Q Fever Endocarditis in Romania: The First Cases Confirmed by Direct Sequencing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Case Definitions
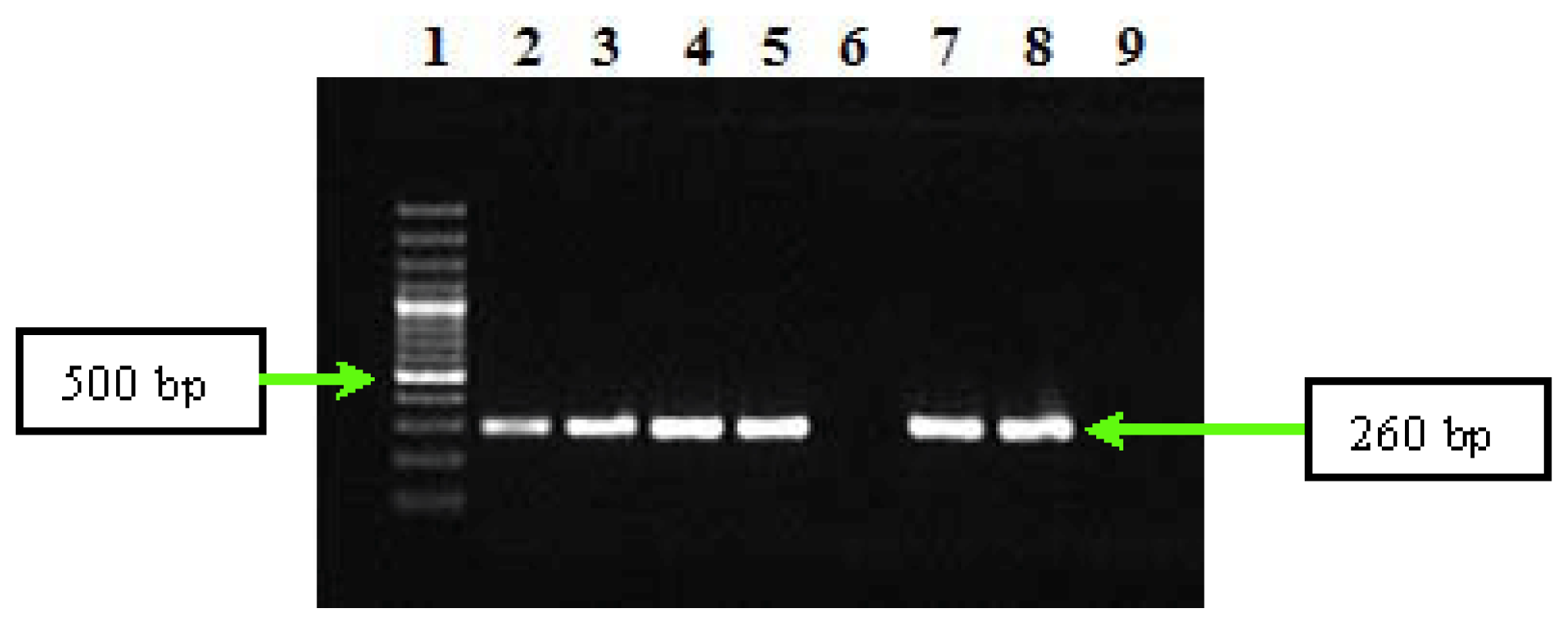
2.2. PCR and Sequencing Results
3. Experimental Section
3.1. Patients
3.2. Indirect Immunofluorescence Assays
3.3. Molecular Methods
3.3.1. DNA Extraction
3.3.2. PCR Assay
3.3.3. Sequencing of PCR Products and Sequence Analysis
4. Conclusions
Acknowledgments
References
- Rolain, J.M.; Lecam, C.; Raoult, D. Simplified serological diagnosis of endocarditis due to Coxiella burnetii and Bartonella. Clin. Diagn. Lab. Immunol 2003, 10, 1147–1148. [Google Scholar]
- Brouqui, P.; Raoult, D. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev 2001, 14, 177–207. [Google Scholar]
- Houpikian, P.; Raoult, D. Diagnostic methods. Current best practices and guidelines for identification of difficult-to-culture pathogens ininfective endocarditis. Cardiol. Clin 2003, 21, 207–217. [Google Scholar]
- Fournier, P.-E.; Thuny, F.; Richet, H.; Lepidi, H.; Casalta, J.-P.; Arzouni, J.-P.; Maurin, M.; Celard, M.; Mainardi, J.-L.; Caus, T.; Collart, F.; Habib, G.; Raoult, D. Comprehensive diagnostic strategy for blood culture-negative endocarditis, a prospective study of 819 new cases. Clin. Infect. Dis 2010, 51, 131–140. [Google Scholar]
- Raoult, D.; Casalta, J.P.; Richet, H.; Khan, M.; Bernit, E.; Rovery, C.; Branger, S.; Gouriet, F.; Imbert, G.; Bothello, E.; Collart, F.; Habib, G. Contribution of systematic serological testing in diagnosis of infective endocarditis. J. Clin. Microbiol 2005, 43, 5238–5242. [Google Scholar]
- Lamas, C.C.; Eykyn, S.J. Blood culture negative endocarditis, analysis of 63 cases presenting over 25 years. Heart 2003, 89, 258–262. [Google Scholar]
- Osler, W. Chronic infectious endocarditis. QJM: Int. J. Med 1909, 2, 219–230. [Google Scholar]
- Siciliano, R.F.; Strabelli, T.M.; Zeigler, R.; Rodrigues, C.; Castelli, J.B.; Grinberg, M.; Colombo, S.; da Silva, L.J.; Mendes do Nascimento, E.M.; Pereira dos Santos, F.C.; Uip, D.E. Infective endocarditis due to Bartonella spp. and Coxiella burnetii experience at a cardiology hospital in Sao Paulo, Brazil. Ann. N.Y. Acad. Sci 2006, 1078, 215–222. [Google Scholar]
- Houpikian, P.; Raoult, D. Blood culture-negative endocarditis in a reference center, etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005, 84, 162–173. [Google Scholar]
- Botelho-Nevers, E.; Fournier, P.-E.; Richet, H.; Fenollar, F.; Lepidi, H.; Foucault, C.; Branchereau, A.; Piquet, P.; Maurin, M.; Raoult, D. Coxiella burnetii infection of aortic aneurysms or vascular grafts, report of 30 new cases and evaluation of outcome. Eur. J. Clin. Microbiol. Infect. Dis 2007, 26, 635–640. [Google Scholar]
- Lepidi, H.; Houpikian, P.; Liang, Z.; Raoult, D. Cardiac valves in patients with Q fever endocarditis, microbiological, molecular, and histologic studies. J. Infect. Dis 2003, 187, 1097–1106. [Google Scholar]
- Landais, C.; Fenollar, F.; Thuny, F.; Raoult, D. From acute Q fever to endocarditis, serological follow-up strategy. Clin. Infect. Dis 2007, 44, 1337–1340. [Google Scholar]
- Healy, B.; Llewelyn, M.; Westmoreland, D.; Lloyd, G.; Brown, N. The value of follow-up after acute Q fever infection. J. Infect 2006, 52, e109–e112. [Google Scholar]
- Raoult, D.; Marrie, T.; Mege, J. Natural history and pathophysiology of Q fever. Lancet Infect. Dis 2005, 5, 219–226. [Google Scholar]
- Kampschreur, L.M.; Oosterheert, J.J.; de Vries Feyens, C.A.; Delsing, C.E.; Hermans, M.H.; van Sluisveld, I.L.; Lestrade, P.J.; Renders, N.H.; Elsman, P.; Wever, P.C. Chronic Q fever-related dual-pathogen endocarditis, case series of three patients. J. Clin. Microbiol 2011, 49, 1692–1694. [Google Scholar]
- Brouqui, P.; Dupont, H.T.; Drancourt, M.; Berland, Y.; Etienne, J.; Leport, C.; Goldstein, F.; Massip, P.; Micoud, M.; Bertrand, A.; et al. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Arch. Intern. Med 1993, 153, 642–648. [Google Scholar]
- Fenollar, F.; Fournier, P.E.; Carrieri, M.P.; Habib, G.; Messana, T.; Raoult, D. Risks factors and prevention of Q fever endocarditis. Clin. Infect. Dis 2001, 33, 312–316. [Google Scholar]
- Parker, N.R.; Barralet, J.H.; Bell, A.M. Q fever. Lancet 2006, 367, 679–688. [Google Scholar]
- Fenollar, F.; Thuny, F.; Xeridat, B.; Lepidi, H.; Raoult, D. Endocarditis after acute Q fever in patients with previously undiagnosed valvulopathies. Clin. Infect. Dis 2006, 42, 818–821. [Google Scholar]
- Hartzell, J.D.; Wood-Morris, R.N.; Martinez, L.J.; Trotta, R.F. Q fever, epidemiology, diagnosis, and treatment. Mayo Clin. Proc 2008, 83, 574–579. [Google Scholar]
- Fenollar, F.; Raoult, D. Molecular genetic methods for the diagnosis of fastidious microorganisms. APMIS 2004, 112, 785–807. [Google Scholar]
- Cracea, E. Q fever epidemiology in Roumania. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1987, 267, 7–9. [Google Scholar]
- Crăcea, E.; Constantinescu, S.; Tofan, N.; Căruntu, F.; Dogaru, D. Q fever urban cases in Romania. Arch. Roum. Pathol. Exp. Microbiol 1989, 48, 13–17. [Google Scholar]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar]
- Habib, G.; Hoen, B.; Tornos, P.; Thuny, F.; Prendergast, B.; Vilacosta, I.; Moreillon, P.; de Jesus Antunes, M.; Thilen, U.; Lekakis, J.; et al. ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The task force on the prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for infection and cancer. Eur. Heart J 2009, 30, 2369–2413. [Google Scholar]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century the international collaboration on endocarditis-prospective cohort study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar]
- Fenollar, F.; Fournier Fenollar, F.; Fournier, P.E.; Raoult, D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J. Clin. Microbiol 2004, 42, 4919–4924. [Google Scholar]
- Deyell, M.W.; Chiu, B.; Ross, D.B.; Alvarez, N. Q fever endocarditis, A case report and review of the literature. Can. J. Cardiol. Vol 2006, 22, 781–785. [Google Scholar]
- Houpikian, P.; Habib, G.; Mesana, T.; Raoult, D. Changing clinical presentation of Q fever endocarditis. Clin. Infect. Dis 2002, 34, E28–E31. [Google Scholar]
- Mesana, T.G.; Collart, F.; Caus, T.; Salamand, A. Q fever endocarditis, A surgical view and a word of caution. J. Thorac. Cardiovasc. Surg 2003, 125, 217–218. [Google Scholar]
- Klee, S.R.; Tyczka, J.; Ellerbrok, H.; Franz, T.; Linke, S.; Baljer, G.; Appel, B. Highly sensitive real-time PCR for specific detection and quantification of. Coxiella burnetii. BMC Microbiol 2006, 6. [Google Scholar] [CrossRef]
- Jhartzell, D.; Wood-Morris, R.N.; Martinez, L.; Trotta, R.F. Q fever, epidemiology, diagnosis, and treatment. Mayo Clin. Proc 2008, 83, 574–579. [Google Scholar]
- Willems, H.; Thiele, D.; Krauss, H. Plasmid based differentiation and detection of Coxiella burnetii in clinical samples. Eur. J. Epidemiol 1993, 9, 411–418. [Google Scholar]
- Kato, K.; Arashima, Y.; Asai, S.; Furuya, Y.; Yoshida, Y.; Murakami, M.; Takahashi, Y.; Hayashi, K.; Katayama, T.; Kumasaka, K.; et al. Detection of Coxiella burnetii specific DNA in blood samples from Japanese patients with chronic nonspecific symptoms by nested polymerase chain reaction. FEMS Immunol. Med. Microbiol 1998, 21, 139–144. [Google Scholar]
- Zhang, G.Q.; Nguyen, S.V.; To, H.; Ogawa, M.; Hotta, A.; Yamaguchi, T.; Kim, H.J.; Fukushi, H.; Hirai, K. Clinical evaluation of a new PCR assay for detection of Coxiella burnetii in human serum samples. J. Clin. Microbiol 1998, 36, 77–80. [Google Scholar]
- Zhang, G.Q.; Hotta, A.; Mizutani, M.; Ho, T.; Yamaguchi, T.; Fukushi, H.; Hirai, K. Direct identification of Coxiella burnetii plasmids in human sera by nested PCR. J. Clin. Microbiol 1998, 36, 2210–2213. [Google Scholar]
- Fournier, P.E.; Raoult, D. Comparison of PCR and serology assays for early diagnosis of acute Q fever. J. Clin. Microbiol 2003, 41, 5094–5098. [Google Scholar]
- Fenollar, F.; Fournier, P.E.; Raoult, D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J. Clin. Microbiol 2004, 42, 4919–4924. [Google Scholar]
- Boulos, A.; Rolain, J.M.; Maurin, M.; Raoult, D. Measurement of the antibiotic susceptibility of Coxiella burnetii using real time PCR. Int. J. Antimicrob. Agents 2004, 23, 169–174. [Google Scholar]
- Seshadri, R.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Nelson, K.E.; Nelson, W.C.; Ward, N.L.; Tettelin, H.; Davidsen, T.M.; Beanan, M.J.; et al. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. USA 2003, 100, 5455–5460. [Google Scholar]
| Major criteria
| |||||
|---|---|---|---|---|---|
| Case | Age (years)/sex | Antiphase I C. burnetii IgG antibody titer >800 | Evidence of endocardial involvement— Echocardiography positive for IE-vegetation | Evidence of endocardial involvement—New valvular regurgitation | Minor criteria |
| 1 | 51/M | Present | MV | MV; AV | Fever ≥ 38 °C |
| 2 | 62/F | Present | - | MV | Fever ≥ 38 °C |
| 3 | 58/M | Present | AV | AV | Fever ≥ 38 °C |
| 4 | 64/M | Present | MV | AV; MV | Fever ≥ 38 °C |
| 5 | 60/M | Present | AV; MV | AV; MV | Fever ≥ 38 °C |
| 6 | 57/M | Present | - | - | Fever ≥ 38 °C |
| 7 | 70/F | Present | MV | AV; MV | Fever ≥ 38 °C |
| 8 | 64/M | Present | AV | AV | Fever ≥ 38 °C |
| 9 | 60/M | Present | MV | AV; MV | Fever ≥ 38 °C |
| The gene | Primer | Nucleotide sequence | Amplicon size (bp) |
|---|---|---|---|
| IS1111 | IS111F1 | 5′-TACTGGGTGTTGATATTGC-3′ | 485 |
| IS111R1 | 5′-CCGTTTCATCCGCGGTG-3′ | ||
| IS111F2 | 5′-GTAAAGTGATCTACACGA-3′ | 260 | |
| IS111R2 | 5′-TTAACAGCGCTTGAACGT-3′ |
| The components used in nested-PCR
| ||||||
|---|---|---|---|---|---|---|
| Primers conc. | MgCl2 conc. | dNTP mix conc. | DNA TaqPol conc. | TaqPol buffer conc. | DNA conc. | Volume/reaction |
| 0.3 μM | 2.5 mM | 0.2 mM | 0.025 U/μL | 1× | 50 ng/μL | 50 μL |
| The amplification program for nested PCR assay
| ||||||
|---|---|---|---|---|---|---|
| Gene | Initial denaturation | No. of cycles | Denaturation in each cycle | Annealing | Primers extension | Final extension |
| IS1111—first round | 94 °C, 5 min | 35 | 94 °C, 1 min | 52 °C, 1 min | 72 °C, 1 min | 72 °C, 5 min |
| IS1111—second round | 48 °C, 1 min | |||||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cotar, A.I.; Badescu, D.; Oprea, M.; Dinu, S.; Banu, O.; Dobreanu, D.; Dobreanu, M.; Ionac, A.; Flonta, M.; Straut, M. Q Fever Endocarditis in Romania: The First Cases Confirmed by Direct Sequencing. Int. J. Mol. Sci. 2011, 12, 9504-9513. https://doi.org/10.3390/ijms12129504
Cotar AI, Badescu D, Oprea M, Dinu S, Banu O, Dobreanu D, Dobreanu M, Ionac A, Flonta M, Straut M. Q Fever Endocarditis in Romania: The First Cases Confirmed by Direct Sequencing. International Journal of Molecular Sciences. 2011; 12(12):9504-9513. https://doi.org/10.3390/ijms12129504
Chicago/Turabian StyleCotar, Ani Ioana, Daniela Badescu, Mihaela Oprea, Sorin Dinu, Otilia Banu, Dan Dobreanu, Minodora Dobreanu, Adina Ionac, Mirela Flonta, and Monica Straut. 2011. "Q Fever Endocarditis in Romania: The First Cases Confirmed by Direct Sequencing" International Journal of Molecular Sciences 12, no. 12: 9504-9513. https://doi.org/10.3390/ijms12129504
APA StyleCotar, A. I., Badescu, D., Oprea, M., Dinu, S., Banu, O., Dobreanu, D., Dobreanu, M., Ionac, A., Flonta, M., & Straut, M. (2011). Q Fever Endocarditis in Romania: The First Cases Confirmed by Direct Sequencing. International Journal of Molecular Sciences, 12(12), 9504-9513. https://doi.org/10.3390/ijms12129504




