Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities
Abstract
:1. Introduction
2. Sources of Tanshinones, Preparative and Analytical Methodologies
2.1. Isolation, Purification and Analytical Methodologies
2.2 Biosynthesis of Tanshinones in S. miltiorrhiza
2.3 Botanical and Alternative Sources of Tanshinones
3. Pharmacokinetics of Tanshinones
3.1. The Pharmacokinetic (PK) Characteristics of Single Administered Agents or as Mixtures
3.2. Structural Modification of Tanshinones
3.3. Improving Pharmacokinetics through Novel Formulations
4. Anti-Cancer Activities of Tanshinones
4.1. Anti-Proliferation and Pro-Apoptosis
4.1.1. Tanshinone I (TI)
4.1.2. Tanshinone IIA (TIIA)
4.1.3. Cryptotanshinone (CT)
4.1.4. Dihydrotanshinone I (DH-TI)
4.2. Pro-Differentiation of Cancer Cells
4.3. Inhibition of Angiogenesis
4.4. Inhibition of Adhesion, Migration, Invasion and Metastasis
4.5. Modulation of Inflammatory and Immune Responses
4.6. Inhibition of Telomerase
4.7. Interaction with DNA Minor Groove and P53 Activation
4.8. Modulation of Androgen Receptor Pathway
4.9. Synergy with Chemotherapy and Radiotherapy
5. Cancer-Related Clinical Studies
6. Novel Tanshinones and Chemical Modifications
7. Summary and Perspective
Acknowledgements
- Conflict of InterestAll authors declare no conflict of interest.
Abbreviations
| APL | acute promyelocytic leukemia |
| CAM | chicken embryo chorioallantoic membrane |
| CCC | countercurrent chromatography |
| CD | cyclodextrins |
| CPP | copalyl diphosphate |
| CR | complete remission |
| CT | cryptotanshinone |
| DAD | diode array |
| DH-TI | dihydrotanshinone I |
| DMAPP | dimethylallyldiphosphate |
| DXP | 1-deoxy-d-xylulose-5-phosphate |
| DXR | 1-deoxy-dxylulose- 5-phosphate reductoisomerase |
| DXS | 1-deoxy-d-xylulose-5-phosphate synthase |
| ECM | extracellular matrix |
| ESI-IT-MS | electrospray ionization quadrupole ion trap mass spectrometry |
| FBS | fetal bovine serum |
| FPP | farnesyl pyrophosphate |
| GA-3P | glyceraldehyde-3-phosphate |
| GGPP | geranylgeranyldiphosphate |
| GPP | geranyldiphosphate |
| HPLC | high-performance liquid chromatography |
| HSCCC | high-speed counter-current chromatography |
| i.m. | intramuscular |
| i.p. | intraperitoneal |
| IPP | isopentenyldiphosphate |
| i.s.i.p. | in situ intestine perfusion |
| i.v. | intravenous |
| LC | liquid chromatography |
| MEP | 2-C-methyl-d-erythritol-4-phosphate |
| MVA | mevalonic acid |
| MVD | microvessel density |
| p.o. | oral adminstration |
| PR | partial remission |
| qTOF-MS | quadrupole time-of-flight mass spectrometry |
| RIF | Realgar-Indigo naturalis formula |
| s.c. | subcutaneous |
| SD | stable disease |
| SLN | solid lipid nanoparticles |
| STS | sodium tanshinone IIA sulfonate |
| TI | tanshinone I |
| TIIA | tanshinone IIA |
| TACE | transcatheter arterial chemoembolization |
| TCM | Traditional Chinese Medicine |
| TLC | thin-layer chromatography |
References
- Zhou, L.; Zuo, Z.; Chow, M.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol 2005, 45, 1345–1359. [Google Scholar]
- Dong, Y.; Morris-Natschke, S.L.; Lee, K.H. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat. Prod. Rep 2011, 28, 529–542. [Google Scholar]
- Nakao, M.; Fukushima, T. On the the chemical composition of Salvia miltiorrhiza (Chinese drug Tan-shen). Yakugaku Zasshi 1934, 54, 844–858. [Google Scholar]
- Wang, X.; Morris-Natschke, S.L.; Lee, K.H. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med. Res. Rev 2007, 27, 133–148. [Google Scholar]
- Bi, H.C.; Zuo, Z.; Chen, X.; Xu, C.S.; Wen, Y.Y.; Sun, H.Y.; Zhao, L.Z.; Pan, Y.; Deng, Y.; Liu, P.Q.; et al. Preclinical factors affecting the pharmacokinetic behaviour of tanshinone IIA, an investigational new drug isolated from Salvia miltiorrhiza for the treatment of ischaemic heart diseases. Xenobiotica 2008, 38, 185–222. [Google Scholar]
- Don, M.J.; Shen, C.C.; Syu, W.J.; Ding, Y.H.; Sun, C.M. Cytotoxic and aromatic constituents from Salvia miltiorrhiza. Phytochemistry 2006, 67, 497–503. [Google Scholar]
- Gu, M.; Zhang, G.; Su, Z.; Ouyang, F. Identification of major active constituents in the fingerprint of Salvia miltiorrhiza Bunge developed by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1041, 239–243. [Google Scholar]
- Wei, Y.J.; Li, S.L.; Li, P. Simultaneous determination of seven active components of Fufang Danshen tablet by high performance liquid chromatography. Biomed. Chromatogr 2007, 21, 1–9. [Google Scholar]
- Yang, M.; Liu, A.; Guan, S.; Sun, J.; Xu, M.; Guo, D. Characterization of tanshinones in the roots of Salvia miltiorrhiza (Dan-shen) by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom 2006, 20, 1266–1280. [Google Scholar]
- Liu, A.H.; Li, L.; Xu, M.; Lin, Y.H.; Guo, H.Z.; Guo, D.A. Simultaneous quantification of six major phenolic acids in the roots of Salvia miltiorrhiza and four related traditional Chinese medicinal preparations by HPLC–DAD method. J. Pharm. Biomed. Anal 2006, 41, 48–56. [Google Scholar]
- Ma, L.; Zhang, X.; Guo, H.; Gan, Y. Determination of four water-soluble compounds in Salvia miltiorrhiza Bunge by high-performance liquid chromatography with a coulometric electrode array system. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 2006, 833, 260–263. [Google Scholar]
- Kong, D.Y. Chemical constituents of Salvia miltiorrhiza (Danshen). Zhongguo Yiyao Gongye Zazhi 1989, 20, 279–285. [Google Scholar]
- Zeng, Y.; Song, J.X.; Shen, X.C. Herbal remedies supply a novel prospect for the treatment of atherosclerosis: A review of current mechanism studies. Phytother. Res 2012, 26, 159–167. [Google Scholar]
- Gao, S.; Liu, Z.; Li, H.; Little, P.J.; Liu, P.; Xu, S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis 2012, 220, 3–10. [Google Scholar]
- Ho, J.H.; Hong, C.Y. Salvianolic acids: Small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci 2011, 18. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, F.; Zhang, S.; Leung, S.W. Is danshen (Salvia miltiorrhiza) dripping pill more effective than isosorbide dinitrate in treating angina pectoris? A systematic review of randomized controlled trials. Int. J. Cardiol 2012, 157, 330–340. [Google Scholar]
- Yuan, S.L.; Wang, X.J.; Wei, Y.Q. Anticancer effect of tanshinone and its mechanisms. Aizheng 2003, 22, 1363–1366. [Google Scholar]
- Yang, M.H.; Blunden, G.; Xu, Y.X.; Nagy, G. MáThé, I. Diterpenoids from Salvia species. Pharm. Pharmacol. Commun 1996, 2, 69–71. [Google Scholar]
- Ikeshiro, Y.; Hashimoto, I.; Iwamoto, Y.; Mase, I.; Tomita, Y. Diterpenoids from Salvia miltiorrhiza. Phytochemistry 1991, 30, 2791–2792. [Google Scholar]
- Honda, G.; Koezuka, Y.; Tabata, M. Isolation of an antidermatophytic substrance from the root of Salvia miltiorrhiza. Chem. Pharm. Bull 1988, 36, 408–411. [Google Scholar]
- Zhao, R.N.; Xie, P.S.; Yin, W.P.; Lu, P.H.; Yan, Y.Z.; Wang, Z.D. Quality analysis of “whitish” radix Salvia mltiorrhiza in Luanchuan region of Henan province by TLC and HPLC. Chin. Tradit. Herbal. Drugs 2006, 37, 119–122. [Google Scholar]
- Li, J.; He, L.Y.; Song, W.Z. Separation and quantitative determination of seven aqueous depsides in Salvia miltiorrhiza by HPTLC scanning. Yaoxue Xuebao 1993, 28, 543–547. [Google Scholar]
- Gu, M.; Su, Z.; Ouyang, F. Fingerprinting of Salvia miltiorrhiza Bunge by thin-layer chromatography scan compared with high speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol 2006, 29, 1503–1514. [Google Scholar]
- Wei, Y.J.; Qi, L.W.; Li, P.; Luo, H.W.; Yi, L.; Sheng, L.H. Improved quality control method for Fufang Danshen preparations through simultaneous determination of phenolic acids, saponins and diterpenoid quinones by HPLC coupled with diode array and evaporative light scattering detectors. J. Pharm. Biomed. Anal 2007, 45, 775–784. [Google Scholar]
- Chang, Q.; Sun, L.; Zhao, R.H.; Chow, M.S.; Zuo, Z. Simultaneous determination of ten active components in traditional Chinese medicinal products containing both gegen (Pueraria iobata) and danshen (Salvia miltiorrhiza) by high-performance liquid chromatography. Phytochem. Anal 2008, 19, 368–375. [Google Scholar]
- Zhu, Z.; Zhang, H.; Zhao, L.; Dong, X.; Li, X.; Chai, Y.; Zhang, G. Rapid separation and identification of phenolic and diterpenoid constituents from Radix Salvia miltiorrhizae by high-performance liquid chromatography diode-array detection, electrospray ionization time-of-flight mass spectrometry and electrospray ionization quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spectrom 2007, 21, 1855–1865. [Google Scholar]
- Zhou, Y.; Xu, G.; Choi, F.F.; Ding, L.S.; Han, Q.B.; Song, J.Z.; Qiao, C.F.; Zhao, Q.S.; Xu, H.X. Qualitative and quantitative analysis of diterpenoids in Salvia species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 4847–4858. [Google Scholar]
- Ito, Y.; Bowman, R.L. Countercurrent chromatography: Liquid-liquid partition chromatography without solid support. Science 1970, 167, 281–283. [Google Scholar]
- Ito, Y.; Conway, W.D. High-speed countercurrent chromatography. Crit. Rev. Anal. Chem 1986, 17, 65–143. [Google Scholar]
- Pauli, G.F.; Pro, S.M.; Friesen, J.B. Countercurrent separation of natural products. J. Nat. Prod 2008, 71, 1489–1508. [Google Scholar]
- Tian, G.; Zhang, Y.; Zhang, T.; Yang, F.; Ito, Y. Separation of tanshinones from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography using stepwise elution. J. Chromatogr. A 2000, 904, 107–111. [Google Scholar]
- Tian, G.; Zhang, T.; Zhang, Y.; Ito, Y. Separation of tanshinones from Salvia miltiorrhiza Bunge by multidimensional counter-current chromatography. J. Chromatogr. A 2002, 945, 281–285. [Google Scholar]
- Rohdich, F.; Kis, K.; Bacher, A.; Eisenreich, W. The non-mevalonate pathway of isoprenoids: Genes, enzymes and intermediates. Curr. Opin. Chem. Biol 2001, 5, 535–540. [Google Scholar]
- Sun, A.; Zhang, Y.; Li, A.; Meng, Z.; Liu, R. Extraction and preparative purification of tanshinones from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography. J. Chromatogr. B 2011, 879, 1899–1904. [Google Scholar]
- Wu, S.; Wu, D.; Liang, J.; Berthod, A. Modeling gradient elution in countercurrent chromatography: Efficient separation of tanshinones from Salvia miltiorrhiza Bunge. J. Sep. Sci 2012, 35, 964–976. [Google Scholar]
- Gu, M.; Ouyang, F.; Su, Z. Comparison of high-speed counter-current chromatography and high-performance liquid chromatography on fingerprinting of Chinese traditional medicine. J. Chromatogr. A 2004, 1022, 139–144. [Google Scholar]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit 2006, 19, 106–180. [Google Scholar]
- Andersson, L.I. Molecular imprinting for drug bioanalysis: A review on the application of imprinted polymers to solid-phase extraction and binding assay. J. Chromatogr. B Biomed. Sci. Appl 2000, 739, 163–173. [Google Scholar]
- Bossi, A.; Bonini, F.; Turner, A.P.; Piletsky, S.A. Molecularly imprinted polymers for the recognition of proteins: The state of the art. Biosens. Bioelectron 2007, 22, 1131–1137. [Google Scholar]
- Cormack, P.A.; Elorza, A.Z. Molecularly imprinted polymers: Synthesis and characterisation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 2004, 804, 173–182. [Google Scholar]
- Jia, X.; Li, H.; Luo, J.; Lu, Q.; Peng, Y.; Shi, L.; Liu, L.; Du, S.; Zhang, G.; Chen, L. Rational design of core–shell molecularly imprinted polymer based on computational simulation and Doehlert experimental optimization: Application to the separation of tanshinone IIA from Salvia miltiorrhiza Bunge. Anal. Bioanal. Chem 2012, 403, 2691–2703. [Google Scholar]
- Seto, H.; Watanabe, H.; Furihata, K. Simultaneous operation of the mevalonate and non-mevalonate pathways in the biosynthesis of isopentenly diphosphate in Streptomyces aeriouvifer. Tetrahedron Lett 1996, 37, 7979–7982. [Google Scholar]
- Arigoni, D.; Sagner, S.; Latzel, C.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Terpenoid biosynthesis from 1-deoxy-d-xylulose in higher plants by intramolecular skeletal rearrangement. Proc. Natl. Acad. Sci. USA 1997, 94, 10600–10605. [Google Scholar]
- Hirai, N.; Yoshida, R.; Todoroki, Y.; Ohigashi, H. Biosynthesis of abscisic acid by the non-mevalonate pathway in plants, and by the mevalonate pathway in Fungi. Biosci. Biotechnol. Biochem 2000, 64, 1448–1458. [Google Scholar]
- Disch, A.; Hemmerlin, A.; Bach, T.J.; Rohmer, M. Mevalonate-derived isopentenyl diphosphate is the biosynthetic precursor of ubiquinone prenyl side chain in tobacco BY-2 cells. Biochem. J 1998, 331, 615–621. [Google Scholar]
- Laule, O.; Fürholz, A.; Chang, H.S.; Zhu, T.; Wang, X.; Heifetz, P.B.; Gruissem, W.; Lange, M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar]
- Schuhr, C.A.; Radykewicz, T.; Sagner, S.; Latzel, C.; Zenk, M.H.; Arigoni, D.; Bacher, A.; Rohdich, F.; Eisenreich, W. Quantitative assessment of crosstalk between the two isoprenoid biosynthesis pathways in plants by NMR spectroscopy. Phytochem. Rev 2003, 2, 3–16. [Google Scholar]
- Wang, J.W.; Wu, J.Y. Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures. Appl. Microbiol. Biotechnol 2010, 88, 437–449. [Google Scholar]
- Estévez, J.M.; Cantero, A.; Reindl, A.; Reichler, S.; León, P. 1-Deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J. Biochem 2001, 276, 22901–22909. [Google Scholar]
- Carretero-Paulet, L.; Ahumada, I.; Cunillera, N.; Rodríguez-Concepción, M.; Ferrer, A.; Boronat, A.; Campos, N. Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-Deoxy-d-Xylulose 5-Phosphate reductoisomerase, the first committed enzyme of the 2-C-Methyl-d-Erythritol 4-Phosphate pathway. Plant Physiol 2002, 129, 1581–1591. [Google Scholar]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep 1999, 16, 565–574. [Google Scholar]
- Cunningham, F.X.; Sun, Z.; Chamovitz, D.; Hirschberg, J.; Gantt, E. Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp. strain PCC7942. Plant Cell 1994, 6, 1107–1121. [Google Scholar]
- Wang, K.; Ohnuma, S. Chain-length determination mechanism of isoprenyl diphosphate synthases and implications for molecular evolution. Trends Biochem. Sci 1999, 24, 445–451. [Google Scholar]
- Gao, W.; Hillwig, M.L.; Huang, L.; Cui, G.; Wang, X.; Kong, J.; Yang, B.; Peters, R.J. A functional genomics approach to tanshinone biosynthesis provides stereochemical insights. Org. Lett 2009, 11, 5170–5173. [Google Scholar]
- Kasimu, R.; Tanaka, K.; Tezuka, Y.; Gong, Z.N.; Li, J.X.; Basnet, P.; Namba, T.; katota, S. Comparative Sstudy of seventeen Salvia plants: Aldose reductase inhibitory activity of water and MeOH extracts and liquid chromatography-mass spectrometry (LC-MS) analysis of water extracts. Chem. Pharma. Bull 1998, 46, 500–504. [Google Scholar]
- Li, B.; Niu, F.D.; Li, Z.W.; Zhang, H.J.; Wang, D.Z.; Sun, H.D. Diterpenoids from the roots of Salvia przewalskii. Phytochemistry 1991, 30, 3815–3817. [Google Scholar]
- Wang, N.; Niwa, M.; Luo, H.W. Triterpenoids from Salvia przewalskii. Phytochemistry 1988, 27, 299–301. [Google Scholar]
- Li, M.H.; Peng, Y.; Xiao, P.G. Distribution of tanshinones in the genus Salvia (family Lamiaceae) from China and its systematic significance. J. Syst. Evol 2010, 48, 118–122. [Google Scholar]
- Yang, H.; Ip, S.P.; Sun, H.D.; Che, C.T. Constituents of Salvia trijuga. Pharm. Biol 2003, 41, 375–378. [Google Scholar]
- Romanova, A.; Pribylova, G.; Patudin, A.; Leskova, E.; Pakaln, D.; Ban’kovskii, A. The quinones of some species of sage. Chem. Nat. Compd 1972, 8, 231–232. [Google Scholar]
- Tezuka, Y.; Kasimu, R.; Li, J.X.; Basnet, P.; Tanaka, K.; Namba, T.; Kadota, S. ChemInform abstract: Constituents of roots of Salvia deserta SCHANG. (Xinjiang-Danshen). ChemInform 1998, 29. [Google Scholar] [CrossRef]
- Qiao, X.; Zhang, Y.T.; Ye, M.; Wang, B.R.; Han, J.; Guo, D. Analysis of chemical constituents and taxonomic similarity of Salvia species in China using LC/MS. Planta Med 2009, 75, 1613–1617. [Google Scholar]
- Chang, H.M.; Cheng, K.P.; Choang, T.F.; Chow, H.F.; Chui, K.Y.; Hon, P.M.; Tan, F.W.; Yang, Y.; Zhong, Z.P. Structure elucidation and total synthesis of new tanshinones isolated from Salvia miltiorrhiza Bunge (Danshen). J. Org. Chem 1990, 55, 3537–3543. [Google Scholar]
- Lee, A.R.; Wu, W.L.; Chang, W.L.; Lin, H.C.; King, M.L. Isolation and bioactivity of new tanshinones. J. Nat. Prod 1987, 50, 157–160. [Google Scholar]
- Matkowski, A.; Zielińska, S.; Oszmiański, J.; Lamer-Zarawska, E. Antioxidant activity of extracts from leaves and roots of Salvia miltiorrhiza Bunge, S. przewalskii Maxim., and S. verticillata L. Bioresour. Technol 2008, 99, 7892–7896. [Google Scholar]
- Adams, J.D.; Wall, M.; Garcia, C. Salvia columbariae contains tanshinones. Evid. Based Complement. Alternat. Med 2005, 2, 107–110. [Google Scholar]
- Li, M.H.; Chen, J.M.; Peng, Y.; Wu, Q.; Xiao, P.G. Investigation of Danshen and related medicinal plants in China. J. Ethnopharmacol 2008, 120, 419–426. [Google Scholar]
- Skała, E.; Wysokińska, H. Tanshinone production in roots of micropropagated Salvia przewalskii Maxim. Z. Naturforsch. C Biochem. Biophys. Biol. Virol 2005, 60, 583–586. [Google Scholar]
- Sun, P.; He, Y.L.; Zhou, L.L.; Qi, J.J.; Rui, Y.; Li, X.E. Effects of genotype and environment on active components of Salviae miltiorrhizae by HPLC. Asian J. Chem 2012, 24, 2146–2150. [Google Scholar]
- Lin, W.; Deng, J.; Lu, M.; Ou, X.; Lin, N.; Yang, G.; Liang, H. The research in production of Danshen in three main cultivations in China. J. Chin. Med. Mater 2008, 31, 338–340. [Google Scholar]
- Song, Z.; Wang, J.; Wang, H.; Zhao, F.; Hao, L. Studied of the floral biology, breeding characters of Salvia miltiorrhiza. Acta Hortic. Sin 2009, 36, 905–910. [Google Scholar]
- Chen, S.; Luo, H.; Yan, S. Preliminary investigation of chemical constituents in callus tissue of Danshen. J. China Pharm. Univ 1981, 16, 6–8. [Google Scholar]
- Hu, Y.; Zhang, R.; Hu, Z.; Wu, Y.; Shen, X.; Zhang, G.; Zhou, X. Callus culture and bioactive ingredients of Salvia miltiorrhiza. Plant Physiol. Commun 1992, 28, 424–425. [Google Scholar]
- Wu, C.T.; Mulabagal, V.; Nalawade, S.M.; Chen, C.L.; Yang, T.F.; Tsay, H.S. Isolation and quantitative analysis of cryptotanshinone, an active quinoid diterpene formed in callus of Salvia miltiorrhiza BUNGE. Pharm. Bull 2003, 26, 845–848. [Google Scholar]
- Zhao, J.; Chen, Z.; Wan, J. Clonic reproduction and plant regeneration from blade of Salvia miltiorrhiza Bunge. J. Cent. China Norm. Univ. Nat. Sci 1999, 33, 108–111. [Google Scholar]
- Tsutomu, N.; Hitoshi, M.; Masao, N.; Hideko, H.; Kaisuke, Y. Production of cryptotanshinone and ferruginol in cultured cells of Salvia miltiorrhiza. Phytochemistry 1983, 22, 721–722. [Google Scholar]
- Miyasaka, H.; Nasu, M.; Yamamoto, T.; Yoneda, K. Production of ferruginol by cell suspension cultures of Salvia miltiorrhiza. Phytochemistry 1985, 24, 1931–1933. [Google Scholar]
- Miyasaka, H.; Nasu, M.; Yamamoto, T.; Shiomi, Y.; Ohno, H.; Endo, Y.; Yoneda, K. Effect of nutritional factors on cryptotanshinone and ferruginol production by cell suspension cultures of Salvia miltiorrhiza. Phytochemistry 1987, 26, 1421–1424. [Google Scholar]
- Zhao, J.L.; Zhou, L.G.; Wu, J.Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol 2010, 87, 137–144. [Google Scholar]
- Miyasaka, H.; Nasu, M.; Yamamoto, T.; Endo, Y.; Yoneda, K. Production of cryptotanshinone and ferruginol by immobilized cultured cells of Salvia miltiorrhiza. Phytochemistry 1986, 25, 1621–1624. [Google Scholar]
- Yuan, J.; Tao, L.; Xu, J. Immobilization of callus tissue cells of Salvia miltiorrhiza and the characteristics of their products. Chin. J. Biotechnol 1990, 6, 199–205. [Google Scholar]
- Shimomura, K.; Kitazawa, T.; Okamura, N.; Yagi, A. Tanshinone production in adventitious roots and regenerates of Salvia miltiorrhiza. J. Nat. Prod 1991, 54, 1583–1587. [Google Scholar]
- Saito, K. Genetic engineering in tissue culture of medicinal plants. Plant Tissue Cult. Lett 1993, 10, 1–8. [Google Scholar]
- Zhi, B.H.; Alfermann, A.W. Diterpenoid production in hairy root cultures of Salvia miltiorrhiza. Phytochemistry 1993, 32, 699–703. [Google Scholar]
- Chen, H.; Chena, F.; Chiu, F.C.; Lo, C.M. The effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza. Enzyme Microb. Technol 2001, 28, 100–105. [Google Scholar]
- Ge, X.; Wu, J. Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Sci 2005, 168, 487–491. [Google Scholar]
- Wang, X.; Cui, G.; Huang, L.; Qiu, D. Effects of methyl jasmonate on accumulation and release of tanshinones in suspension cultures of Salvia miltiorrhiza hairy root. Zhongguo Zhongyao Zazhi 2007, 32, 300–302. [Google Scholar]
- Kai, G.; Xu, H.; Zhou, C.; Liao, P.; Xiao, J.; Luo, X.; You, L.; Zhang, L. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng 2011, 13, 319–327. [Google Scholar]
- Zhang, Y.; Song, J.; Zhao, B.; Liu, H. Crown gall culture and production of tanshinone in Salvia miltiorrhiza. Chin. J. Biotechnol 1995, 11, 137–141. [Google Scholar]
- Chen, H.; Yuan, J.P.; Chen, F.; Zhang, Y.L.; Song, J.Y. Tanshinone production in Ti-transformed Salvia miltiorrhiza cell suspension cultures. J. Biotechnol 1997, 58, 147–156. [Google Scholar]
- Chen, H.; Chen, F. Effects of yeast elicitor on the growth and secondary metabolism of a high-tanshinone-producing line of the Ti transformed Salvia miltiorrhiza cells in suspension culture. Process Biochem 2000, 35, 837–840. [Google Scholar]
- Song, J.; Qi, J.; Ren, C.; Fu, J.; Zhang, Y. Dynamics of growth and total tanshinones accumulation in crown gall cultures of Salvia miltiorrhiza. Yaoxue Xuebao 2000, 35, 929–931. [Google Scholar]
- Ming, Q.; Han, T.; Li, W.; Zhang, Q.; Zhang, H.; Zheng, C.; Huang, F.; Rahman, K.; Qin, L. Tanshinone IIA and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine 2012, 19, 330–333. [Google Scholar]
- Zhang, J.; Huang, M.; Guan, S.; Bi, H.C.; Pan, Y.; Duan, W.; Chan, S.Y.; Chen, X.; Hong, Y.H.; Bian, J.S.; et al. A mechanistic study of the intestinal absorption of cryptotanshinone, the major active Cconstituent of Salvia miltiorrhiza. J. Pharmacol. Exp. Ther 2006, 317, 1285–1294. [Google Scholar]
- Qiao, J.; Hou, P.; Li, Y.; Abliz, Z. Determination of tanshinone IIA in rat plasma and the pharmacokinetics by RP-HPLC method. Yaoxue Xuebao 2003, 38, 368–370. [Google Scholar]
- Hao, H.; Wang, G.; Cui, N.; Li, J.; Xie, L.; Ding, Z. Pharmacokinetics, absorption and tissue distribution of tanshinone IIA solid dispersion. Planta Med 2006, 72, 1311–1317. [Google Scholar]
- Guo, Z.J.; Zhang, Y.; Tang, X.; Li, H.; Sun, Q.S. Pharmacokinetic interaction between tanshinones and polyphenolic extracts of Salvia miltinorrhiza Bunge after intravenous administration in rats. Biol. Pharm. Bull 2008, 31, 1469–1474. [Google Scholar]
- Li, X.; Li, X.; Wang, L.; Li, Y.; Xu, Y.; Xue, M. Simultaneous determination of danshensu, ferulic acid, cryptotanshinone and tanshinone IIA in rabbit plasma by HPLC and their pharmacokinetic application in danxiongfang. J. Pharm. Biomed. Anal 2007, 44, 1106–1112. [Google Scholar]
- Li, X.; Wang, L.; Li, Y.; Xu, Y.; Xue, M. Pharmacokinetics of cryptotanshinone used alone or combined with danxiongfang in rabbits. Chin. Pharmacol. Bull 2007, 23, 1102–1105. [Google Scholar]
- Xue, M.; Cui, Y.; Wang, H.; Luo, Y.; Zhang, B.; Zhou, Z. Pharmacokinetics of cryptotanshinone and its metabolite in pigs. Acta Pharm. Sin 1999, 34, 81–84. [Google Scholar]
- Hao, H.; Wang, G.; Li, P.; Li, J.; Ding, Z. Simultaneous quantification of cryptotanshinone and its active metabolite tanshinone IIA in plasma by liquid chromatography/tandem mass spectrometry (LC–MS/MS). J. Pharm. Biomed. Anal 2006, 40, 382–388. [Google Scholar]
- Song, M.; Hang, T.J.; Zhang, Z.X.; Du, R.; Chen, J. Determination of cryptotanshinone and its metabolite in rat plasma by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 2005, 827, 205–209. [Google Scholar]
- Song, M.; Hang, T.J.; Zhang, Z.; Chen, H.Y. Effects of the coexisting diterpenoid tanshinones on the pharmacokinetics of cryptotanshinone and tanshinone IIA in rat. Eur. J. Pharm. Sci 2007, 32, 247–253. [Google Scholar]
- Li, J.; Wang, G.; Li, P.; Hao, H. Simultaneous determination of tanshinone IIA and cryptotanshinone in rat plasma by liquid chromatography-electrospray ionisation-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 2005, 826, 26–30. [Google Scholar]
- Park, E.J.; Ji, H.Y.; Kim, N.J.; Song, W.Y.; Kim, Y.H.; Kim, Y.C.; Sohn, D.H.; Lee, H.S. Simultaneous determination of tanshinone I, dihydrotanshinone I, tanshinone IIA and cryptotanshinone in rat plasma by liquid chromatography–tandem mass spectrometry: Application to a pharmacokinetic study of a standardized fraction of Salvia miltiorrhiza, PF2401-SF. Biomed. Chromatogr 2008, 22, 548–555. [Google Scholar]
- Liu, Y.; Li, X.; Li, Y.; Wang, L.; Xue, M. Simultaneous determination of danshensu, rosmarinic acid, cryptotanshinone, tanshinone IIA, tanshinone I and dihydrotanshinone I by liquid chromatographic–mass spectrometry and the application to pharmacokinetics in rats. J. Pharm. Biomed. Anal 2010, 53, 698–704. [Google Scholar]
- Yang, S.; Zhang, K.; Lin, X.; Miao, Y.; Meng, L.; Chen, W.; Tang, X. Pharmacokinetic comparisons of single herb extract of Fufang Danshen preparation with different combinations of its constituent herbs in rats. J. Pharm. Biomed. Anal 2012, 67–68, 77–85. [Google Scholar]
- Yuan, Y.; Jiang, X.H.; Zhou, J.; Yang, J.Y.; Wang, C.X. The absorption mechanism of tanshinon IIa in rat small intestine. West China J. Pharm. Sci 2002, 17, 248–250. [Google Scholar]
- Yan, H.; Wu, Q.; Du, S.; Yang, Y.; Zhou, L.; Li, X. Absorption mechanism of tanshinone II A, cryptotanshinone, tanshinone I and tanshinones extract in rat small intestine in vivo. Zhongguo Zhongyao Zazhi 2010, 35, 2917–2922. [Google Scholar]
- Nizamutdinova, I.T.; Lee, G.W.; Lee, J.S.; Cho, M.K.; Son, K.H.; Jeon, S.J.; Kang, S.S.; Kim, Y.S.; Lee, J.H.; Seo, H.G.; et al. Tanshinone I suppresses growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion molecules. Carcinogenesis 2008, 29, 1885–1892. [Google Scholar]
- Lee, D.S.; Lee, S.H.; Noh, J.G.; Hong, S.D. Antibacterial activities of cryptotanshinone and dihydrotanshinone I from a medicinal herb, Salvia miltiorrhiza Bunge. Biosci. Biotechnol. Biochem 1999, 63, 2236–2239. [Google Scholar]
- Chen, Y.; Tu, J.H.; He, Y.J.; Zhang, W.; Wang, G.; Tan, Z.R.; Zhou, G.; Fan, L.; Zhou, H.H. Effect of sodium tanshinone II A sulfonate on the activity of CYP1A2 in healthy volunteers. Xenobiotica 2009, 39, 508–513. [Google Scholar]
- Tan, X.; Yang, Y.; Cheng, J.; Li, P.; Inoue, I.; Zeng, X. Unique action of sodium tanshinone II-A sulfonate (DS-201) on the Ca2+ dependent BKCa activation in mouse cerebral arterial smooth muscle cells. Eur. J. Pharm 2011, 656, 27–32. [Google Scholar]
- Zhang, K.Q.; Bao, Y.; Wu, P.; Rosen, R.T.; Ho, C.T. Antioxidative components of tanshen (Salvia miltiorhiza Bung). J. Agric. Food Chem 1990, 38, 1194–1197. [Google Scholar]
- Kang, B.Y.; Chung, S.W.; Kim, S.H.; Ryu, S.Y.; Kim, T.S. Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology 2000, 49, 355–361. [Google Scholar]
- Tian, H.L.; Yu, T.; Xu, N.N.; Feng, C.; Zhou, L.Y.; Luo, H.W.; Chang, D.C.; Le, X.F.; Luo, K.Q. A novel compound modified from tanshinone inhibits tumor growth in vivo via activation of the intrinsic apoptotic pathway. Cancer Lett 2010, 297, 18–30. [Google Scholar]
- Weng, X.C.; Gordon, M.H. Antioxidant activity of quinones extracted from tanshen (Salvia miltiorrhiza Bunge). J. Agric. Food Chem 1992, 40, 1331–1336. [Google Scholar]
- Liu, J.; Zhu, J.; Du, Z.; Qin, B. Preparation and pharmacokinetic evaluation of Tashinone IIA solid lipid nanoparticles. Drug Dev. Ind. Pharm 2005, 31, 551–556. [Google Scholar]
- Zhang, W.; Liu, J.; Liu, X.; Chen, Z. Stealth tanshinone IIA-loaded solid lipid nanoparticles: Effects of poloxamer 188 coating on in vitro phagocytosis and in vivo pharmacokinetics in rats. Yaoxue Xuebao 2009, 44, 1421–1428. [Google Scholar]
- Li, Q.; Wang, Y.; Feng, N.; Fan, Z.; Sun, J.; Nan, Y. Novel polymeric nanoparticles containing tanshinone IIA for the treatment of hepatoma. J. Drug Targeting 2008, 16, 725–732. [Google Scholar]
- Hu, L.; Xing, Q.; Meng, J.; Shang, C. Preparation and enhanced oral bioavailability of cryptotanshinone-loaded solid lipid nanoparticles. AAPS PharmSciTech 2010, 11, 582–587. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem 2004, 39, 1033–1046. [Google Scholar]
- Yuexian, F.; Junfen, L.; Chuan, D. Preparation and study on the inclusion complexes of two tanshinone compounds with β-cyclodextrin. Spectrochim Acta Part A 2005, 61, 135–140. [Google Scholar]
- Su, C.C.; Chen, G.W.; Lin, J.G. Growth inhibition and apoptosis induction by tanshinone I in human colon cancer Colo 205 cells. Int. J. Mol. Med 2008, 22, 613–618. [Google Scholar]
- Zhou, X.; Jia, M.; Zhang, Y. The effect of Tanshinone I on proliferation and apoptosis of human gastric adenocarcinoma cell line SGC-7901. J. Mod. Oncol 2011, 19, 23–27. [Google Scholar]
- Nizamutdinova, I.T.; Lee, G.W.; Son, K.H.; Jeon, S.J.; Kang, S.S.; Kim, Y.S.; Lee, J.H.; Seo, H.G.; Chang, K.C.; Kim, H.J. Tanshinone I effectively induces apoptosis in estrogen receptor-positive (MCF-7) and estrogen receptor-negative (MDA-MB-231) breast cancer cells. Int. J. Oncol 2008, 33, 485–491. [Google Scholar]
- Gong, Y.; Li, Y.; Abdolmaleky, H.M.; Li, L.; Zhou, J.R. Tanshinones inhibit the growth of breast cancer cells through epigenetic modification of Aurora A expression and function. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Zheng, G.; Li, Z. Study on the anti-tumor effect and mechanism of tanshinone I. J. Prac. Oncol 2005, 94, 33–35. [Google Scholar]
- Gong, Y.; Li, Y.; Lu, Y.; Li, L.; Abdolmaleky, H.; Blackburn, G.L.; Zhou, J.R. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int. J. Cancer 2011, 129, 1042–1052. [Google Scholar]
- Li, Y.; Gong, Y.; Li, L.; Abdolmaleky, H.M.; Zhou, J.R. Bioactive tanshinone I inhibits the growth of lung cancer in part via downregulation of Aurora A function. Mol. Carcinog 2012. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, W.D.; Yang, H.Z.; Zhang, Y.; Fang, Z.G.; Liu, P.Q.; Lin, D.J.; Xiao, R.Z.; Hu, Y.; Wang, C.Z.; et al. Inactivation of PI3k/Akt signaling pathway and activation of caspase-3 are involved in tanshinone I-induced apoptosis in myeloid leukemia cells in vitro. Ann. Hematol 2010, 89, 1089–1097. [Google Scholar]
- Won, S.H.; Lee, H.J.; Jeong, S.J.; Lu, J.; Kim, S.H. Activation of p53 signaling and inhibition of androgen receptor mediate tanshinone IIA induced G1 arrest in LNCaP prostate cancer cells. Phytother. Res 2012, 26, 669–674. [Google Scholar]
- Won, S.H.; Lee, H.J.; Jeong, S.J.; Lee, E.O.; Jung, D.B.; Shin, J.M.; Kwon, T.R.; Yun, S.M.; Lee, M.H.; Choi, S.H.; et al. Tanshinone IIA induces mitochondria dependent apoptosis in prostate cancer cells in association with an inhibition of phosphoinositide 3-kinase/AKT pathway. Biol. Pharm. Bull 2010, 33, 1828–1834. [Google Scholar]
- Su, C.C.; Lin, Y.H. Tanshinone IIA inhibits human breast cancer cells through increased Bax to Bcl-xL ratios. Int. J. Mol. Med 2008, 22, 357–361. [Google Scholar]
- Yan, M.Y.; Chien, S.Y.; Kuo, S.J.; Chen, D.R.; Su, C.C. Tanshinone IIA inhibits BT-20 human breast cancer cell proliferation through increasing caspase 12, GADD153 and phospho-p38 protein expression. Int. J. Mol. Med 2012, 29, 855–863. [Google Scholar]
- Wang, X.; Wei, Y.; Yuan, S.; Liu, G.; Lu, Y.; Zhang, J.; Wang, W. Potential anticancer activity of tanshinone IIA against human breast cancer. Int. J. Cancer 2005, 116, 799–807. [Google Scholar]
- Lu, Q.; Zhang, P.; Zhang, X.; Chen, J. Experimental study of the anti-cancer mechanism of tanshinone IIA against human breast cancer. Int. J. Mol. Med 2009, 24, 773–780. [Google Scholar]
- Zhang, X.; Zhang, P.; Chen, J.; Lu, Q. A study on the effect of Tanshinone IIA against human breast cancer in vivo. Sichuan Daxue Xuebao Yixueban 2010, 41, 62–67. [Google Scholar]
- Du, R.; Zheng, H.; Wang, Y.; Yuan, S. Reversal of the malignant phenotypes by tanshinone IIA in human breast cancer MCF-7 cell lines and its molecular mechanism. West China West China J. Pharm. Sci 2009, 24, 42–45. [Google Scholar]
- Jing, J.; Zheng, H.; Wang, J.; Lin, P.; Zhang, J.; Xiong, Z.; Wu, Y.; Ren, J.; Yang, H.; Wang, X. Growth inhibition and multidrug resistance-reversing effect of Tanshinone IIA on human breast cancer cell with estrogen receptor negative. Sichuan Daxue Xuebao Yixueban 2007, 38, 391–395. [Google Scholar]
- Su, C.C.; Lin, Y.H. Tanshinone IIA down-regulates the protein expression of ErbB-2 and up-regulates TNF-alpha in colon cancer cells in vitro and in vivo. Int. J. Mol. Med 2008, 22, 847–851. [Google Scholar]
- Chiu, T.L.; Su, C.C. Tanshinone IIA induces apoptosis in human lung cancer A549 cells through the induction of reactive oxygen species and decreasing the mitochondrial membrane potential. Int. J. Mol. Med 2010, 25, 231–236. [Google Scholar]
- Cheng, C.Y.; Su, C.C. Tanshinone IIA may inhibit the growth of small cell lung cancer H146 cells by up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial membrane potential. Mol. Med. Rep 2010, 3, 645–650. [Google Scholar]
- Wang, J.; Zhou, X.; Yuan, H.; Huang, X.; Wu, L.; Zhang, F.; Liu, X. Effect of tanshinone IIA on proliferation and apoptosis of human lung cancer cell line A549/CDDP. J. Prac. Oncol 2010, 25, 684–688. [Google Scholar]
- Dai, Z.; Shi, J.; Wu, Q.; Yu, L.; Xu, Q. Apoptosis inducing effect of Tanshinone IIA onhuman lung adenocarcinoma A549 cells. Chin. Pharmacol. Bull 2010, 26, 111–114. [Google Scholar]
- Dai, Z.K.; Qin, J.K.; Huang, J.E.; Luo, Y.; Xu, Q.; Zhao, H.L. Tanshinone IIA activates calcium-dependent apoptosis signaling pathway in human hepatoma cells. J. Nat. Med 2012, 66, 192–201. [Google Scholar]
- Cheng, C.Y.; Su, C.C. Tanshinone IIA inhibits Hep-J5 cells by increasing calreticulin, caspase 12 and GADD153 protein expression. Int. J. Mol. Med 2010, 26, 379–385. [Google Scholar]
- Chien, S.Y.; Kuo, S.J.; Chen, Y.L.; Chen, D.R.; Cheng, C.; Su, C.C. Tanshinone IIA inhibits human hepatocellular carcinoma J5 cell growth by increasing Bax and caspase 3 and decreasing CD31 expression in vivo. Mol. Med. Rep 2012, 5, 282–286. [Google Scholar]
- Lee, W.Y.; Chiu, L.C.; Yeung, J.H. Cytotoxicity of major tanshinones isolated from Danshen (Salvia miltiorrhiza) on HepG2 cells in relation to glutathione perturbation. Food Chem. Toxicol 2008, 46, 328–338. [Google Scholar]
- Yuan, S.L.; Wei, Y.Q.; Wang, X.J.; Xiao, F.; Li, S.F.; Zhang, J. Growth inhibition and apoptosis induction of tanshinone II-A on human hepatocellular carcinoma cells. World J. Gastroenterol 2004, 10, 2024–2028. [Google Scholar]
- Tang, Z.Z.; Tang, Y.; Fu, L.B. Effect of tanshinone IIA on the growth behavior of human hepatoma cell line BEL-7402 in vitro and its mechanism. Diyi Junyi Daxue Xuebao 2003, 23. [Google Scholar]
- Zhai, X.; He, S.; Ren, M.; Chen, J.; Wang, Z.; Han, M.; Hou, H. Effect of Tanshinone IIA on expression of EGF and EGFR in hepatocellular carcinoma cell line SMMC-7721. Zhejiang Daxue Xuebao Yixueban 2009, 38, 163–169. [Google Scholar]
- Zhao, G.; He, S.; Fu, H.; Xu, J.; Wang, Y.; Ren, M. Effect of Tanshinone IIA on SMMC-7721 gene expression of TGF-β1. Chin. J. Gastroenterol. Hepatol 2006, 15, 396–398. [Google Scholar]
- Li, Q.; Li, C.; Huang, Z.; Luo, Y.; Wei, G.; Mei, G. The effect of three composition of Salviae miltiorrhizae on growth of Bel-7402 and peripheral blood stem cells. Prac. J. Cancer 2001, 16, 467–468. [Google Scholar]
- Chen, J.; Shi, D.Y.; Liu, S.L.; Zhong, L. Tanshinone IIA induces growth inhibition and apoptosis in gastric cancer in vitro and in vivo. Oncol. Rep 2012, 27, 523–528. [Google Scholar]
- Dong, X.; Dong, J.; Peng, G. Growth-inhibiting and apoptosis-inducing effects of Tanshinone II A on human gastric carcinoma cells. J. Huazhong Univ. Sci. Technol. Med. Sci 2007, 27, 706–709. [Google Scholar]
- Zhou, X.; Song, Z.; Wang, X. The effect of Tanshinone IIA on proliferation and apoptosis of human gastric adenocarcinoma cell line SGC-7901. J. Xi’an Jiaotong Univ. (Med. Sci.) 2007, 28. [Google Scholar]
- Dai, Z.; Huang, J.; Luo, W.; Xu, Q. Anticancer effect of Tanshinone IIA on human gastric carcinoma MGC-803 cells. Chin. Pharm. J 2011, 46, 1491–1495. [Google Scholar]
- Chen, J.; Zhong, L.; Qiang, L.; Hua, Z.; Lin, G.; Liu, S. Apoptosis of SGC7901 gastric cancer cell induced by Tanshinone IIA and the primary mechanisms. Fudan Univ. J. Med. Sci 2007, 34, 57–61. [Google Scholar]
- Wang, Y.; Li, Q.; Fan, Z.; Wang, Y.; Qiu, Y.; Jin, B.; Chen, X.; Yin, P. Tanshinone IIA induces apoptosis of pancreatic cancer cells via the SAPK/JNK signal pathway. World Chin. J. Digestol 2011, 19, 1028–1033. [Google Scholar]
- Qi, W.; Huang, Q.; Wang, C.; Qiu, L.; Yu, J. Significance and effect of tanshinone IIA on the expression of surviving in human bile duct carcinoma cell line. Chin. J. Hepatobiliary Surg 2008, 16, 452–454. [Google Scholar]
- Wei, X.; Zhou, L.; Hu, L.; Huang, Y. Tanshinone IIA arrests cell cycle and induces apoptosis in 786-O human renal cell carcinoma cells. Oncol. Lett 2012, 3, 1144–1148. [Google Scholar]
- Jiao, J.W.; Wen, F. Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Oncol. Rep 2011, 25, 781–788. [Google Scholar]
- Zhu, M.; Chen, P.; Chen, D.; Shi, X.; Kong, X.; Guo, L.; Zheng, F. The effects of inhibition and inducing apoptosis of Tanshinone II A on human ovarian cancer A2780 cell lines. J. Emerg. Tradit. Chin. Med 2009, 18, 596–597. [Google Scholar]
- Zhuang, Y.; Wang, H.; Du, R.; Zhang, C. The studies of apoptosis effect and its mechanisms of Tanshinone IIA on human ovarian cancer cell. J. Int. Obstet. Gynecol 2011, 38, 328–331. [Google Scholar]
- Meng, S.; Lu, X. Induction of apoptosis by tanshinone IIA in HeLa cells. J. Qiqihar Univ. Med 2011, 32, 175–177. [Google Scholar]
- Zhao, J.; Xu, Q.; Sun, J.; Xu, X. Tanshinone IIA induces apoptosis in human nasopharyngeal carcinoma CNE1 cells. Acad. J. Second Mil. Med. Univ 2011, 32, 879–883. [Google Scholar]
- Dai, Z.; Huang, D.; Shi, J.; Yu, L.; Wu, Q.; Xu, Q. Apoptosis inducing effect of tanshinone II(A) on human nasopharyngeal carcinoma CNE cells. Zhongguo Zhongyao Zazhi 2011, 36, 2129–2133. [Google Scholar]
- Wang, J.; Wang, X.; Jiang, S.; Yuan, S.; Lin, P.; Zhang, J.; Lu, Y.; Wang, Q.; Xiong, Z.; Wu, Y.; et al. Growth inhibition and induction of apoptosis and differentiation of tanshinone IIA in human glioma cells. J. Neurooncol 2007, 82, 11–21. [Google Scholar]
- Zhang, Y.; Wei, R.X.; Zhu, X.B.; Cai, L.; Jin, W.; Hu, H. Tanshinone IIA induces apoptosis and inhibits the proliferation, migration, and invasion of the osteosarcoma MG-63 cell line in vitro. Anticancer. Drugs 2012, 23, 212–219. [Google Scholar]
- Liu, J.J.; Zhang, Y.; Lin, D.J.; Xiao, R.Z. Tanshinone IIA inhibits leukemia THP-1 cell growth by induction of apoptosis. Oncol. Rep 2009, 21, 1075–1081. [Google Scholar]
- Mosaddik, M.A. In vitro cytotoxicity of tanshinones isolated from Salvia miltiorrhiza Bunge against P388 lymphocytic leukemia cells. Phytomedicine 2003, 10, 682–685. [Google Scholar]
- Zhang, K.; Li, J.; Meng, W.; Xing, H.; Yang, Y. C/EBPbeta and CHOP participate in tanshinone IIA-induced differentiation and apoptosis of acute promyelocytic leukemia cells in vitro. Int. J. Hematol 2010, 92, 571–578. [Google Scholar]
- Huang, R.; Yuan, S.; Song, Y.; Huang, G. Apoptosis of human leukemic HL-60 cells induced by Tanshinone IIA. J. Cancer 1998, 17, 164–166. [Google Scholar]
- Shan, D.; Gong, Y.; Guo, Y.; Lin, J.; Zhou, R.; Yang, X. Anti-tumor effect of tanshinone II, tetrandrine, honokiol, curcumin, oridonin and paeonol on leukemia cell lines. Sichuan Daxue Xuebao Yixueban 2012, 43, 362–366. [Google Scholar]
- Zhou, L.; Chan, W.K.; Xu, N.; Xiao, K.; Luo, H.; Luo, K.Q.; Chang, D.C. Tanshinone IIA, an isolated compound from Salvia miltiorrhiza Bunge, induces apoptosis in HeLa cells through mitotic arrest. Life Sci 2008, 83, 394–403. [Google Scholar]
- Liu, Z.; Yan, R.; Al-Salman, A.; Shen, Y.; Bu, Y.; Ma, J.; Luo, D.X.; Huang, C.; Jiang, Y.; Wilber, A.; et al. Epidermal growth factor induces tumour marker AKR1B10 expression through activator protein-1 signalling in hepatocellular carcinoma cells. Biochem. J 2012, 442, 273–282. [Google Scholar]
- Ye, Y.; Xu, W.; Zhong, W. Effects of cryptotanshinone on proliferation and apoptosis of Hela cell line of cervical cancer. Zhongguo Zhongyao Zazhi 2010, 35, 118–121. [Google Scholar]
- Chen, C.; Gong, J.; Miao, H.; Lin, H.; Lin, J. The effects of cryptotanshinone on the expression of survivin in human cholangiocarcinoma cell of HCCC-9810. Guangdong Med. J 2001, 32, 3028–3031. [Google Scholar]
- Chen, L.; Zheng, S.Z.; Sun, Z.G.; Wang, A.Y.; Huang, C.H.; Punchard, N.A.; Huang, S.L.; Gao, X.; Lu, Y. Cryptotanshinone has diverse effects on cell cycle events in melanoma cell lines with different metastatic capacity. Cancer Chemother. Pharmacol 2011, 68, 17–27. [Google Scholar]
- Chen, W.; Luo, Y.; Liu, L.; Zhou, H.; Xu, B.; Han, X.; Shen, T.; Liu, Z.; Lu, Y.; Huang, S. Cryptotanshinone inhibits cancer cell proliferation by suppressing Mammalian target of rapamycin-mediated cyclin D1 expression and Rb phosphorylation. Cancer Prev. Res 2010, 3, 1015–1025. [Google Scholar]
- Ge, Y.; Cheng, R.; Zhou, Y.; Shen, J.; Peng, L.; Xu, X.; Dai, Q.; Liu, P.; Wang, H.; Ma, X.; et al. Cryptotanshinone induces cell cycle arrest and apoptosis of multidrug resistant human chronic myeloid leukemia cells by inhibiting the activity of eukaryotic initiation factor 4E. Mol. Cell. Biochem 2012, 368, 17–25. [Google Scholar]
- Shin, D.S.; Kim, H.N.; Shin, K.D.; Yoon, Y.J.; Kim, S.J.; Han, D.C.; Kwon, B.M. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res 2009, 69, 193–202. [Google Scholar]
- Aggarwal, B.B.; Sethi, G.; Ahn, K.S.; Sandur, S.K.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Ichikawa, H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann. NY Acad. Sci 2006, 1091, 151–169. [Google Scholar]
- Catlett-Falcone, R.; Dalton, W.S.; Jove, R. STAT proteins as novel targets for cancer therapy. Signal transducer an activator of transcription. Curr. Opin. Oncol 1999, 11, 490–496. [Google Scholar]
- Sinibaldi, D.; Wharton, W.; Turkson, J.; Bowman, T.; Pledger, W.J.; Jove, R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: Role of activated STAT3 signaling. Oncogene 2000, 19, 5419–5427. [Google Scholar]
- Aoki, Y.; Feldman, G.M.; Tosato, G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 2003, 101, 1535–1542. [Google Scholar]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar]
- Tang, C.; Xue, H.L.; Huang, H.B.; Wang, X.G. Tanshinone IIA inhibits constitutive STAT3 activation, suppresses proliferation, and induces apoptosis in rat C6 glioma cells. Neurosci. Lett 2010, 470, 126–129. [Google Scholar]
- Park, I.J.; Kim, M.J.; Park, O.J.; Park, M.G.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett 2010, 298, 88–98. [Google Scholar]
- Shi, H.; Zhang, Q.; Li, H.; Chu, T.; Jin, H.; Mao, S. Growth inhibition of tanshinones on SPC-A-1 cell line and their structure-activity relationship. Zhongguo Feiai Zazhi 2011, 14, 7–12. [Google Scholar]
- Lee, W.Y.; Liu, K.W.; Yeung, J.H. Reactive oxygen species-mediated kinase activation by dihydrotanshinone in tanshinones-induced apoptosis in HepG2 cells. Cancer Lett 2009, 285, 46–57. [Google Scholar]
- Tsai, S.L.; Suk, F.M.; Wang, C.I.; Liu, D.Z.; Hou, W.C.; Lin, P.J.; Hung, L.F.; Liang, Y.C. Anti-tumor potential of 15,16-dihydrotanshinone I against breast adenocarcinoma through inducing G1 arrest and apoptosis. Biochem. Pharmacol 2007, 74, 1575–1586. [Google Scholar]
- Chuang, M.T.; Ho, F.M.; Wu, C.C.; Zhuang, S.Y.; Lin, S.Y.; Suk, F.M.; Liang, Y.C. 15,16-Dihydrotanshinone I, a compound of Salvia miltiorrhiza Bunge, induces apoptosis through inducing endoplasmic reticular stress in human prostate carcinoma cells. Evid. Based Complement. Alternat. Med 2011. [Google Scholar] [CrossRef]
- Breccia, M.; Lo-Coco, F. Arsenic trioxide for management of acute promyelocytic leukemia: Current evidence on its role in front-line therapy and recurrent disease. Expert Opin. Pharmacother 2012, 13, 1031–1043. [Google Scholar]
- Mi, J. Current treatment strategy of acute promyelocytic leukemia. Front. Med 2011, 5, 341–347. [Google Scholar]
- Petrie, K.; Zelent, A.; Waxman, S. Differentiation therapy of acute myeloid leukemia: Past, present and future. Curr. Opin. Hematol 2009, 16, 84–91. [Google Scholar]
- Yuan, S.; Huang, R.; Song, Y.; Wang, X.; Huang, G.; Yang, Y. Tanshinone II A induced differentiation of HL-60 cell line in vitro. J. Pract. Oncol 1997, 11, 253–256. [Google Scholar]
- Liang, Y.; Yang, Y.; Yuan, S.; Meng, W.; Liu, T.; Jia, Y. Acute promyelocytic leukemia cell differentiation induced by tanshinone II A and its molecular mechanism. Zhonghua Xueyexue Zazhi 2000, 21, 23–26. [Google Scholar]
- Du, R.; Zheng, H.; Wang, Y.P.; Meng, W.T.; Qin, H.; Yuan, S.L. Study of molecular mechanism of tanshinone II A inducing differentiation in acute promyelocytic leukemia NB4 cells. Zhongguo Zhongyao Zazhi 2008, 33, 2954–2958. [Google Scholar]
- Wu, Y.; Yang, Y.; Meng, W.; Li, Y.; Jia, Y.; Liu, T. Study on the differentiation of K562 cell-line induced by Tanshinone II A. Huaxi Yike Daxue Xuebao 2002, 33, 80–83. [Google Scholar]
- Liang, Y.; Song, W.X.; Wang, J.; Jing, L.P.; Qu, W.; Fu, R.; Wu, X.Z. A study on the cell differentiation induced by tanshinone IIA and its molecular mechanism in retinoic acid: Resistant acute promyelocytic leukemia. Zhonghua Neike Zazhi 2005, 44, 366–369. [Google Scholar]
- Liang, Y.; Yang, Y.; Yuan, S.; Liu, T.; Jia, Y.; Xu, C.; Niu, T.; Qin, H.; Qin, P. Terminal differentiation of human acute promyelocytic leukemia (APL) cells induced by Tanshinone II A in primary culture. Huaxi Yike Daxue Xuebao 2000, 31, 207–210. [Google Scholar]
- Liu, Z.G.; Yang, Y.M.; Meng, W.T.; Huang, C.L. Differentiation and apoptosis of NB4 cells synergistically induced by Tanshinone II A and all-trans retinoic acid. Sichuan Daxue Xuebao Yi Xue Ban 2004, 35, 788–791. [Google Scholar]
- Ramji, D.P.; Foka, P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J 2002, 365, 561–575. [Google Scholar]
- Popernack, P.M.; Truong, L.T.; Kamphuis, M.; Henderson, A.J. Ectopic expression of CCAAT/enhancer binding protein beta (C/EBPbeta) in long-term bone marrow cultures induces granulopoiesis and alters stromal cell function. J. Hematother. Stem Cell Res 2001, 10, 631–642. [Google Scholar]
- Ron, D.; Habener, J.F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 1992, 6, 439–453. [Google Scholar]
- Zhou, R.; Skalli, O. TGF-alpha differentially regulates GFAP, vimentin, and nestin gene expression in U-373 MG glioblastoma cells: Correlation with cell shape and motility. Exp. Cell Res 2000, 254, 269–278. [Google Scholar]
- Cuevas, P.; Diaz-Gonzalez, D.; Dujovny, M. Differentiation-inducing activity of neomycin in cultured rat glioma cells. Neurochem. Res 2004, 26, 401–403. [Google Scholar]
- Huang, G.; Yuan, S.; Zhou, H.; Jiang, Y. Tanshinone-induced differentiation in human cervical carcinoma cell line ME180. Chin. J. Pharmacol. Toxicol 1996, 10, 285–289. [Google Scholar]
- Shi, S.; Wang, G.; Ma, Z.; Shi, J.; Lin, C.; Li, Q. The effect of TanshinoneIIA on the morphology and terminal differentiation of MG-63 cell. Prog. Mod. Biomed 2008, 8, 801–804. [Google Scholar]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol 2002, 29, 15–18. [Google Scholar]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar]
- Wang, H.; Gao, X.; Zhang, B. Tanshinone: An inhibitor of proliferation of vascular smooth muscle cells. J. Ethnopharmacol 2005, 99, 93–98. [Google Scholar]
- Tsai, M.Y.; Yang, R.C.; Wu, H.T.; Pang, J.H.; Huang, S.T. Anti-angiogenic effect of Tanshinone IIA involves inhibition of matrix invasion and modification of MMP-2/TIMP-2 secretion in vascular endothelial cells. Cancer Lett 2011, 310, 198–206. [Google Scholar]
- Xu, C.; Xi, T. Anti-migration and anti-angiogenic effects of tanshinone IIA on breast cancer cell MDA-MB-435. J. Chin. Pharm. Univ 2009, 40, 565–570. [Google Scholar]
- Gao, L.; Xu, C.; He, Y.; Xi, T. Inhibitary effect of Tanshinone IIA on vasculogenic mimicry in human breast cancer cell line MDA-MB-231 in vitro. Pharm. Clin. Res 2011, 19, 315–317. [Google Scholar]
- Fu, H.; He, S.; Xu, J.; Zhao, G.; Wang, Y.; Ren, M. Effect of Tanshinone IIA on vascular endothelial growth factor expression in hepatocellular carcinoma cell line SMMC-7721. J. Xi’an Jiaotong Univ. (Med. Sci.) 2009, 30, 115–118. [Google Scholar]
- Zhou, J.; Zheng, J.; Xiao, W.; Liu, Y. Influence of Tanshinone IIA on cell proliferation and vascular endothelial growth factor expression in human bile duct carcinoma cell line HCCC-9810. J. Chongqing Med. Univ 2010, 35, 1798–1801. [Google Scholar]
- Zong, X.; Feng, Y.; Wang, X.; Xing, G.; Wu, M.; Zhu, Y. Effects of Tan II A on the proliferation, apoptosis and expression of HIF-1α of human gastric cancer cell line SGC7901 under hypoxia. World Chin. J. Digestol 2009, 17, 642–646. [Google Scholar]
- Feng, Y.; Zong, X.; Xing, G.; Wu, M.; Zhu, Y. Effects of Tan IIA on the expression of HIF-1α and c-Myc in gastric cancer SGC-7901 cell under hypoxia. Shandong Med. J 2010, 50, 7–8. [Google Scholar]
- Zhou, L.; Liu, X.; Wang, Y.; Fan, Z.; Sun, Y.; Li, Q. Tanshinone IIA inhibits angiogenesis in subcutaneous colorectal cancer xenografts in mice. World Chin. J. Digestol 2009, 17, 3203–3209. [Google Scholar]
- Hur, J.M.; Shim, J.S.; Jung, H.J.; Kwon, H.J. Cryptotanshinone but not tanshinone IIA inhibits angiogenesis in vitro. Exp. Mol. Med 2005, 37, 133–137. [Google Scholar]
- Bian, W.; Chen, F.; Bai, L.; Zhang, P.; Qin, W. Dihydrotanshinone I inhibits angiogenesis both in vitro and in vivo. Acta Biochim. Biophys. Sin. (Shanghai) 2008, 40, 1–6. [Google Scholar]
- Dat, N.T.; Jin, X.; Lee, J.H.; Lee, D.; Hong, Y.S.; Lee, K.; Kim, Y.H.; Lee, J.J. Abietane diterpenes from Salvia miltiorrhiza inhibit the activation of hypoxia-inducible factor-1. J. Nat. Prod 2007, 70, 1093–1097. [Google Scholar]
- Bian, W.; Xu, Y.; Wang, L.; Chen, F. The antiangiogenesis effect of cryptotanshinone on chick embryo chorioallantoic membrane. J. Chin. Microcirc 2007, 12, 23–26. [Google Scholar]
- Zhang, P.; Pei, Y.; Qi, X.; Pu, B. Effects of certain drugs on the expression of antigens on human pulmonary giant cell carcinoma. Chin. Tranit. Herbal Drugs 1999, 30, 352–355. [Google Scholar]
- Liu, J.J.; Lin, D.J.; Liu, P.Q.; Huang, M.; Li, X.D.; Huang, R.W. Induction of apoptosis and inhibition of cell adhesive and invasive effects by tanshinone IIA in acute promyelocytic leukemia cells in vitro. J. Biomed. Sci 2006, 13, 813–823. [Google Scholar]
- Shan, Y.F.; Shen, X.; Xie, Y.K.; Chen, J.C.; Shi, H.Q.; Yu, Z.P.; Song, Q.T.; Zhou, M.T.; Zhang, Q.Y. Inhibitory effects of tanshinone II-A on invasion and metastasis of human colon carcinoma cells. Acta Pharmacol. Sin 2009, 30, 1537–1542. [Google Scholar]
- Xu, Y.; Tian, F.; Li, R.; Liu, Z. Tanshinone II-A inhibits invasion and metastasis of human hepatocellular carcinoma cells in vitro and in vivo. Tumori 2009, 95, 789–795. [Google Scholar]
- Ye, Z.; Ye, P.; Yang, Q. Inhibitory action of transhinone II A on human gastric cancer cell line MKN-45. Chin. J. Surg. Integr. Tradit. West. Med 2009, 15, 294–298. [Google Scholar]
- Lee, C.Y.; Sher, H.F.; Chen, H.W.; Liu, C.C.; Chen, C.H.; Lin, C.S.; Yang, P.C.; Tsay, H.S.; Chen, J.J. Anticancer effects of tanshinone I in human non-small cell lung cancer. Mol. Cancer Ther 2008, 7, 3527–3538. [Google Scholar]
- Qin, X.Y.; Li, T.; Yan, L.; Liu, Q.S.; Tian, Y. Tanshinone IIA protects against immune-mediated liver injury through activation of T-cell subsets and regulation of cytokines. Immunopharmacol. Immunotoxicol 2010, 32, 51–55. [Google Scholar]
- Gao, Y.G. Anti-inflammatory actions of tanshinone. Zhong Xiyi Jiehe Zazhi 1983, 3, 300–301. [Google Scholar]
- Kim, S.Y.; Moon, T.C.; Chang, H.W.; Son, K.H.; Kang, S.S.; Kim, H.P. Effects of tanshinone I isolated from Salvia miltiorrhiza bunge on arachidonic acid metabolism and in vivo inflammatory responses. Phytother. Res 2002, 16, 616–620. [Google Scholar]
- Jang, S.I.; Jeong, S.I.; Kim, K.J.; Kim, H.J.; Yu, H.H.; Park, R.; Kim, H.M.; You, Y.O. Tanshinone IIA from Salvia miltiorrhiza inhibits inducible nitric oxide synthase expression and production of TNF-alpha, IL-1beta and IL-6 in activated RAW 264.7 cells. Planta Med 2003, 69, 1057–1059. [Google Scholar]
- Autexier, C.; Lue, N.F. The structure and function of telomerase reverse transcriptase. Annu. Rev. Biochem 2006, 75, 493–517. [Google Scholar]
- Cao, Y.; Li, H.; Deb, S.; Liu, J.P. TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene 2002, 21, 3130–3138. [Google Scholar]
- Rahman, R.; Latonen, L.; Wiman, K.G. hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene 2005, 24, 1320–1327. [Google Scholar]
- Park, J.I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009, 460, 66–72. [Google Scholar]
- Qin, P.; Yang, Y.; Qu, Y.; Yuan, S.; Meng, W.; Liang, Y.; Liu, T.; Jia, Y. Change of telomerase activities before and after the leukemia cell differentiation induced by Tanshinone. J. West China Univ. Med. Sci 2002, 33, 397–400. [Google Scholar]
- Li, H.; Liu, X.; Wang, H.; Wang, J.; Qu, Y.; Mu, D. Effect of Tanshinone IIA on expression of human telomerase reverse transcriptase in HL-60 cells. J. Appl. Clin. Pediatr 2012, 27, 170–172. [Google Scholar]
- Song, Y.; Yuan, S.L.; Yang, Y.M.; Wang, X.J.; Huang, G.Q. Alteration of activities of telomerase in tanshinone IIA inducing apoptosis of the leukemia cells. Zhongguo Zhongyao Zazhi 2005, 30, 207–211. [Google Scholar]
- Liu, X.D.; Fan, R.F.; Zhang, Y.; Yang, H.Z.; Fang, Z.G.; Guan, W.B.; Lin, D.J.; Xiao, R.Z.; Huang, R.W.; Huang, H.Q.; et al. Down-regulation of telomerase activity and activation of caspase-3 are responsible for tanshinone I-induced apoptosis in monocyte leukemia cells in vitro. Int. J. Mol. Sci 2010, 11, 2267–2280. [Google Scholar]
- Soares, J.; Keppler, B.R.; Wang, X.; Lee, K.H.; Jarstfer, M.B. ortho-Quinone tanshinones directly inhibit telomerase through an oxidative mechanism mediated by hydrogen peroxide. Bioorg. Med. Chem. Lett 2011, 21, 7474–7478. [Google Scholar]
- Keppler, B.R.; Jarstfer, M.B. Inhibition of telomerase activity by preventing proper assemblage. Biochemistry 2004, 43, 334–343. [Google Scholar]
- Holt, S.E.; Aisner, D.L.; Baur, J.; Tesmer, V.M.; Dy, M.; Ouellette, M.; Trager, J.B.; Morin, G.B.; Toft, D.O.; Shay, J.W.; et al. Functional requirement of p23 and Hsp90 in telomerase complexes. Gene Dev 1999, 13, 817–826. [Google Scholar]
- Keppler, B.R.; Grady, A.T.; Jarstfer, M.B. The biochemical role of the heat shock protein 90 chaperone complex in establishing human telomerase activity. J. Biol. Chem 2006, 281, 19840–19848. [Google Scholar]
- Zhang, Z.; Zhang, J.; Jin, L.; Song, T.; Wu, G.; Gao, J. Tanshinone IIA interacts with DNA by minor groove-binding. Biol. Pharm. Bull 2008, 31, 2342–2345. [Google Scholar]
- Zhang, Z.; Gao, J.; Wang, Y.; Song, T.; Zhang, J.; Wu, G.; Zhang, T.; Du, G. Tanshinone IIA triggers p53 responses and apoptosis by RNA polymerase II upon DNA minor groove binding. Biochem. Pharmacol 2009, 78, 1316–1322. [Google Scholar]
- Scicchitano, D.A.; Olesnicky, E.C.; Dimitri, A. Transcription and DNA adducts: What happens when the message gets cut off? DNA repair (Amst) 2004, 3, 1537–1548. [Google Scholar]
- Straney, D.C.; Crothers, D.M. Effect of drug-DNA interactions upon transcription initiation at the lac promoter. Biochemistry 1987, 26, 1987–1995. [Google Scholar]
- Mote, J.; Ghanouni, P.; Reines, D. A DNA minor groove-binding ligand both potentiates and arrests transcription by RNA polymerase II. Elongation factor SII enables readthrough at arrest sites. J. Mol. Biol 1994, 236, 725–737. [Google Scholar]
- Zhang, Z.; Wang, Y.; Song, T.; Gao, J.; Wu, G.; Zhang, J.; Qian, X. DNA double helix unwinding triggers transcription block-dependent apoptosis: A semiquantitative probe of the response of ATM, RNAPII, and p53 to two DNA intercalators. Chem. Res. Toxicol 2009, 22, 483–491. [Google Scholar]
- Luo, Z.; Zheng, J.; Lu, Y.; Bregman, D.B. Ultraviolet radiation alters the phosphorylation of RNA polymerase II large subunit and accelerates its proteasome-dependent degradation. Mutat. Res 2001, 486, 259–274. [Google Scholar]
- Ljungman, M.; Lane, D.P. Transcription—Guarding the genome by sensing DNA damage. Nat. Rev. Cancer 2004, 4, 727–737. [Google Scholar]
- Zhang, Y.; Won, S.H.; Jiang, C.; Lee, H.J.; Jeong, S.J.; Lee, E.O.; Zhang, J.; Ye, M.; Kim, S.H.; Lu, J. Tanshinones from Chinese medicinal herb Danshen (Salvia miltiorrhiza Bunge) suppress prostate cancer growth and androgen receptor signaling. Pharm. Res 2012, 29, 1595–1608. [Google Scholar]
- Wang, D.B.; Liu, A.S. Tanshinone in the treatment of acne (a primary report of 20 cases). Zhongguo Yixue Kexueyuan Xuebao 1980, 2, 187–188. [Google Scholar]
- Ju, Q.; Yin, X.; Shi, J.; Xin, Y.; Kang, X.; Chen, Y.; Cui, P.; Cao, Y.; Xia, L. Effects of cryptotanshinone and tanshinone A on proliferation, lipid synthesis and expression of androgen receptor mRNA in human sebocytes in vitro. Chin. J. Dermatol 2005, 38, 98–101. [Google Scholar]
- Gao, Y.; Wang, L.; Tang, C. Sex Hormone-like activity of tanshinone. Zhongguo Yixue Kexueyuan Xuebao 1980, 2, 189–191. [Google Scholar]
- Xu, D.; Lin, T.H.; Li, S.; Da, J.; Wen, X.Q.; Ding, J.; Chang, C.; Yeh, S. Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett 2012, 316, 11–22. [Google Scholar]
- Xu, D.; Lin, T.H.; Zhang, C.; Tsai, Y.C.; Li, S.; Zhang, J.; Yin, M.; Yeh, S.; Chang, C. The selective inhibitory effect of a synthetic tanshinone derivative on prostate cancer cells. Prostate 2012, 72, 803–816. [Google Scholar]
- Wu, C.Y.; Hsieh, C.Y.; Huang, K.E.; Chang, C.; Kang, H.Y. Cryptotanshinone down-regulates androgen receptor signaling by modulating lysine-specific demethylase 1 function. Int. J. Cancer 2011, 131, 1423–1434. [Google Scholar]
- Liu, W.; Zhou, J.; Geng, G.; Shi, Q.; Sauriol, F.; Wu, J.H. Antiandrogenic, maspin induction, and antiprostate cancer activities of tanshinone IIA and its novel derivatives with modification in ring A. J. Med. Chem 2012, 55, 971–975. [Google Scholar]
- Li, X.H.; Yang, X.M.; Wu, X.K. Effects of cryptotanshinone in lowering androgens synthesis for the prenatally androgenized male rats. Zhong Xiyi Jiehe Zazhi 2008, 28, 1001–1004. [Google Scholar]
- Yang, X.; Zhang, Y.; Wu, X.; Bae, C.S.; Hou, L.; Kuang, H.; Wang, Y.; Stener-Victorin, E. Cryptotanshinone reverses reproductive and metabolic disturbances in prenatally androgenized rats via regulation of ovarian signaling mechanisms and androgen synthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol 2011, 300, R869–R875. [Google Scholar]
- Zhao, L.L.; Zhang, Y.H.; Wang, N.M.; Wu, X.K.; Hou, L.H. Impact of cryptotanshinone on the reproductivity and metabolism of male mice with Akt2 deletion. Zhonghua Nankexue 2011, 17, 662–668. [Google Scholar]
- Qiu, F.; Zhang, R.; Wang, G.; Gao, C.; Sun, J.; Jiang, J.; Ma, Y. Activation of CYP3A-mediated testosterone 6beta-hydroxylation by tanshinone IIA and midazolam 1-hydroxylation by cryptotanshinone in human liver microsomes. Xenobiotica 2010, 40, 800–806. [Google Scholar]
- Wang, X.; Yeung, J.H. Inhibitory effect of tanshinones on rat CYP3A2 and CYP2C11 activity and its structure-activity relationship. Fitoterapia 2011, 82, 539–545. [Google Scholar]
- Zhou, A.; Hu, P.; Ren, Q.; Zhou, Y.; Huang, G.; Kong, X.; Guo, L.; Zheng, F. Effect of Tanshinone II A combined with cisplatin on the growth and p53 protein expression of Hela cells. J. Yunyang Med. Coll 2007, 25. [Google Scholar]
- Zhou, A.; Hu, P.; Huang, G.; Duan, Y.; Ren, Q.; Yang, B.; Zhou, Y.; Huang, Y. Effect of tanshinone II A combined with cisplatin on apoptosis of Hela cells. J. Xinxiang Med. Coll 2010, 27, 129–131. [Google Scholar]
- Wang, D.; Tian, Y.; Jiang, Y.; Ren, B.; Fang, Q.; Li, Y. Study on apoptosis and inhibited growth of human cervical carcinoma HeLa cell induced by Tanshinon II A and 5-FU. Chin J. Mod. Med 2008, 18, 3237–3240. [Google Scholar]
- Gao, J.; Zheng, S.; Liang, T.; Geng, L.; Wu, L. Effect of Tanshinone IIA combined with oxaliplatin on human hepatocellular carcinoma cell line SMMC-7721. Chin. Pharm. J 2007, 42, 995–998. [Google Scholar]
- He, X.; Zeng, B.; Liu, H. Effect of tanshinone II A on acquired multi-drug resistance of S180’S tumor and expression of P-gp, LRP and TOPO II in mice. J. Tradit. Chin. Med. Univ. Hunan 2010, 30, 16–18. [Google Scholar]
- Kim, J.H.; Jeong, S.J.; Kwon, T.R.; Yun, S.M.; Jung, J.H.; Kim, M.; Lee, H.J.; Lee, M.H.; Ko, S.G.; Chen, C.Y.; et al. Cryptotanshinone enhances TNF-alpha-induced apoptosis in chronic myeloid leukemia KBM-5 cells. Apoptosis 2011, 16, 696–707. [Google Scholar]
- Park, I.J.; Kim, M.J.; Park, O.J.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Cryptotanshinone induces ER stress-mediated apoptosis in HepG2 and MCF7 cells. Apoptosis 2012, 17, 248–257. [Google Scholar]
- Ye, Y.; Xu, W.; Zhong, W.; Li, Y.; Wang, C. Combination treatment with dihydrotanshinone I and irradiation enhances apoptotic effects in human cervical cancer by HPV E6 down-regulation and caspases activation. Mol. Cell. Biochem 2012, 363, 191–202. [Google Scholar]
- Yang, Y.M.; Liu, T. Complete remission of acute promyelocytic leukemia resisting all-trans retinoic acid of one case treated by tanshinone II A. Sichuan Daxue Xuebao Yixueban 2006, 37, 965–967. [Google Scholar]
- Yang, L.; Gong, Y.P.; Yang, Y.M.; Luo, S. A successful case of tanshinone II A treatment for relapsed acute promyelocytic leukemia after maintainance therapy of all-trans retinoic acid and arsenic trioxide. Sichuan Daxue Xuebao Yixueban 2010, 41, 1065–1067. [Google Scholar]
- Ji, B.; Wang, S.; Jing, B. Study of composite Salviae miltiorrhizae injection on acute leukemia. Mod. J. Integr. Tradit. Chin. West. Med 2004, 28, 2958–2959. [Google Scholar]
- Jiang, D.; Li, Q.; Ding, H.; Huang, J.; Wang, X.; Shi, L. Clinical research on prevention of recurrence by trans-umbilical-portal vein perfusion of FufangDanshen in patients with hepatocellular carcinoma. Pract. J. Cancer 2007, 22, 365–367. [Google Scholar]
- Li, Y.; Zhu, J.; Li, Y.; Wang, Y.; Zhang, W. The efficiencies of hepatic artery infusion with the liquid compound of Radix Salvia miltiorrhizae and segmental hepatic artery chemoembolization in HCC. Chin. Imaging J. Integr. Tradit. West. Med 2006, 4, 352–355. [Google Scholar]
- Chen, X.; Liang, Q.; Li, X.; Zhang, Y.; Li, J.; Liang, Z.; Zhang, Y. Effect of composite Salviae dropping pill combined with chemotherapy in 41 cases with pancreatic carcinoma. J. Oncol 2005, 13, 49–51. [Google Scholar]
- Qian, L.; Zhao, Y. The cooperation group of phase II clinical trial of compound huangdai tablet: Phase II clinical trial of compound Huangdai tablet in newly diagnosed acute promyelocytic leukemia. Chin. J. Hematol 2006, 27, 801–804. [Google Scholar]
- Xiang, Y.; Huang, S.; Guo, A.; Wei, A.; Zhang, L.; Sun, S.; Chen, N.; Cheng, Y.; Wang, D. The influence on long-term survey of the patients with acute promyelocytic leukemia treated alternatively with compound huangdai tablets and chemotherapy. J. Clin. Hematol 2003, 16, 204–206. [Google Scholar]
- Huang, S.; Guo, A.; Xiang, Y.; Wang, X.; Lin, H.; Fu, L. Clincal study on the treatment of acute promyelocytic leukemia mainly with composite indigo naturalis tablets. Chin. J. Hematol 1995, 16, 26–28. [Google Scholar]
- Cheng, Y.; Huang, S.; Xing, Y.; Chen, N.; Sun, F.; Guo, A.; Wei, A.; Zhang, L. The clinical Sstudy of relapsed acute promyelocytic leukemia treated with compound Huangdai tablets. J. Emerg. Tradit. Chin. Med 2007, 16. [Google Scholar]
- Sun, F.; Chen, N.; Cheng, Y. Compound realgar and natural indigo tablets in treatment of acute promyelocytic leukemia: A summary of experience in 204 cases. Zhongxiyi Jiehe Xue Bao 2008, 6, 639–642. [Google Scholar]
- Xiang, Y.; Huang, S.; Guo, A.; Wang, Q.; Zhang, C.; Yan, J.; Chen, A. The analysis of therapeutic efficiency on treating acute promyelocytic leukemia (APL) with compound Huangdai tablets. J. Clin. Hematol 2000, 13, 11–12. [Google Scholar]
- Zhang, C.; Huang, S.; Xiang, Y.; Guo, A. Induction of apoptosis by compound “Huang Dai” tablet In acute promyelocytic leukemia. Med. J. Chin. People’s Liberation Army 2003, 28, 556–557. [Google Scholar]
- Wang, L.; Zhou, G.B.; Liu, P.; Song, J.H.; Liang, Y.; Yan, X.J.; Xu, F.; Wang, B.S.; Mao, J.H.; Shen, Z.X.; et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 4826–4831. [Google Scholar]
- Wang, X.; Nakagawa-Goto, K.; Bastow, K.F.; Don, M.J.; Lin, Y.L.; Wu, T.S.; Lee, K.H. Antitumor agents. 254. Synthesis and biological evaluation of novel neo-tanshinlactone analogues as potent anti-breast cancer agents. J. Med. Chem 2006, 49, 5631–5634. [Google Scholar]
- Sashidhara, K.V.; Rosaiah, J.N.; Kumar, M.; Gara, R.K.; Nayak, L.V.; Srivastava, K.; Bid, H.K.; Konwar, R. Neo-tanshinlactone inspired synthesis, in vitro evaluation of novel substituted benzocoumarin derivatives as potent anti-breast cancer agents. Bioorg. Med. Chem. Lett 2010, 20, 7127–7131. [Google Scholar]
- Dong, Y.; Shi, Q.; Pai, H.C.; Peng, C.Y.; Pan, S.L.; Teng, C.M.; Nakagawa-Goto, K.; Yu, D.; Liu, Y.N.; Wu, P.C.; et al. Antitumor agents. 272. Structure–Activity relationships and in vivo selective anti-breast cancer activity of novel neo-tanshinlactone analogues. J. Med. Chem 2010, 53, 2299–2308. [Google Scholar]
- Dong, Y.; Nakagawa-Goto, K.; Lai, C.Y.; Morris-Natschke, S.L.; Bastow, K.F.; Lee, K.H. Antitumor agents 287. Substituted 4-amino-2H-pyran-2-one (APO) analogs reveal a new scaffold from neo-tanshinlactone with in vitro anticancer activity. Bioorg. Med. Chem. Lett 2011, 21, 2341–2344. [Google Scholar]
- Wang, X.; Bastow, K.F.; Sun, C.M.; Lin, Y.L.; Yu, H.J.; Don, M.J.; Wu, T.S.; Nakamura, S.; Lee, K.H. Antitumor agents. 239. Isolation, structure elucidation, total synthesis, and anti-breast cancer activity of neo-tanshinlactone from Salvia miltiorrhiza. J. Med. Chem 2004, 47, 5816–5819. [Google Scholar]
- Anonymous. Therapeutic effect of sodium tanshinone IIA sulfonate in patients with coronary heart disease. A double blind study. Shanghai Cooperative Group for the Study of Tanshinone IIA. J. Tradit. Chin. Med 1984, 4, 20–24. [Google Scholar]
- Chen, K. The therapeutic effect of purified coronary heart II tablets on 112 cases of angina pectoris by double blind method. Zhonghua Xinxueguanbing Zazhi 1982, 10, 85–89. [Google Scholar]
- Shi, Y. Comparative Study of composite Danshen droplet pills and sordi in treatment of patients with chronic stable angina. Zhongguo Zhongxiyi Jiehe Zazhi 1997, 17, 23–25. [Google Scholar]
- Chan, S.E.; Lai, H.W.; Su, C.C.; Kuo, S.J.; Chien, S.Y.; Lin, H.Y.; Chen, D.R. Effect of supplementation of Tanshinone IIA and sodium Tanshinone IIA sulfonate on the anticancer effect of epirubicin: An in vitro study. Evid. Based Complement. Alternat. Med 2011. [Google Scholar] [CrossRef]
- Tian, H.; Ip, L.; Luo, H.; Chang, D.C.; Luo, K.Q. A high throughput drug screen based on fluorescence resonance energy transfer (FRET) for anticancer activity of compounds from herbal medicine. Br. J. Pharmacol 2007, 150, 321–334. [Google Scholar]
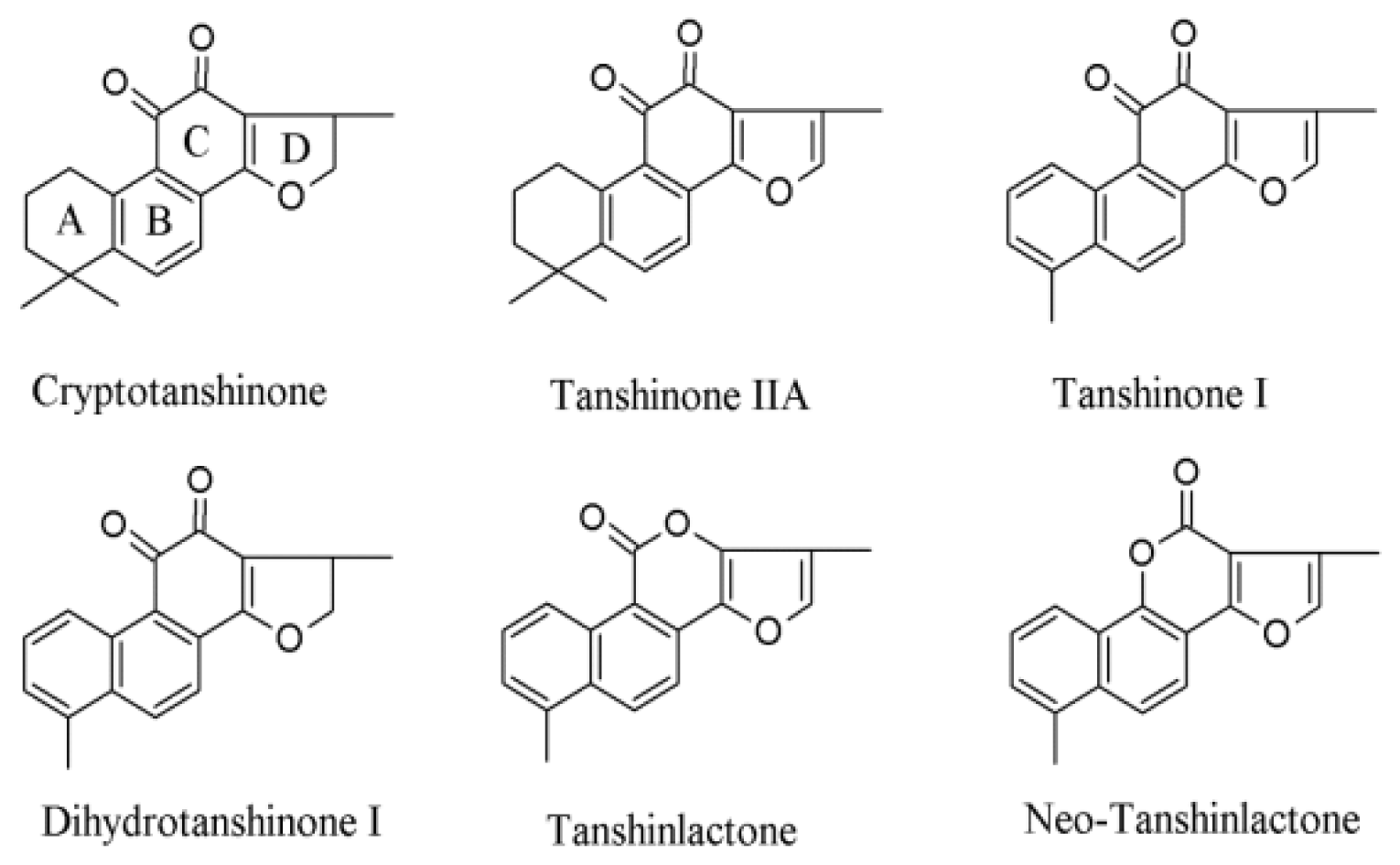
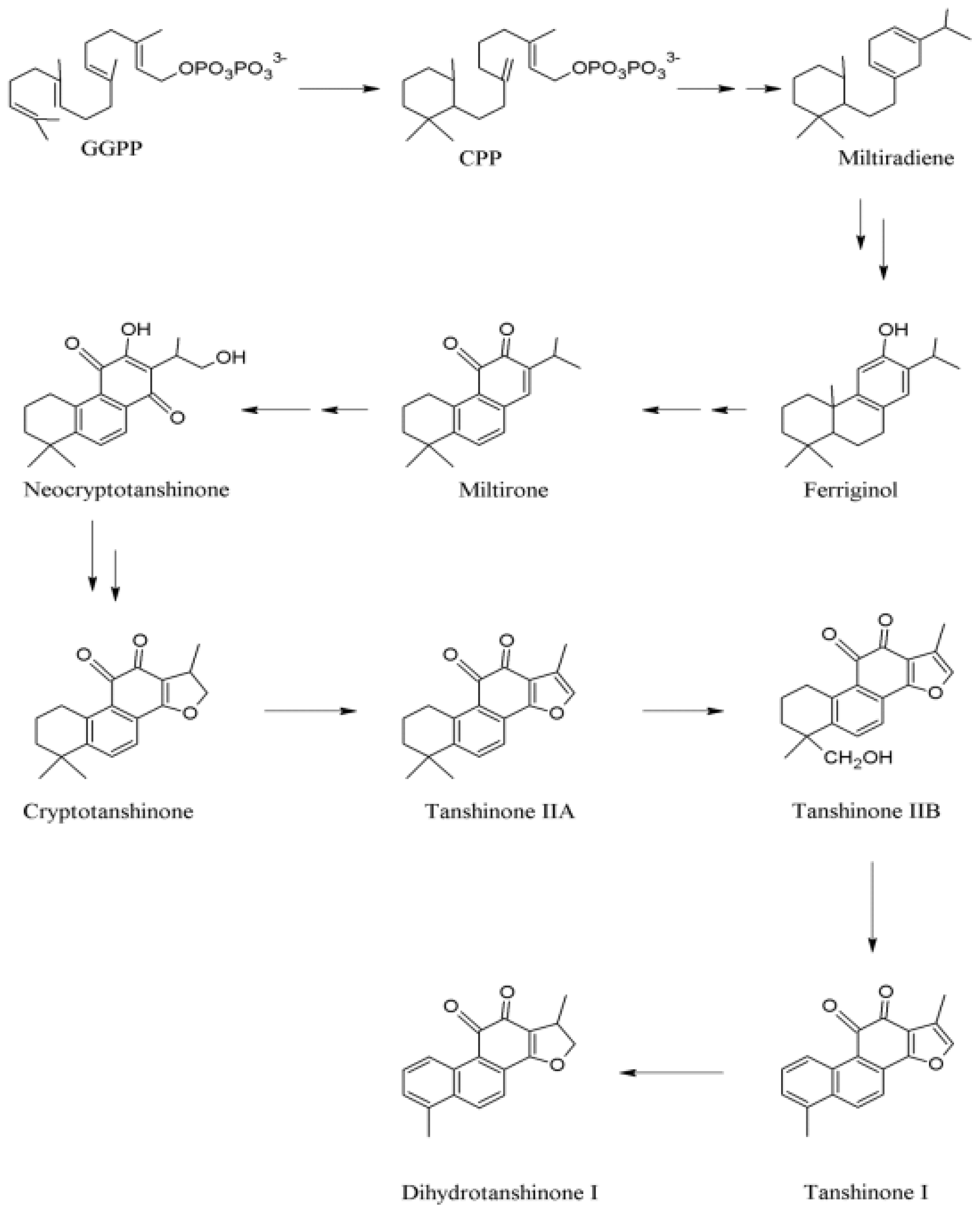
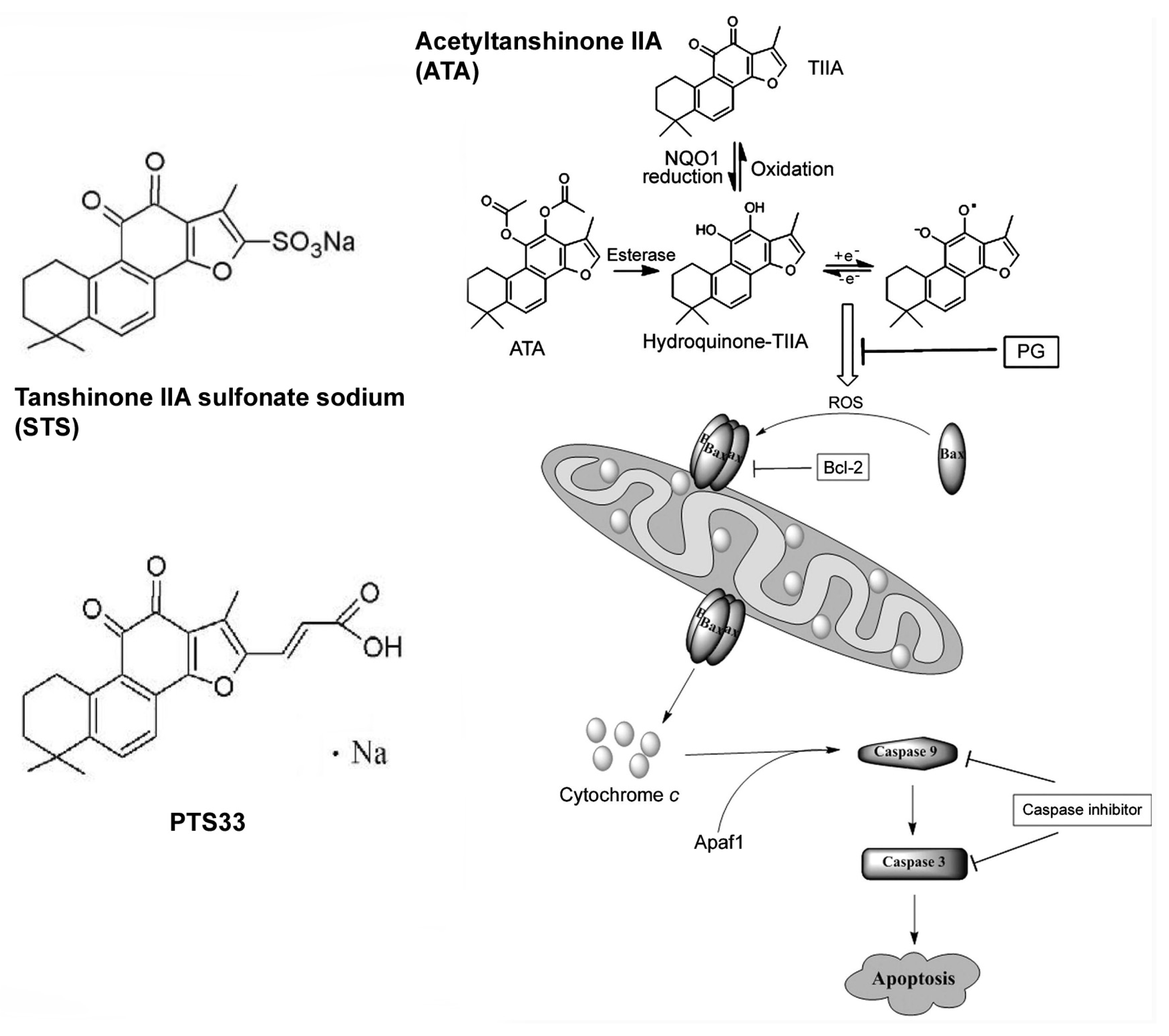
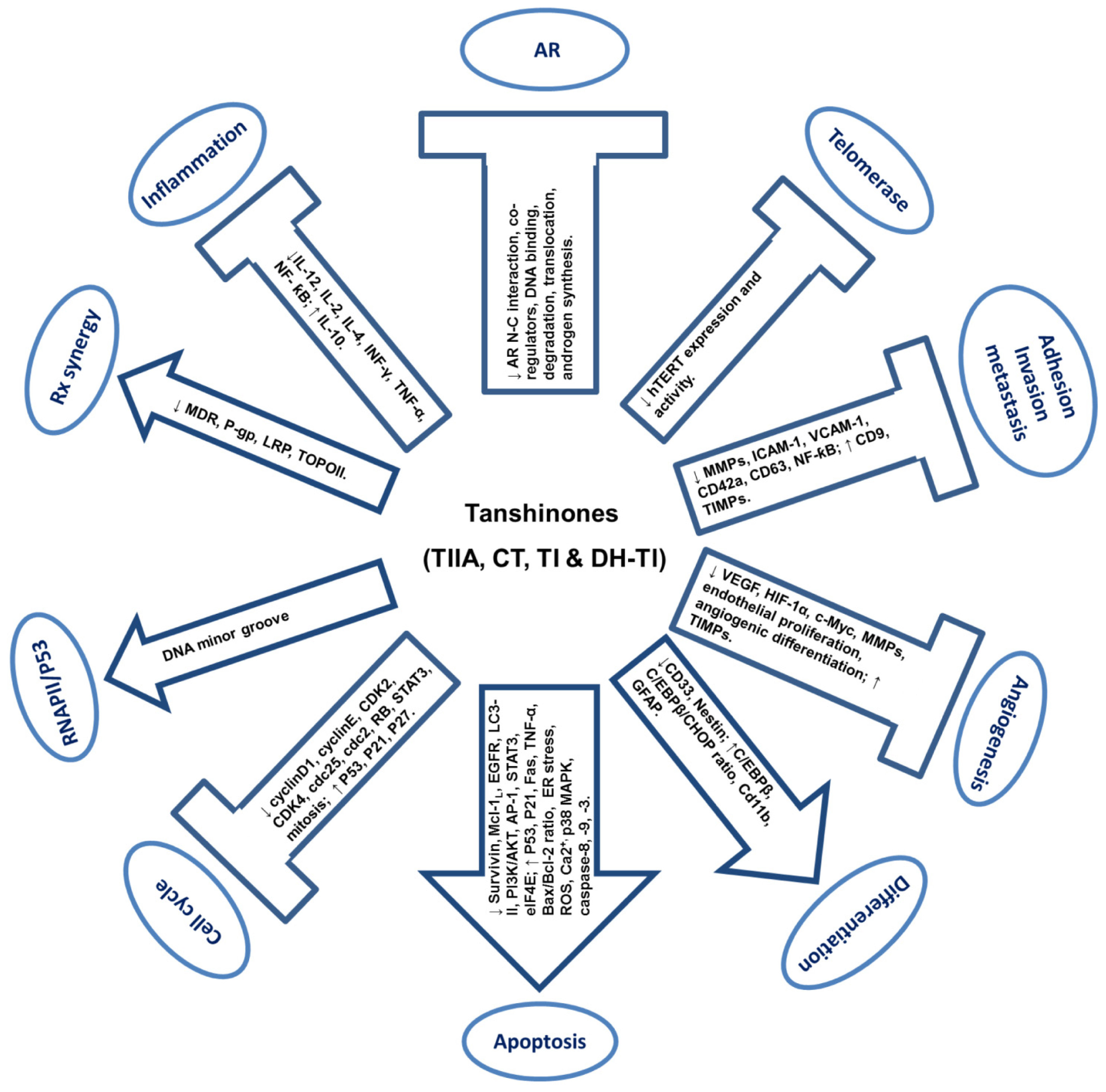
| Alternative Source | Characteristics | Products | References |
|---|---|---|---|
| Callus culture (root, stem, leaf blade, and petiole) | Well established conventional strategy, potential to form a whole plant, and easy maintenance of tissue in culture | Cryptotanshinone, tanshinone I, and tanshinone IIA | [72–75] |
| Cell suspension culture | Reliable, sustainable, free of adverse environment factors, automatic control, and scalable for commercial production | Tanshinone I, tanshinone IIA, cryptotanshinone, and ferruginol | [76–79] |
| Cell immobilization culture | High cell densities, continuous removal of secreted products, reuse of biocatalysts, and protection for shear-sensitive cells by matrix | Tanshinone IIA, cryptotanshinone, and ferruginol | [80,81] |
| Rhizogenesis | Genetic and biosynthetic stability, plant hormone-independent growth, multi-enzyme biosynthetic potential, and relatively low cost | Tanshinone I, tanshinone IIA, tanshinone IIB, tanshinone V, dihydrotanshinone I, cryptotanshinone, tanshinone VI, and diterpene ferruginol | [82–88] |
| Crown gall cultures | Fast growth rate, independent of exogenous phytohormones, high productivity of secondary metabolites that are low in normal cell cultures | Cryptotanshinone, tanshinone I, tanshinone IIA, rosmarinic acid, and lithospermic acid B | [89–92] |
| Endophytic fungi | Economic, reproducible, ecology-friendly, and easy to scale up | Tanshinone I and tanshinone IIA | [93] |
| Species | Administered drug/mixture/extracts (marker compound) | Route | Dose mg/kg | PK for | CA+B, Cmax or Ct (μM) | t1/2β (h) | t1/2γ (h) | References |
|---|---|---|---|---|---|---|---|---|
| Rat | Danshen extract-lipid emulsion (TIIA) | i.v. | 2 | TIIA | 0.144 (C5min) | 2.27 | [97] | |
| 4 | TIIA | 0.20 (C5min) | 2.35 | |||||
| 8 | TIIA | 0.932 (C5min) | 2.17 | |||||
| Danshen extract-lipid emulsion (TIIA) Plus polyphenolic extract | i.v. | 2 | TIIA | 0.81 (C5min) | 3.13 | |||
| 4 | TIIA | 1.17 (C5min) | 3.43 | |||||
| 8 | TIIA | 22.78 (C5min) | 4.79 | |||||
| Rabbit | Danxiongfang formula in Tween80/saline (TIIA) | i.v. | 2.5 | TIIA | 10.99 (CA + CB) | 0.041 | 2.25 | [98] |
| Danxiongfang formula Tween80/saline (CT) | i.v. | 4.5 | CT | 15.10 (CA + CB) | 0.039 | 1.42 | ||
| Rabbit | CT in Tween80/saline | i.v. | 4.5 | CT | 11.89 (CA + CB) | 0.036 | 1.16 | [99] |
| Danxiongfang formula (CT) | i.v. | 4.5 | CT | 15.10 (CA + CB) | 0.039 | 1.42 | ||
| Pig | CT in isopropanol solution | i.v. | 10 | CT | 10.44 (CA + CB) | 0.040 | 1.08 | [100] |
| TIIA | 2.10 (@tmax = 4.6 min) | 3.15 | ||||||
| p.o. | 40 | CT | 0.15 (@1 h) | |||||
| i.m. | 20 | CT | 0.19 (@20 min) | |||||
| Rat | CT in aqueous solution | i.v. | 20 | CT | 9.57 | 1.06 | [94] | |
| CT in aqueous solution | i.p. | 100 | CT | 2.22 (@tmax 1.91 h) (10.6% iv AUC) | 6.88 | |||
| CT in aqueous solution | p.o. | 100 | CT | 0.305 (@tmax 5.19 h) (2.1% iv AUC) | 6.64 | |||
| Rat | CT as dispersion | p.o. | 20 | CT | 0.085 (@tmax 4 h) | ~4 | [101] | |
| TIIA | 0.041 (@tmax 4 h) | ~5 | ||||||
| Rat | CT suspension in 1% Tween80 | p.o. | 5.7 | CT | 0.037 (@tmax 0.50 h) | ~0.05 | ~3.9 | [102] |
| TIIA | 0.009 (@tmax 0.50 h) | |||||||
| Rat | TIIA | p.o. | 15 | TIIA | 18.9 (@tmax 0.85 h) | 0.55 | 3.63 | [95] |
| Rat | TIIA in Tween80 suspension | p.o. | 8 | TIIA | 0.012 (@tmax 0.32 h) | 3.84 | [103] | |
| CT in Tween80 suspension | p.o. | 5.7 | CT | 0.022 (@tmax 0.56 h) | 2.83 | |||
| CT in Tween80 suspension | p.o. | 5.7 | TIIA | 0.012 (@tmax 0.42 h) | 3.12 | |||
| Danshen EtOH Extract (TIIA) in Tween80 suspension | p.o. | 8 | TIIA | 0.121 (@tmax 0.64 h) | 5.12 | |||
| Danshen EtOH Extract (CT) in Tween80 suspension | p.o. | 5.7 | CT | 0.189 (@tmax 0.58h) | 4.80 | |||
| Rat | Tanshinones Mixture (TIIA) | p.o. | 18 | TIIA | 0.06 (@tmax 4h) | [104] | ||
| Tanshinones Mixture (CT) | p.o. | 18 | CT | 0.027 (@tmax 4 h) | ||||
| Rat | Tanshinones Mixture (TIIA) | p.o. | 4.1 | TIIA | 0.009 (@tmax 0.54 h) | 2.07 | [105] | |
| Tanshinones Mixture (CT) | p.o. | 1.91 | CT | 0.002 (@tmax 0.42 h) | 1.13 | |||
| Tanshinones Mixture (TI) | p.o. | 1.1 | TI | 0.006 (@tmax 0.42 h) | 3.00 | |||
| Tanshinones Mixture (DH-TI) | p.o. | 1.91 | DH-TI | 0.012 (@tmax 0.79 h) | 1.69 | |||
| Rat | Tanshinones Mixture (TIIA) | p.o. | 5.79 | TIIA | 0.076(@tmax 0.61 h) | 0.40 | 3.70 | [106] |
| Tanshinones Mixture (CT) | p.o. | 9.82 | CT | 0.145 (@tmax 0.86 h) | 0.69 | 2.81 | ||
| Tanshinones Mixture (TI) | p.o. | 3.9 | TI | 0.198 (@tmax 0.60 h) | 0.94 | 4.72 | ||
| Tanshinones Mixture (DH-TI) | p.o. | 3.58 | DH-TI | 0.041(@tmax 0.74 h) | 0.54 | 3.65 | ||
| Rat | Danshen tanshinone extract (TIIA) in CMC 0.5% | p.o. | 20 | TIIA | 0.057 (@tmax 6.67 h) | 7.04 | [107] | |
| tanshinone extract + salvianolic acid B extract | p.o. | 20 | TIIA | 0.060 (@tmax 4.35 h) | 5.86 | |||
| tanshinone extract + notoginseng extract | p.o. | 20 | TIIA | 0.054 (@tmax 4.33 h) | 6.90 | |||
| Tanshinone extract + borneol (Bingpian) | p.o. | 20 | TIIA | 0.066 (@tmax 2.00 h) | 0.032 | 6.28 | ||
| All extracts combined | p.o. | 20 | TIIA | 0.075 (@tmax 3.67 h) | 0.041 | 6.02 |
| Tanshinone tested/Tanshinone containing formula | Cancers | Treatment(s) | Number of patients | Clinical benefit | References |
|---|---|---|---|---|---|
| TIIA | Leukemia | TIIA (80 mg, i.v. once per day) | Single case report | CR | [280] |
| TIIA | Leukemia | TIIA (30 mg, p.o., twice per day) | Single case report | CR | [279] |
| Fufang Danshen Injection | Leukemia | Control: chemotherapy only; Treatment: chemotherapy plus Fufang Danshen Injection (20–30 mL, i.v., once per day) | Control: 46 Treatment: 86 | Fufang Danshen slightly increased CR rate, but significantly attenuate the side effects of chemotherapy. | [281] |
| Fufang Danshen Injection | Liver carcinoma | Control: surgical resection only; Treatment: surgical resection plus chemotherapy and Fufang Danshen Injection (250 mL, TUV perfusion, once per day for 7 days, repeat every 3–4 week ) | Control: 30 Treatment: 30 | 1- and 2-year recurrence rates (control vs. treatment): 60.7% vs. 15.3% (p < 0.05) and 75.1% vs. 30.0% (p < 0.05). | [282] |
| Fufang Danshen Injection | Liver carcinoma | Control: TACE only; Treatment: TACE plus Fufang Danshen Injection (16 mL, i.v. once per day for 7 days) | Control: 37 Treatment: 53 | 1-, 2- and 3-year survival rate (control vs. treatment): 72.97% vs. 79.25%, 43.24% vs. 66.04% (p < 0.05) and 24.32% vs. 45.28% (p < 0.05). | [283] |
| Fufang Danshen Dripping Pill | Pancreatic carcinoma | Control: chemotherapy only; Treatment: chemotherapy plus Fufang Danshen Dripping Pill (250 mg, p.o. 3 times per day) | Control: 40 Treatment: 41 | CR + PR and CR + PR + SD rates (control vs. treatment): 35.0% vs. 46.3% and 50.0% vs. 73.2% (p < 0.05). | [284] |
| RIF (formula) | Leukemia | Control: ATRA (30 mg per day) plus placebo for RIF. Treatment: RIF (2.25–7.5 g per day, p.o.) plus placebo for ATRA | Control: 59 (placebo controlled) Treatment: 61 | CR rate (control vs. treatment): 94.9% vs. 96.7% (p > 0.05). | [285] |
| RIF (formula) | Leukemia | Alternating treatments with chemotherapy and RIF (6.0–7.5 g per day, p.o. 30 days of a cycle) | Treatment: 62 (no control group) | 3-, 5-, 7- and 10-year relapsefree survival rates: 68.41%, 48.15%, 38.89%, 18.52%, respectively. 3-year and 5 to 10-year survival rates: 88.52% and 86.88%. | [286] |
| RIF (formula) | Leukemia | RIF (3.75–9 g per day, p.o.) | Multiple cases reports over many years: n = 18 to 204/report | CR rate: 91.67%-100%. | [287–291] |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.-H.; Jiang, C.; Lü, J. Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities. Int. J. Mol. Sci. 2012, 13, 13621-13666. https://doi.org/10.3390/ijms131013621
Zhang Y, Jiang P, Ye M, Kim S-H, Jiang C, Lü J. Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities. International Journal of Molecular Sciences. 2012; 13(10):13621-13666. https://doi.org/10.3390/ijms131013621
Chicago/Turabian StyleZhang, Yong, Peixin Jiang, Min Ye, Sung-Hoon Kim, Cheng Jiang, and Junxuan Lü. 2012. "Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities" International Journal of Molecular Sciences 13, no. 10: 13621-13666. https://doi.org/10.3390/ijms131013621
APA StyleZhang, Y., Jiang, P., Ye, M., Kim, S.-H., Jiang, C., & Lü, J. (2012). Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities. International Journal of Molecular Sciences, 13(10), 13621-13666. https://doi.org/10.3390/ijms131013621






