Release of Bacteriocins from Nanofibers Prepared with Combinations of Poly(D,L-lactide) (PDLLA) and Poly(Ethylene Oxide) (PEO)
Abstract
:1. Introduction
2. Results
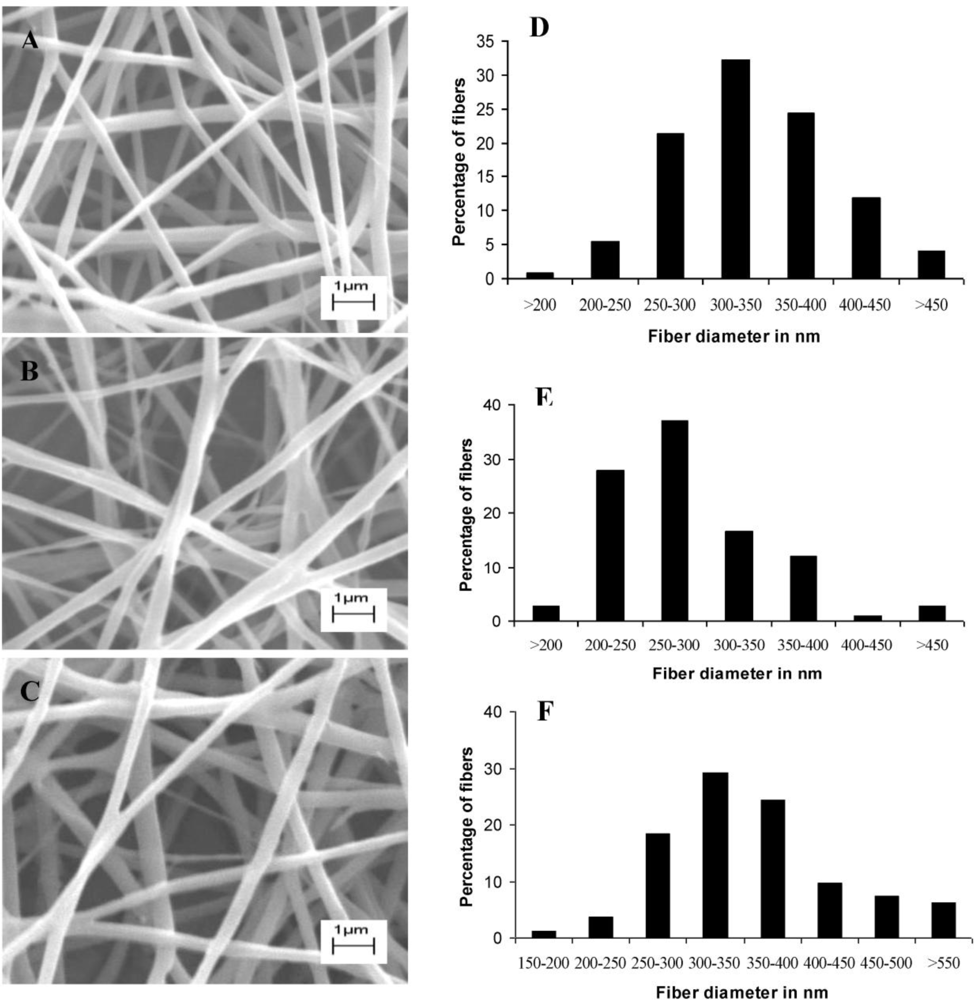
2.1. Electrospinning of Nanofibers
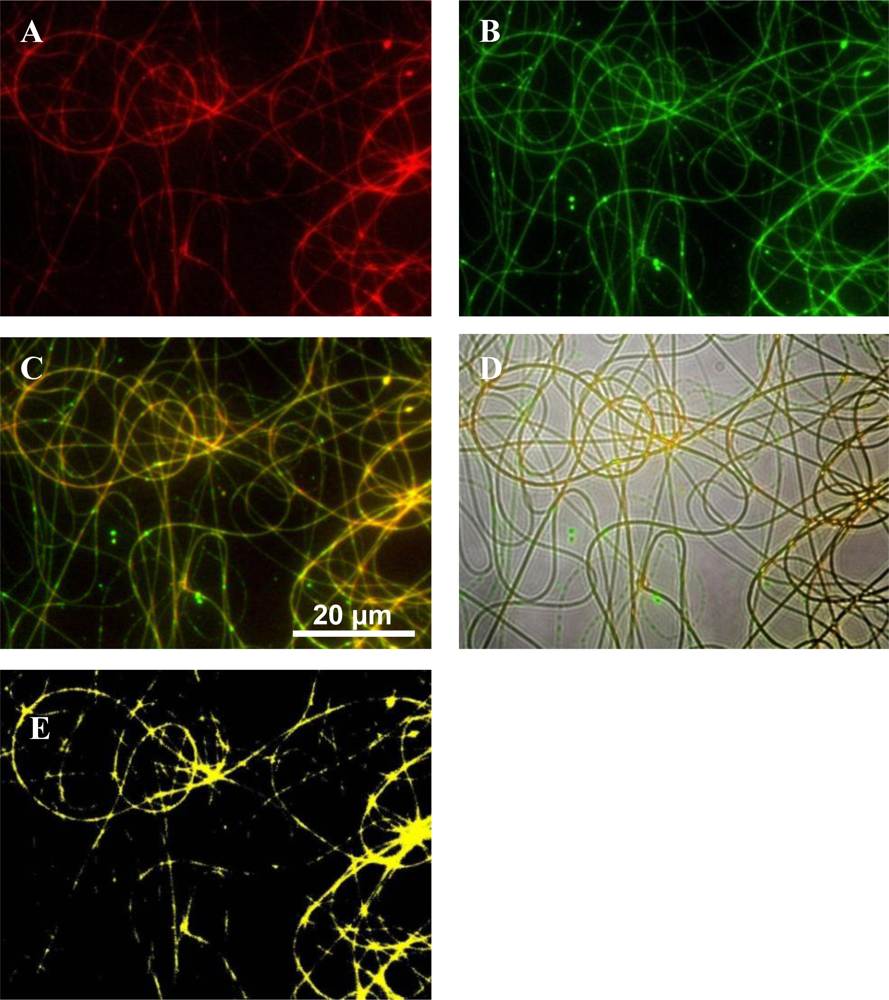
2.2. Fluorescent Microscopy Images of Polymers
2.3. DSC Analysis
2.4. Fluorescent Images of Labeled Polymers and Peptide
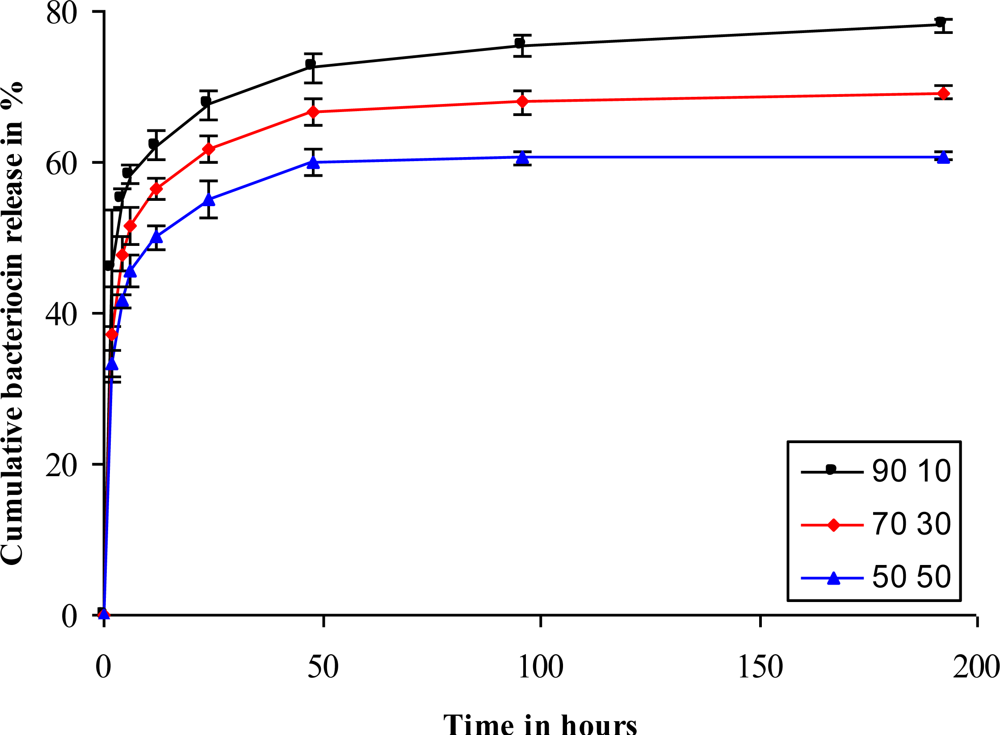
2.5. Release of Antimicrobial Peptide
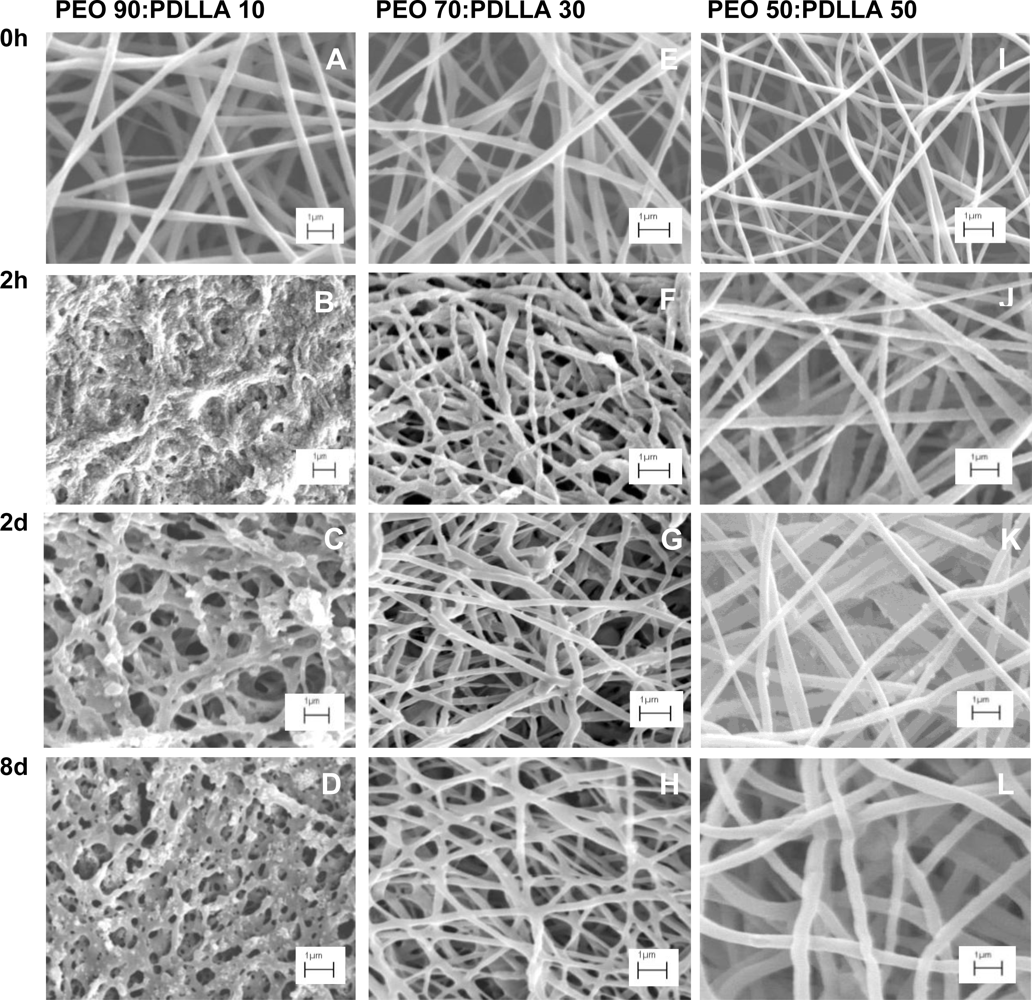
2.6. Morphological Changes and Weight Loss
2.7. Antimicrobial Activity Tests
3. Experimental Section
3.1. Isolation of Antimicrobial Peptides
3.2. Electrospinning of Nanofibers
3.3. Scanning Electron Microscopy of Electrospun Fibers
3.4. Fluorescent Labeling of Antimicrobial Peptides and Polymers
3.5. Differential Scanning Calorimetry (DSC)
3.6. Release of Antimicrobial Peptides and Degradation of Nanofibers
3.7. Antimicrobial Activity Tests
4. Discussion
5. Conclusion
Acknowledgments
References
- Cotter, PD; Hill, C; Ross, RP. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol 2005, 3, 777–788. [Google Scholar]
- Dicks, LMT; Knoetze, H; van Reenen, CA. Otitis media: A review, with a focus on alternative treatments. Probiotics Antimicrob. Prot 2009, 1, 45–59. [Google Scholar]
- Todorov, SD; Dicks, LMT. Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria. Enzyme Microb. Technol 2005, 36, 318–326. [Google Scholar]
- Bauer, R; Dicks, LMT. Mode of action of lipid II-targeting lantibiotics. Int. J. Food Microbiol 2005, 10, 201–216. [Google Scholar]
- Knoetze, H; Todorov, SD; Dicks, LMT. A class IIa peptide from Enterococcus mundtii inhibits bacteria associated with otitis media. Int. J. Antimicrob. Agents 2008, 31, 228–234. [Google Scholar]
- Jack, RW; Tagg, JR; Ray, B. Bacteriocins of gram-positive bacteria. Microbiol. Rev 1995, 59, 171–200. [Google Scholar]
- Klaenhammer, TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev 1993, 12, 39–86. [Google Scholar]
- McAuliffe, O; Ross, RP; Hill, C. Lantibiotics: Structure, biosynthesis and mode of action. FEMS Microbiol. Rev 2001, 25, 285–308. [Google Scholar]
- Kim, JY. Understanding the evolution of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Newsl 2009, 31, 17–23. [Google Scholar]
- Chatterjee, S; Chatterjee, DK; Jani, RH; Blumbach, J; Ganguli, BN; Klesel, N; Limbert, M; Seibert, G. Mersacidin, a new antibiotic from Bacillus: in vitro and in vivo antibacterial activity. J. Antibiot 1992, 45, 839–845. [Google Scholar]
- Galvin, M; Hill, C; Ross, RP. Lacticin 3147 displays activity in buffer against gram-positive bacterial pathogens which appear insensitive in standard plate assays. Lett. Appl. Microbiol 1999, 28, 355–358. [Google Scholar]
- Dicks, LMT; Ten Doeschate, K. Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 alleviated symptoms of Salmonella infection, as determined in Wistar rats challenged with Salmonella enterica Serovar typhimurium. Curr. Microbiol 2010, 61, 184–189. [Google Scholar]
- Ramiah, K; Ten Doeschate, K; Smith, R; Dicks, LMT. Safety assessment of Lactobacillus plantarum 423 and Enterococcus mundtii ST4SA determined in trials with Wistar rats. Probiotics Antimicrob. Prot 2009, 1, 15–23. [Google Scholar]
- Granger, M; van Reenen, CA; Dicks, LMT. Effect of gastro-intestinal conditions on Enterococcus mundtii ST4SA and production of bacteriocin ST4SA, as recorded by real-time PCR. Int. J. Food Microbiol 2008, 123, 277–280. [Google Scholar]
- de Kwaadsteniet, M; Ten Doeschate, K; Dicks, LMT. Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett. Appl. Microbiol 2009, 48, 65–70. [Google Scholar]
- Ramiah, K; Van Reenen, CA; Dicks, LMT. Surface-bound proteins of Lactobacillus plantarum 423 that contribute to adhesion of Caco-2 cells, and their role in competitive exclusion and displacement of Clostridium sporogenes and Enterococcus faecalis. Res. Microbiol 2008, 159, 470–475. [Google Scholar]
- van Reenen, CA; Dicks, LMT; Chikindas, ML. Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J. Appl. Microbiol 1998, 84, 1131–1137. [Google Scholar]
- Heunis, TDJ; Dicks, LMT. Nanofibers offer alternative ways to the treatment of skin infections. J Biomed Biotechnol 2010, 510682:1–510682:10. [Google Scholar]
- Chew, SY; Wen, J; Yim, EKF; Leong, KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules 2005, 6, 2017–2024. [Google Scholar]
- Heunis, TDJ; Botes, M; Dicks, LMT. Encapsulation of Lactobacillus plantarum 423 and its bacteriocin in nanofibers. Probiotics Antimicrob. Prot 2010, 2, 46–51. [Google Scholar]
- Kenawy, E-R; Bowlin, GL; Mansfield, K; Layman, J; Simpson, DG; Sanders, EH; Wnek, GE. Release of tetracycline hydrochloride from electrospun poly(ethylene-covinylacetate), poly(lactic acid), and a blend. J. Control Release 2002, 81, 57–64. [Google Scholar]
- Kim, TG; Lee, DS; Park, TG. Controlled protein release from electrospun biodegradable fiber mesh composed of poly(ɛ-caprolactone) and poly(ethylene oxide). Int. J. Pharm 2007, 338, 276–283. [Google Scholar]
- Rujitanaroj, P; Pimpha, N; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar]
- López -Rubio, A; Sanchez, E; Sanz, Y; Lagaron, JM. Encapsulation of living Bifidobacteria in ultrathin PVOH electrospun fibers. Biomacromolecules 2009, 10, 2823–2829. [Google Scholar]
- Luu, YK; Kim, K; Hsiao, BS; Chu, B; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J. Control Release 2003, 89, 341–353. [Google Scholar]
- Maretschek, S; Greiner, A; Kissel, T. Electrospun biodegradable nanofiber nonwovens for controlled release of proteins. J. Control Release 2008, 127, 180–187. [Google Scholar]
- Zeng, J; Xu, X; Chen, X; Liang, Q; Bian, X; Yang, L; Jing, X. Biodegradable electrospun fibers for drug delivery. J. Control Release 2003, 92, 227–231. [Google Scholar]
- Li, W-J; Laurencin, CT; Caterson, EJ; Tuan, RS; Ko, FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mat. Res 2002, 60, 613–621. [Google Scholar]
- Smith, LA; Ma, PX. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B 2004, 39, 125–131. [Google Scholar]
- Yang, F; Murugan, R; Wang, S; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar]
- Zhou, Y; Yang, D; Chen, X; Xu, Q; Lu, F; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar]
- Sambrook, JE; Fritsch, F; Maniatis, J. Molecular Cloning: A Laboratory Manual, 2nd ed; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Min, B-M; Lee, SW; Lim, JN; You, Y; Lee, TS; Kang, PH; Park, WH. Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer 2004, 45, 7137–7142. [Google Scholar]
- Nijenhuis, AJ; Colstee, E; Grijpma, DW; Pennings, AJ. High molecular weight poly(l-lactide) and poly(ethylene oxide) blends: Thermal characterization and physical properties. Polymer 1996, 37, 5849–5857. [Google Scholar]




© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Heunis, T.; Bshena, O.; Klumperman, B.; Dicks, L. Release of Bacteriocins from Nanofibers Prepared with Combinations of Poly(D,L-lactide) (PDLLA) and Poly(Ethylene Oxide) (PEO). Int. J. Mol. Sci. 2011, 12, 2158-2173. https://doi.org/10.3390/ijms12042158
Heunis T, Bshena O, Klumperman B, Dicks L. Release of Bacteriocins from Nanofibers Prepared with Combinations of Poly(D,L-lactide) (PDLLA) and Poly(Ethylene Oxide) (PEO). International Journal of Molecular Sciences. 2011; 12(4):2158-2173. https://doi.org/10.3390/ijms12042158
Chicago/Turabian StyleHeunis, Tiaan, Osama Bshena, Bert Klumperman, and Leon Dicks. 2011. "Release of Bacteriocins from Nanofibers Prepared with Combinations of Poly(D,L-lactide) (PDLLA) and Poly(Ethylene Oxide) (PEO)" International Journal of Molecular Sciences 12, no. 4: 2158-2173. https://doi.org/10.3390/ijms12042158
APA StyleHeunis, T., Bshena, O., Klumperman, B., & Dicks, L. (2011). Release of Bacteriocins from Nanofibers Prepared with Combinations of Poly(D,L-lactide) (PDLLA) and Poly(Ethylene Oxide) (PEO). International Journal of Molecular Sciences, 12(4), 2158-2173. https://doi.org/10.3390/ijms12042158



