yamaguchi, H.; Kanzawa, T.; Hirano, T. Living ring-opening polymerization of epsilon-caprolactone with combinations of tert-butyllithium and bilky aluminium phenoxides. Polym. Bull 2000, 45, 97–104. [Google Scholar]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci 2010, 35, 1217–1256. [Google Scholar] [Green Version]
- Wu, C.-S. Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym. Degrad. Stabil 2003, 80, 127–134. [Google Scholar]
- Elzubair, A.; Elias, C.N. The physical characterization of a thermoplastic polymer for endodontic obturation. J. Dent 2006, 34, 784–789. [Google Scholar]
- Eda, K. Zinc oxide varistors. IEEE Electric. Insul. Mag 1989, 5, 28–30. [Google Scholar]
- Kusy, A.; Kleinpenning, T.G.M. Conduction mechanism and 1/f noise in ZnO varistors. J. Appl. Phys 1983, 54, 2900–2906. [Google Scholar]
- Nasar, G. Structural study of PVA composites with inorganic salts by X-ray diffraction. J. Pak. Mater. Soc 2009, 3, 67–70. [Google Scholar]
- Rizwan, Z.; Zakaria, A.; Ghazali, M.S.M. Photopyroelectric spectroscopic studies of ZnO-MnO2-Co3O4-V2O5 ceramics. Int. J. Mol. Sci 2011, 12, 1625–1632. [Google Scholar]
- Wang, Q.; Qin, Y.; Xu, G.J.; Chen, L.; Li, Y.; Duan, L.; Li, Z.X.; Li, Y.; Cui, P. Low-voltage ZnO varistor fabricated by the solution-coating method. Ceram. Int 2008, 34, 1697–1701. [Google Scholar]
- Palilla, F.C. Process for the Preparation of Homogeneous Metal Oxide Varistors. U.S. Patent US 4575440, 11 March 1986. [Google Scholar]
| Ingredients | Varistor nanocomposite | Varistor nano-sized powder |
|---|---|---|
| 2θ (degree) | 2θ (degree) | |
| Bismuth Zinc Oxide | 39.51, 45.17, 48.99, 62.91, 77.01 | 39.55, 63.01, 65.71, 72.73 |
| Zinc Cobalt Oxide | 49.11, 59.29, 77.08 | 48.99, 62.91, 74.14 |
| Zinc Manganese Oxide | 30.34, 43.69, 62.91 | 38.85, 56.73, 60.86, 68.08, 69.21 |
| Composition | PCL/20 wt% filler | PCL/50 wt% filler | PCL/70 wt% filler | Pure PCL |
|---|---|---|---|---|
| Alpha | 1.71 | 2.21 | 3.57 | 1.11 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ahmad, M.B.; Fatehi, A.; Zakaria, A.; Mahmud, S.; Mohammadi, S.A. Fabrication of an Electrically-Resistive, Varistor-Polymer Composite. Int. J. Mol. Sci. 2012, 13, 15640-15652. https://doi.org/10.3390/ijms131215640
Ahmad MB, Fatehi A, Zakaria A, Mahmud S, Mohammadi SA. Fabrication of an Electrically-Resistive, Varistor-Polymer Composite. International Journal of Molecular Sciences. 2012; 13(12):15640-15652. https://doi.org/10.3390/ijms131215640
Chicago/Turabian StyleAhmad, Mansor Bin, Asma Fatehi, Azmi Zakaria, Shahrom Mahmud, and Sanaz A. Mohammadi. 2012. "Fabrication of an Electrically-Resistive, Varistor-Polymer Composite" International Journal of Molecular Sciences 13, no. 12: 15640-15652. https://doi.org/10.3390/ijms131215640
APA StyleAhmad, M. B., Fatehi, A., Zakaria, A., Mahmud, S., & Mohammadi, S. A. (2012). Fabrication of an Electrically-Resistive, Varistor-Polymer Composite. International Journal of Molecular Sciences, 13(12), 15640-15652. https://doi.org/10.3390/ijms131215640