Inherently Chiral Calixarenes: Synthesis, Optical Resolution, Chiral Recognition and Asymmetric Catalysis
Abstract
:1. Introduction
2. Synthesis of Inherently Chiral Calixarenes
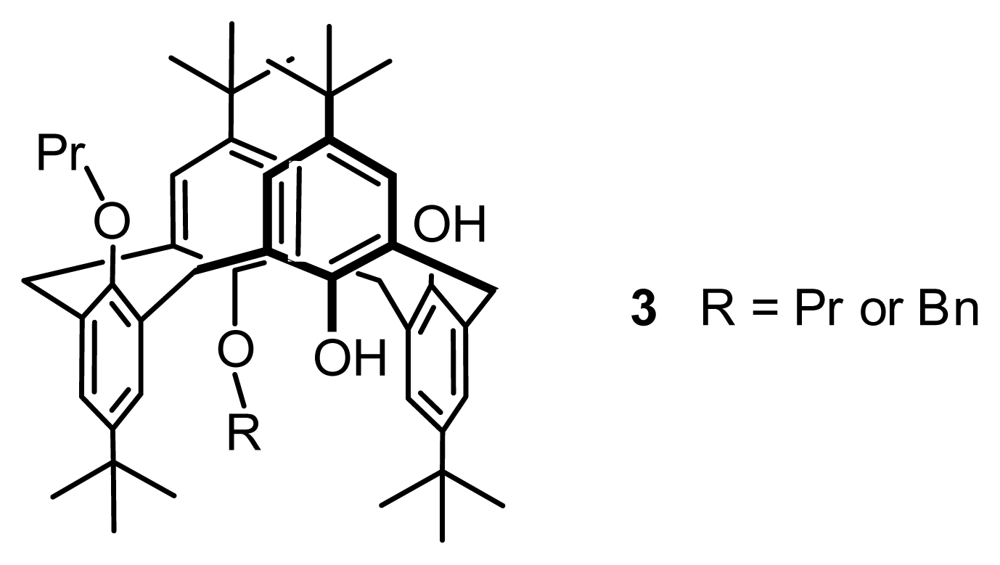
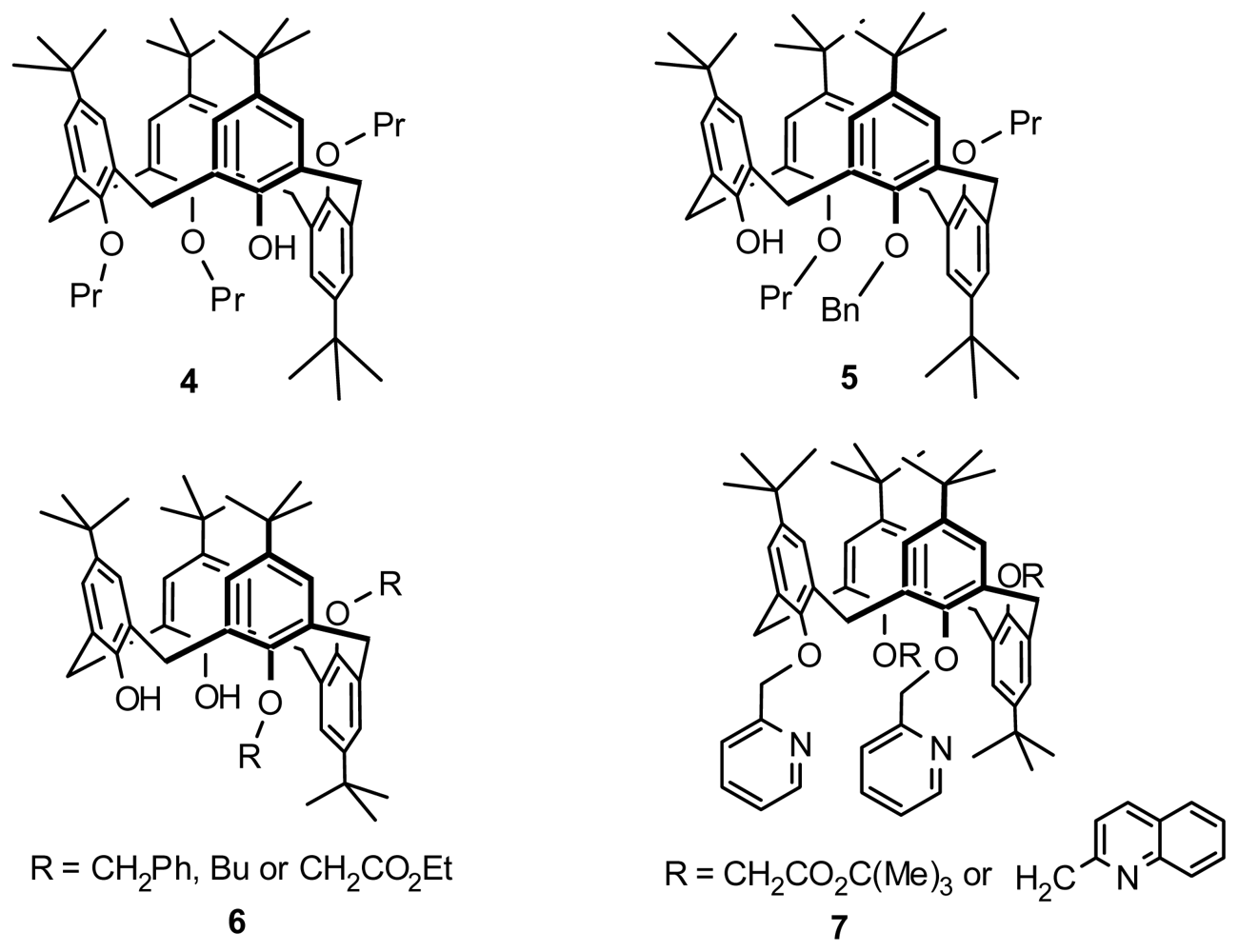
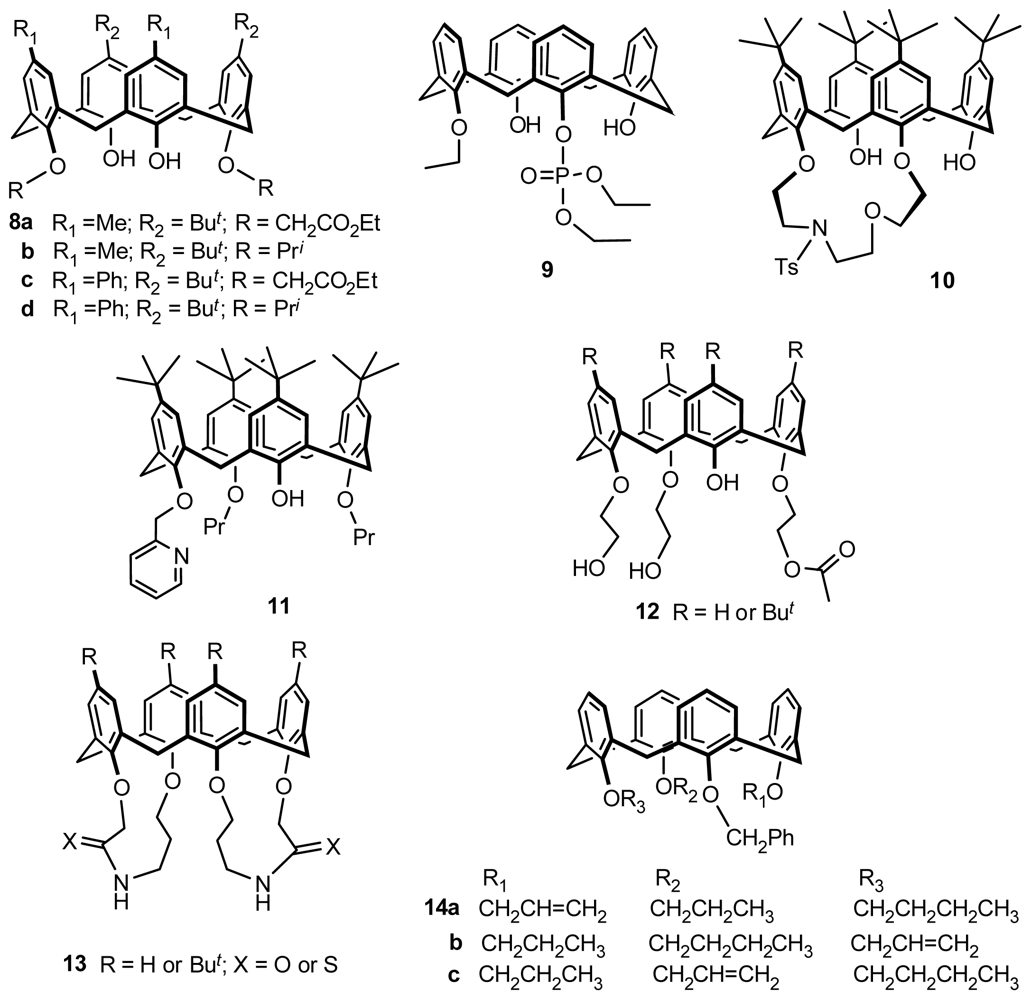
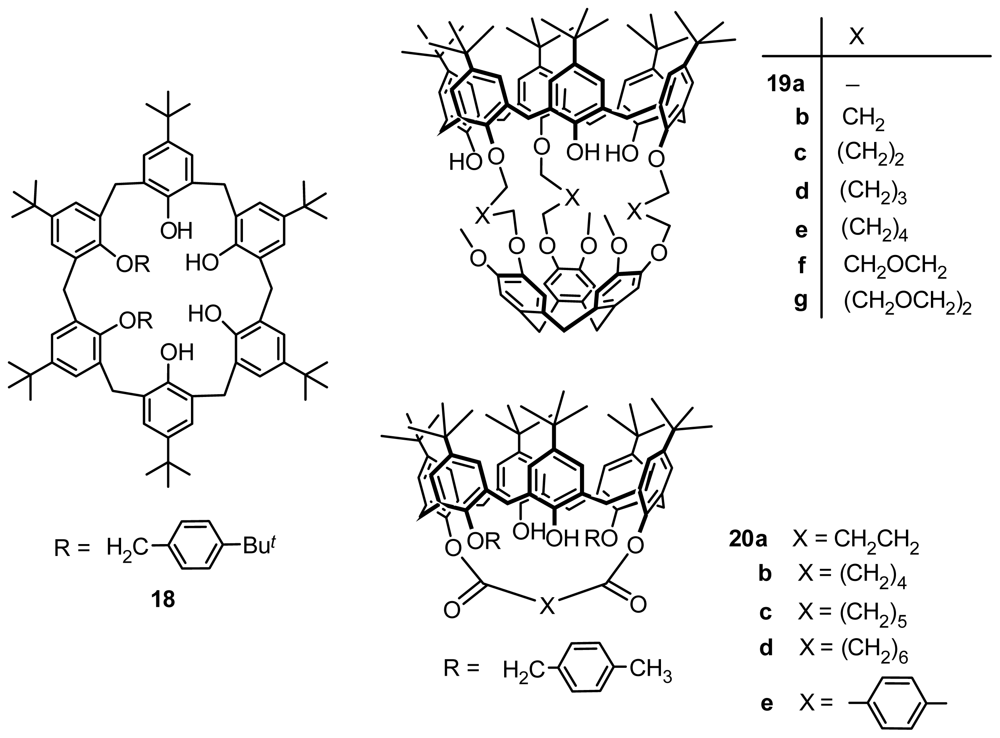
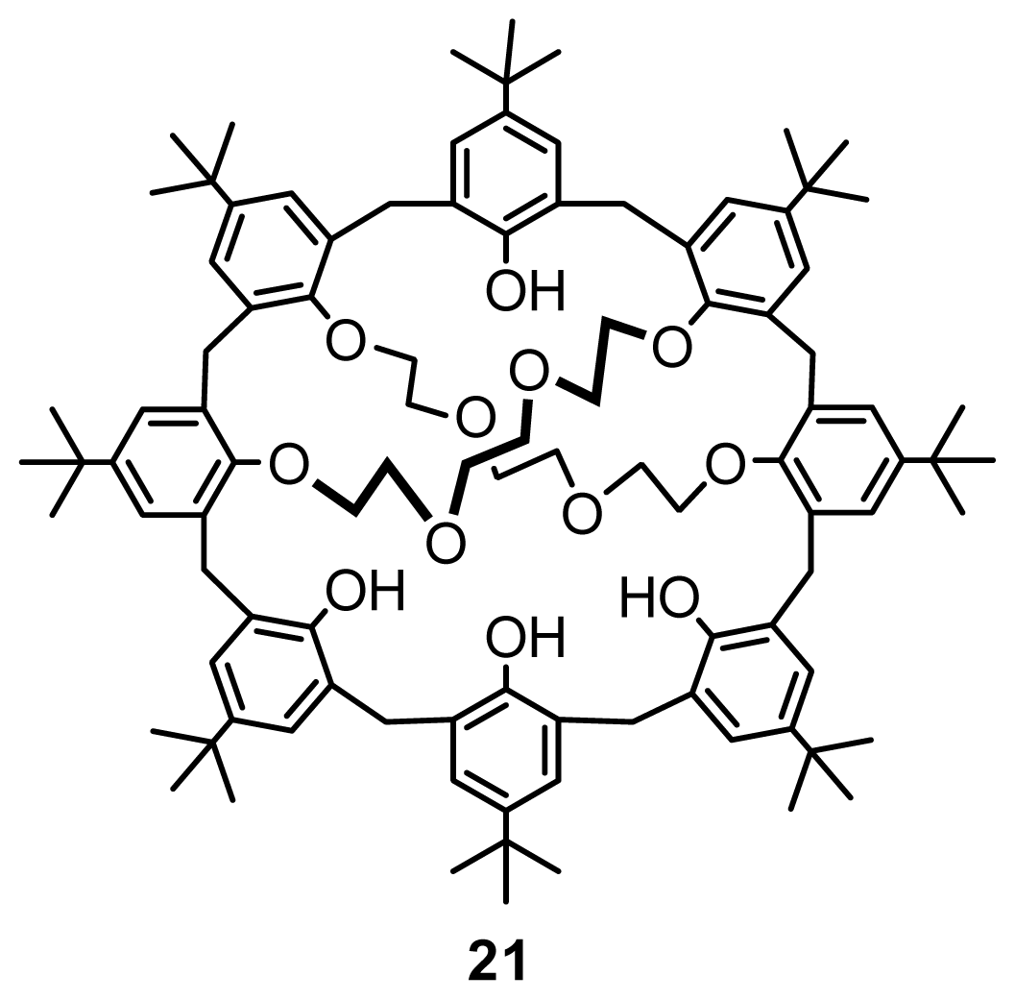
2.1. Fragment Condensation
2.2. Asymmetric Functionalization
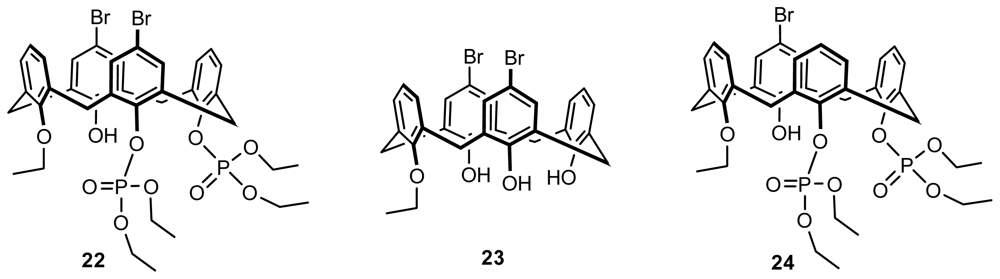
2.2.1. Asymmetric Functionalization on the Lower Rim
2.2.2. Asymmetric Functionalization on the Lower and Upper Rims
2.2.3. Asymmetric Functionalization on the Upper Rim
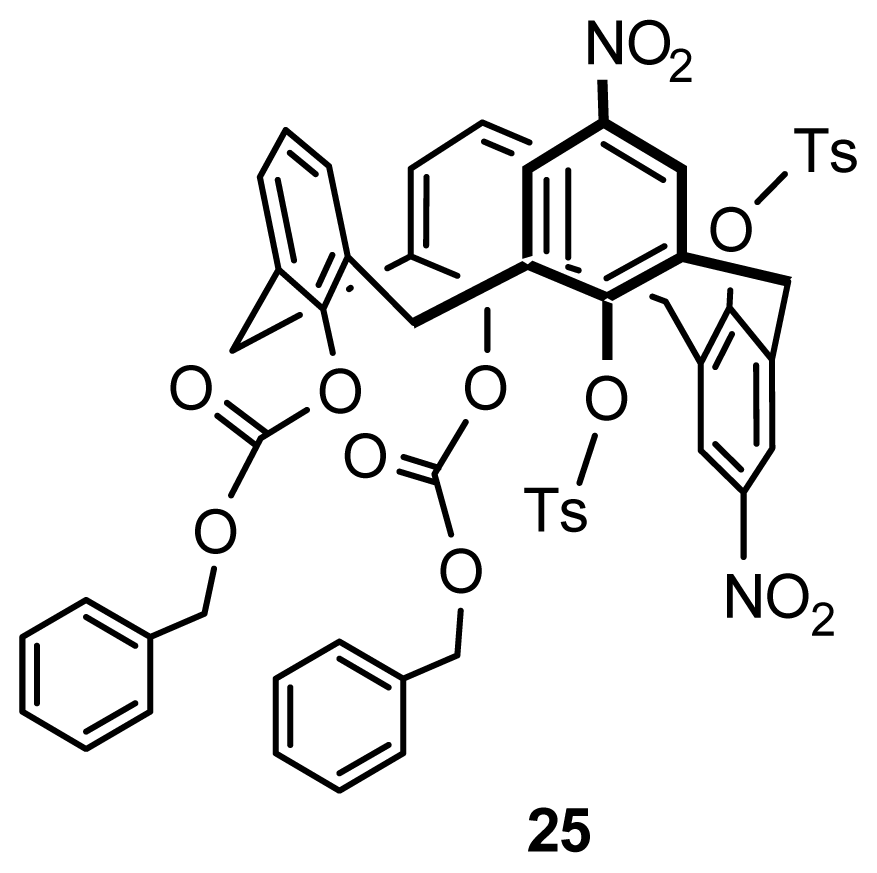
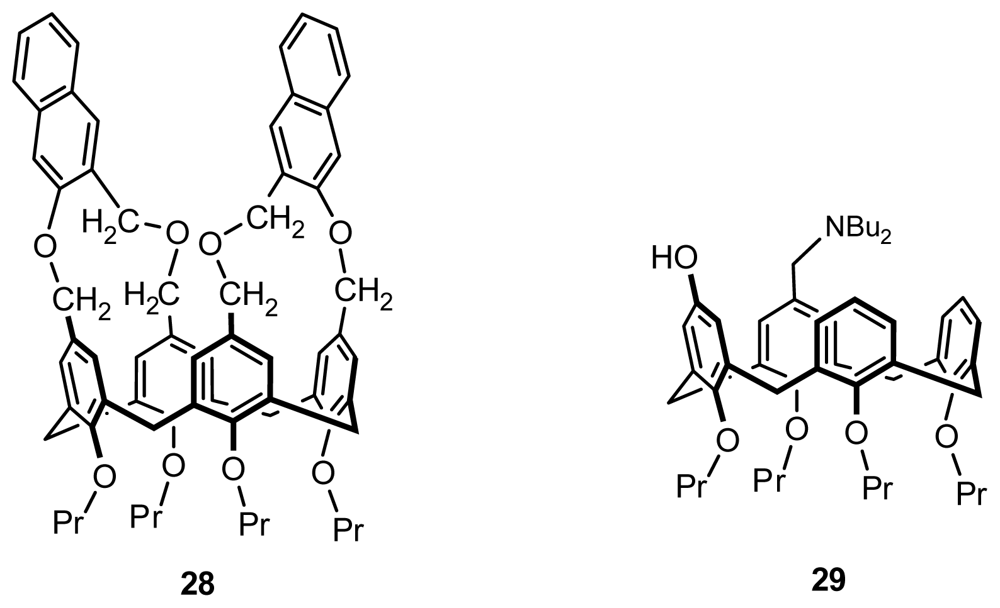
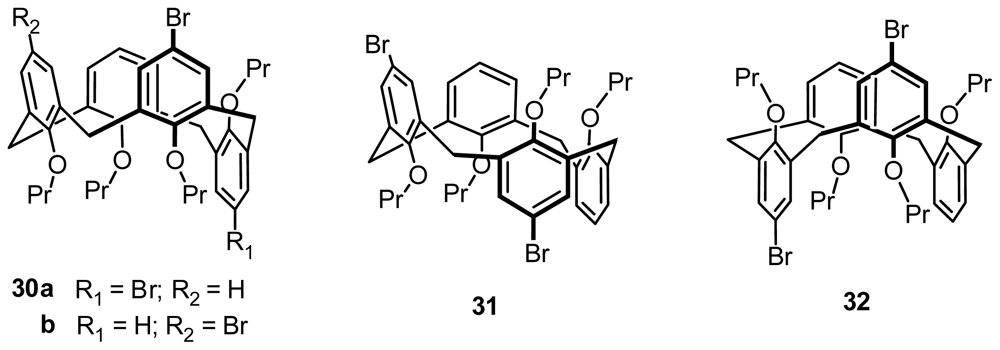
2.2.4. Asymmetric Functionalization on Phenolic Meta-Position
3. Optical Resolution of Inherently Chiral Calixarenes
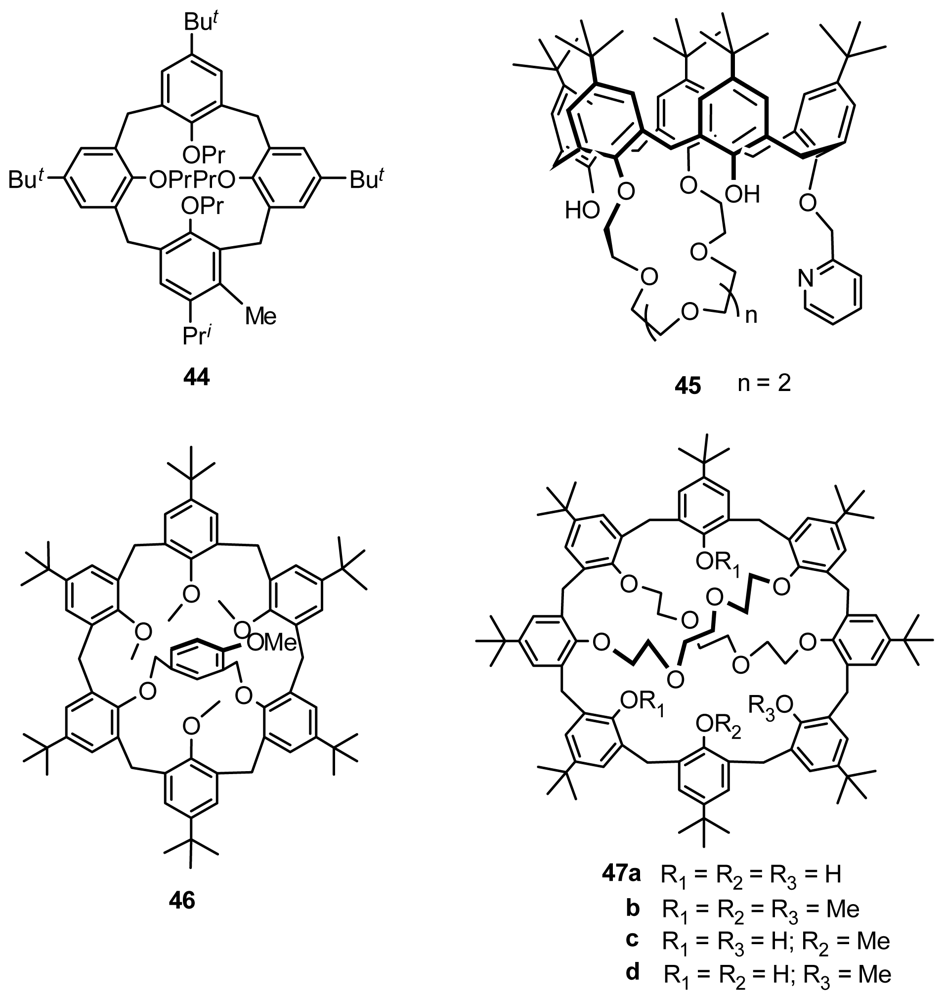
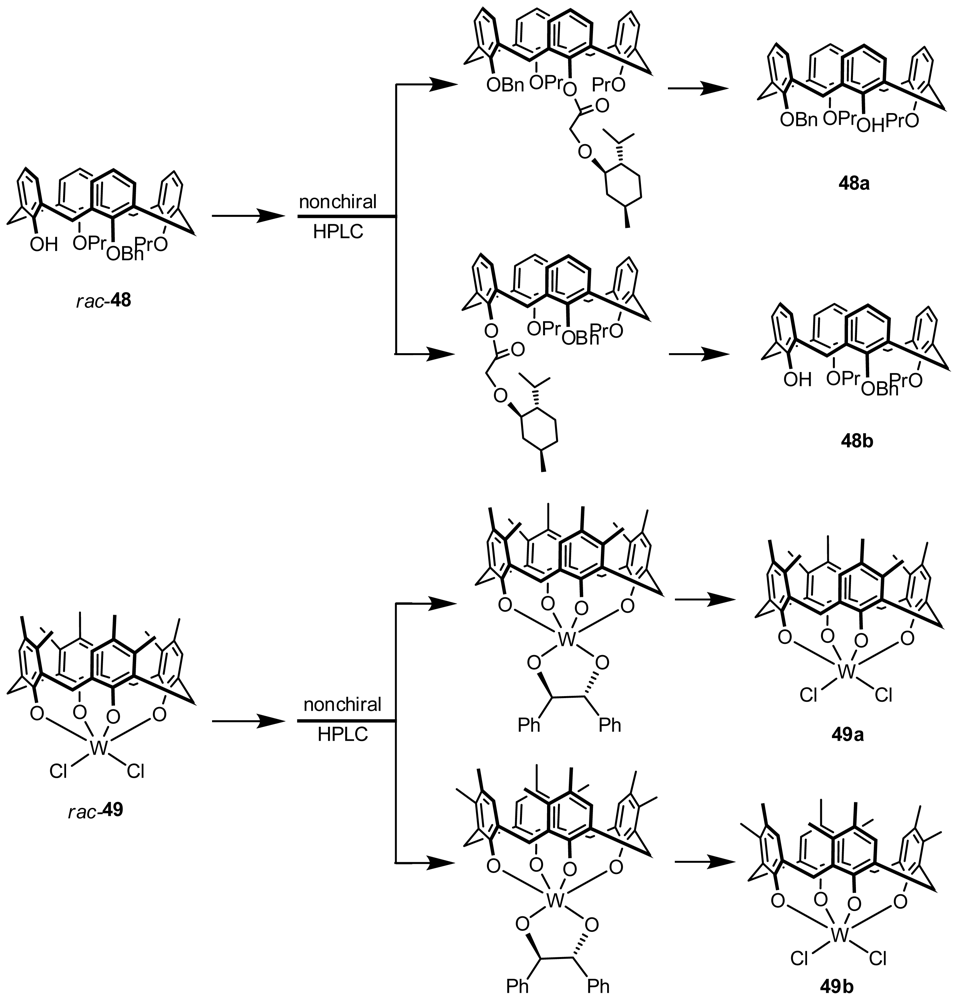
3.1. Resolution by Chiral Column Chromatography
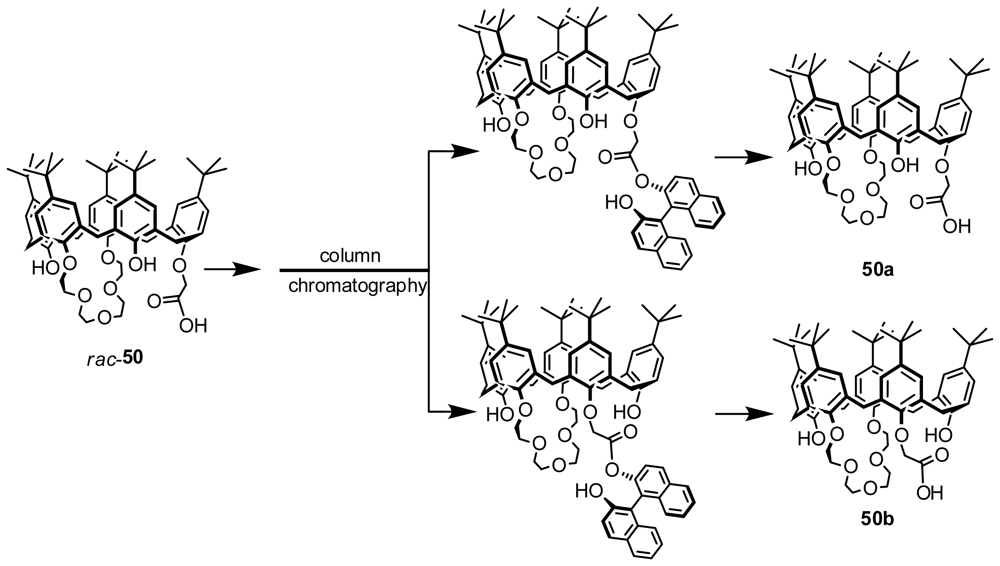
3.2. Resolution from Diastereomers by Conventional Chromatography
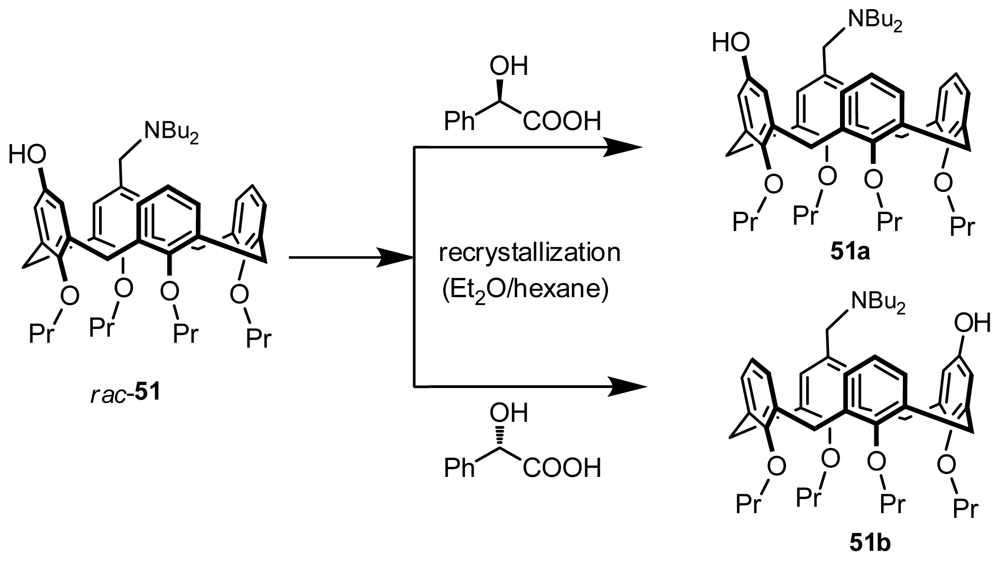
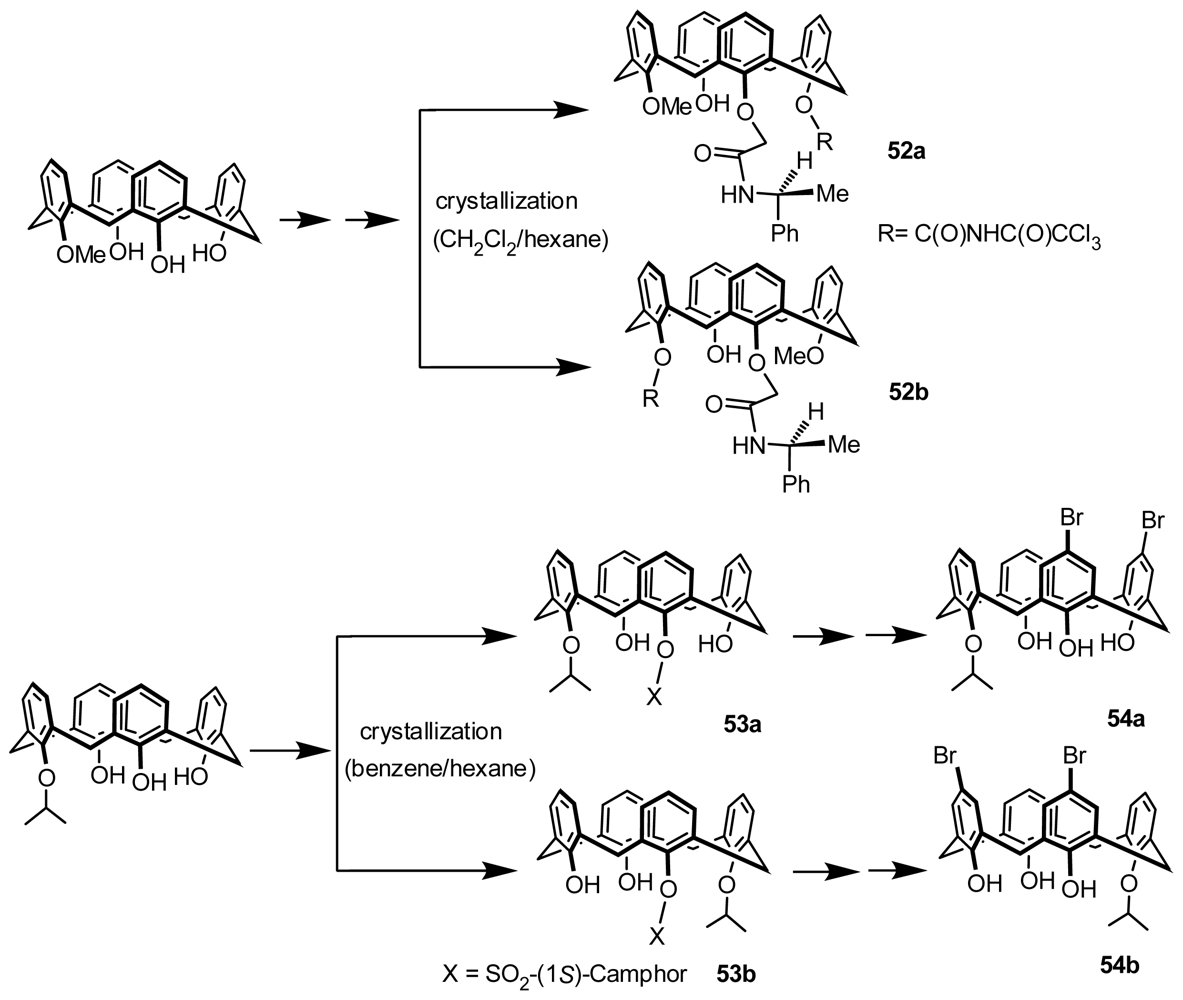
3.3. Resolution through Recrystallization with a Chiral Auxiliary
3.4. Resolution through Recrystallization of Diastereomers
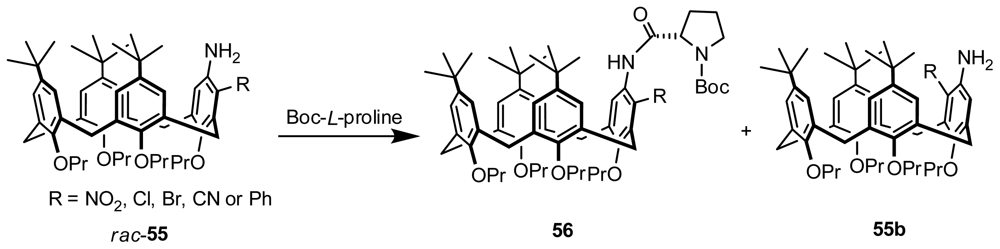
3.5. Kinetic Resolution
4. Applications of Inherently Chiral Calixarenes
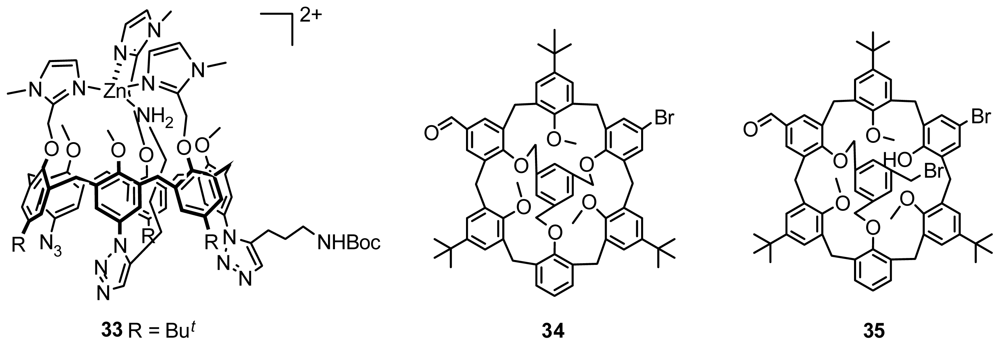
4.1. Chiral Recognition of Inherently Chiral Calixarenes
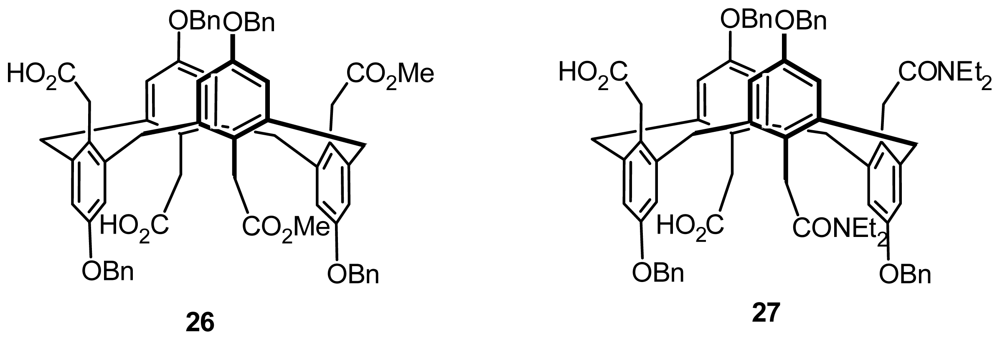
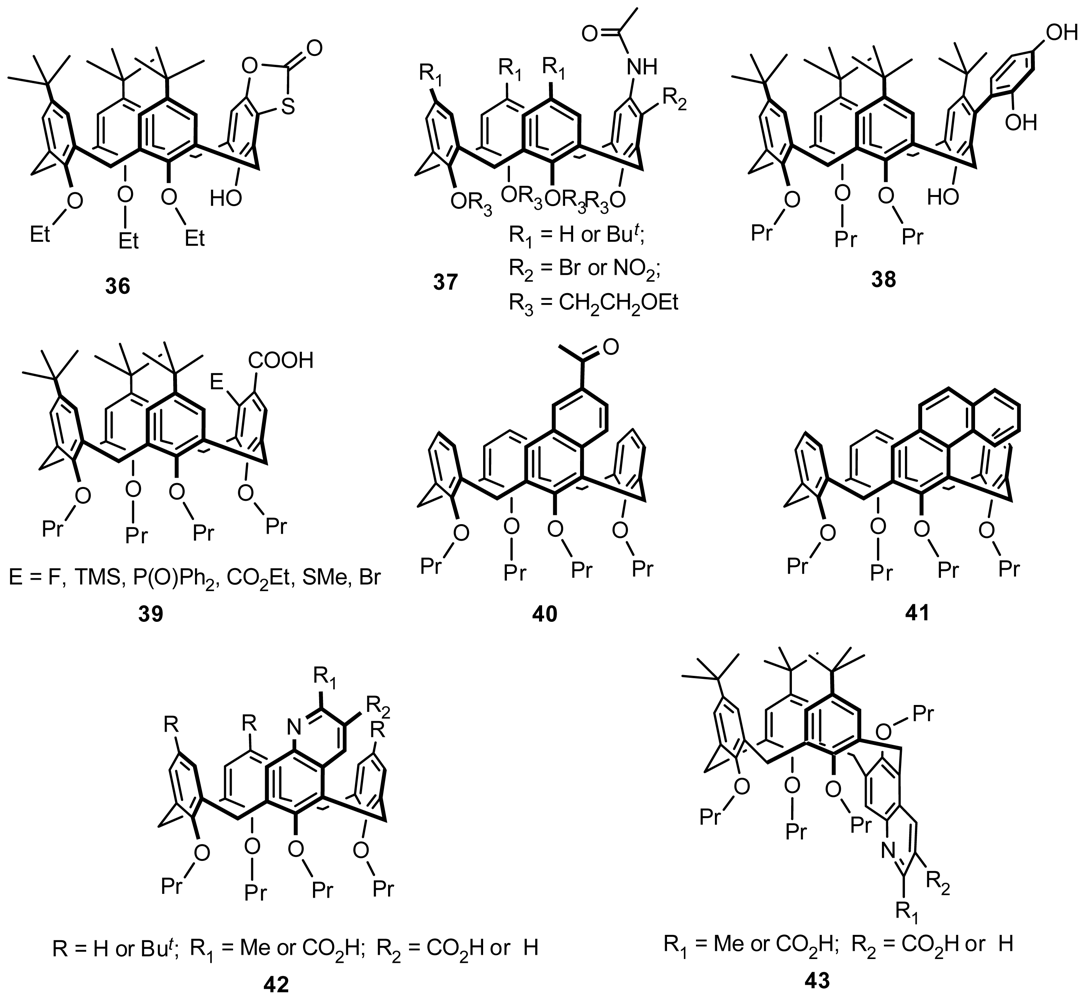
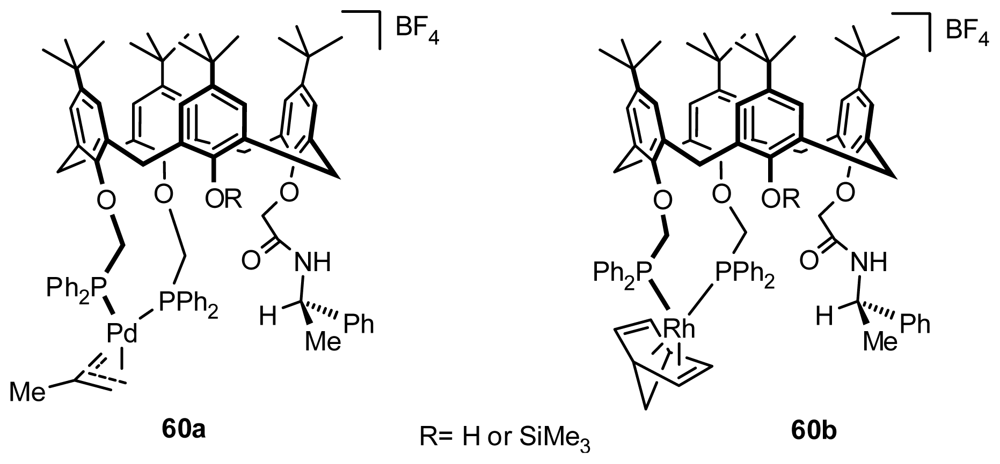
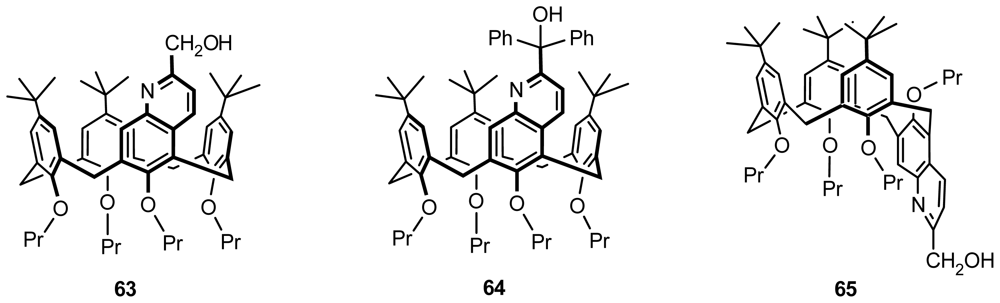
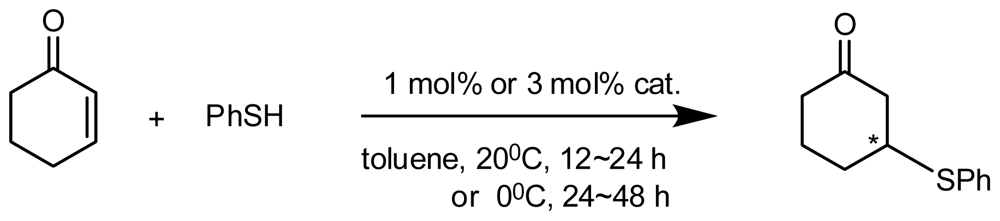
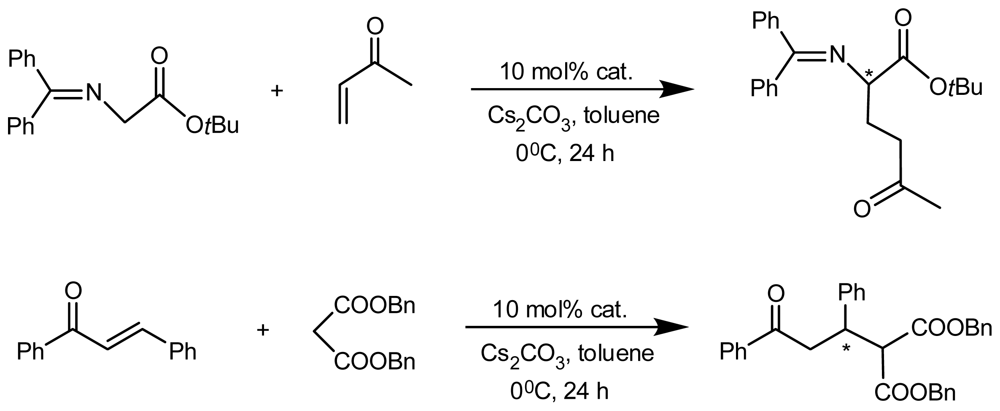
4.2. Asymmetric Catalysis of Inherently Chiral Calixarenes
4. Conclusions
Acknowledgements
References
- Gutsche, DC. Calixarenes: An Introduction; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 209–237. [Google Scholar]
- Kubo, Y; Maeda, S; Tokita, S; Kubo, M. Colorimetric Chiral Recogniton by a molecular Sensor. Nature 1996, 382, 522–524. [Google Scholar]
- Pinkhassik, E; Stibor, I; Casnati, A; Ungaro, R. Synthesis of Upper and Lower Rim Binaphthyl Bridged Calix[4]arenes: New Potential Chiral Hosts for Molecular Recognition and Catalysis. J. Org. Chem 1997, 62, 8654–8659. [Google Scholar]
- Castellano, RK; Nuckolls, C; Rebek, J, Jr. Transfer of Chiral Information through Molecular Assembly. J. Am. Chem. Soc 1999, 121, 11156–11163. [Google Scholar]
- Prins, LJ; Jong, FD; Timmerman, P; Reinhoudt, DN. An Enantiomerically Pure Hydrogen-bonded Assembly. Nature 2000, 408, 181–184. [Google Scholar]
- Zheng, Y-S; Zhang, C. Exceptional Chiral Recognition of Racemic Carboxylic Acids by Calix[4]arenes Bearing Optically Pure α,β-Amino Alcohol Groups. Org. Lett 2004, 6, 1189–1192. [Google Scholar]
- Cherenok, S; Vovk, A; Muravyova, I; Shivanyuk, A; Kukhar, V; Lipkowski, J; Kalchenko, V. Calix[4]arene α-Aminophosphonic Acids: Asymmetric Synthesis and Enantioselective Inhibition of an Alkaline Phosphatase. Org. Lett 2006, 8, 549–552. [Google Scholar]
- Bonini, C; Chiummiento, L; Funicello, M; Teresa Lopardo, M; Lupattelli, P; Laurita, A; Cornia, A. Novel Chiral Calix[4]arenes by Direct Asymmetric Epoxidation Reaction. J. Org. Chem 2008, 73, 4233–4236. [Google Scholar]
- Gaeta, C; de Rosa, M; Fruilo, M; Soriente, A; Neri, P. Synthesis of Calix[4]arene Derivatives Bearing Chiral Pendant Groups as Ligands for Enantioselective Catalysis. Tetrahedron: Asymmetry 2005, 16, 2333–2340. [Google Scholar]
- Quintard, A; Darbost, U; Vocanson, F; Pellet-Rostaing, S; Lemaire, M. Synthesis of New Calix[4]arene Based Chiral Ligands Bearing β-Amino Alcohol Groups and Their Application in Asymmetric Transfer Hydrogenation. Tetrahedron: Asymmetry 2007, 18, 1926–1933. [Google Scholar]
- Marson, A; Freixa, Z; Kamer, PCJ; van Leeuwen, PWNM. Chiral Calix[4]arene-Based Diphosphites as Ligands in the Asymmetric Hydrogenation of Prochiral Olefins. Eur J Inorg Chem 2007, 4587–4591. [Google Scholar]
- Böhmer, V; Kraft, D; Tabatabai, M. Inherently Chiral Calixarenes. J. Incl. Phenom. Macro. Chem 1994, 19, 17–39. [Google Scholar]
- Cort, AD; Mandolini, L; Pasquini, C; Schiaffino, L. “Inherent chirality” and curvature. New J. Chem 2004, 28, 1198–1199. [Google Scholar]
- Szumna, A. Inherently Chiral Concave Molecules—from Synthesis to Applications. Chem. Soc. Rev 2010, 39, 4274–4285. [Google Scholar]
- No, KH; Gutsche, CD. Calixarenes. 8. Short, Stepwise Synthesis of p-Phenylcalix[4]arene, p-Phenyl-p-tert-butylcalix[4]arene, and Derived Products. J. Org. Chem 1982, 47, 2713–2719. [Google Scholar]
- Luo, J; Zheng, Q; Chen, C; Huang, Z. Progress in Inherently Chiral Calixarenes. Prog. Chem 2006, 18, 897–906. [Google Scholar]
- McIldowie, MJ; Mocerino, M; Ogden, MI. A Brief Review of Cn-Symmetric Calixarenes and Resorcinarenes. Supramol. Chem 2010, 22, 13–39. [Google Scholar]
- Böhmer, V; Merkel, L; Kunz, U. Asymmetrically-Substituted Calix[4]arenes. J Chem Soc, Chem Commun 1987, 896–897. [Google Scholar]
- Casabianca, H; Royer, J; Satrallah, A; Taty-C, A; Vicens, J. Synthesis of Calix[4]arenes Presenting No Plane of Symmetry. Tetrahedron Lett 1987, 28, 6595–6596. [Google Scholar]
- Andreetti, GD; Böhmer, V; Jordon, JG; Tabatabai, M; Ugozzoli, F; Vogt, W; Wolfft, A. Dissymmetric Calix[4]arenes with C4- and C2-Symmetry. Synthesis, X-ray Structures, Conformational Fixation, and lH NMR Spectroscopic Studies. J. Org. Chem 1993, 58, 4023–4032. [Google Scholar]
- Iwamoto, K; Shimizu, H; Araki, K; Shinkai, S. Syntheses and Optical Resolution of Calix[4]arenes with Molecular Asymmetry. Systematic Classification of All Possible Chiral Isomers Derivable from Calix[4]arene. J. Am. Chem. Soc 1993, 115, 3997–4006. [Google Scholar]
- Narumi, F; Yamabuki, W; Hattori, T; Kameyama, H; Miyano, S. Synthesis and Optical Resolution of an anti-O,O′-Dialkylated Calix[4]arene. Chem. Lett 2003, 32, 320–321. [Google Scholar]
- Pappalardo, S; Caccamese, S; Giunta, L. Stereoselective Synthesis and Optical Resolution of Chiral Calix[4]arenes with Mixed Ligating Functionalities. Tetrahedron Lett 1991, 32, 7747–7750. [Google Scholar]
- Böhmer, V; Wolff, A; Vogt, W. A New Route to Chiral Calix[4]arenes via 1,3-Derivatization. J. Chem. Soc., Chem. Commun 1990, 14, 968–970. [Google Scholar]
- Vysotsky, MO; Tairov, MO; Pirozhenko, VV; Kalchenko, VI. Inherently Chiral Calix[4]arenes with Asymmetrical Superposition of Substituents at the Lower and the Upper Rims of Macrocycle. Tetrahedron Lett 1998, 39, 6057–6060. [Google Scholar]
- Li, SY; Sun, B; Xiao, ZC; Huang, MH; Xie, WX; Liu, JM. One-Step Synthesis of Inherently Chiral p-tert-Butylcalix[4]azacrown. Chin. Chem. Lett 2009, 20, 640–642. [Google Scholar]
- Iwamoto, K; Yanagi, A; Arimura, T; Matsuda, T; Shinkai, S. Synthesis and Optical Resolution of a Chiral Calix[4]arene Asymmetrically-Substituted on the Lower Rim. Chem. Lett 1990, 19, 1901–1904. [Google Scholar]
- Browne, JK; McKervey, MA; Pitarch, M; Russell, JA. Enzymatic Synthesis of Nonracemic Inherently Chiral Calix[4]arenes by Lipase-Catalysed Transesterification. Tetrahedron Lett 1998, 39, 1787–1790. [Google Scholar]
- Bitter, I; Grün, A; Tóth, G; Balázs, B; Horváth, G; Tóke, L. Studies on Calix(aza)crowms, II.1 Synthesis of Novel Proximal Doubly Bridged Calix[4]arenes by Intramolecular Ring Closure of syn-l,3- and 1,2-ω-Chloroalkylamides. Tetrahedron 1998, 54, 3857–3870. [Google Scholar]
- Shu, C-M; Chung, W-S. Synthesis of Calix[4]arenes with Four Different “Lower Rim” Substituents. J. Org. Chem 1999, 64, 2673–2679. [Google Scholar]
- Arnecke, R; Böhmer, V; Fergwon, G; Pappalardo, S. Inherently Chiral Derivatives of Calix[5]crowns. Tetrahedron Lett 1996, 37, 1497–1500. [Google Scholar]
- No, K; Kwon, KM; Kim, BH. Asymmetrically Substituted Calix[5]arene Derivatives. Bull. Korean Chem. Soc 1998, 19, 1395–1398. [Google Scholar]
- Neri, P; Rocco, C; Consoli, GML; Piattelli, M. Conformational Isomerism of Calix[6]arenes: Isolation of Two Conformational Isomers of the 1,2-Bis(p-tert-butylbenzyl) Ether of p-tert- Butylcalix[6]arene. J. Org. Chem 1993, 58, 6535–6537. [Google Scholar]
- Janssen, RG; Verboom, W; van Duynhoven, JPM; van Velzen, EJJ; Reinhoudt, DN. Cryptocalix[6]arenes; Molecules with a Larger Cavity. Tetrahedron Lett 1994, 35, 6555–6558. [Google Scholar]
- Kanamathareddy, S; Gutsche, CD. Synthesis and Conformational Properties of Calix[6]arenes Bridged on the Lower Rim: Self-Anchored Rotaxanes. J. Am. Chem. Soc 1993, 115, 6572–6579. [Google Scholar]
- Corrada Geraci, C; Piattelli, M; Neri, P. Inherent Chirality in Calix[8]arenes Exploiting the Steric Constraint of Two Intercrossing Polyether Chains. Tetrahedron Lett 1996, 37, 7627–7630. [Google Scholar]
- Hesek, D; Inoue, Y; Drew, MGB; Beer, PD; Hembury, GA; Ishida, H; Aoki, F. Acid-Promoted Rearrangement of Carbonate Functionality Anchored to the Lower Rim of a Calix[4]arene Skeleton: A New Class of Chiral Calix[4]arene and Its Chiroptical Properties. Org. Lett 2000, 2, 2237–2240. [Google Scholar]
- Sharma, SK; Gutsche, CD. Synthesis and Reactions of Calix[4]arene Bisanhydrides. J. Org. Chem 1999, 64, 3507–3512. [Google Scholar]
- Ikeda, A; Yoshimura, M; Lhotak, P; Shinkai, S. Synthesis and Optical Resolution of Naphthalene-Containing Inherently Chiral Calix[4]arenes Derived by Intramolecular Ring Closure or Stapling of Proximal Phenyl Units. J Chem Soc, Perkin Trans 1996, 1945–1950. [Google Scholar]
- Shirakawa, S; Moriyama, A; Shimizu, S. Design of a Novel Inherently Chiral Calix[4]arene for Chiral Molecular Recognition. Org. Lett 2007, 9, 3117–3119. [Google Scholar]
- Shimizu, S; Moriyama, A; Kito, K; Sasaki, Y. Selective Synthesis and Isolation of All Possible Conformational Isomers of Proximally para-Disubstituted Calix[4]arene. J. Org. Chem 2003, 68, 2187–2194. [Google Scholar]
- Colasson, B; Reinaud, O. Selective Hetero-Trisfunctionalization of the Large Rim of a Biomimetic Calix[6]arene Using Host-Guest Chemistry as a Synthetic Tool. J. Am. Chem. Soc 2008, 130, 15226–15227. [Google Scholar]
- Liu, J-M; Shi, J-Y; Xu, Y-W; Su, C-Y; Li, S-Y. Synthesis of Inherently Chiral Wide Rim ABC-Substituted Calix[6]arene Derivatives. Supramol Chem 544733. [CrossRef]
- Reddy, PA; Gutache, CD. Calixarenes. 32. Reactions of Calix[4]quinines. J. Org. Chem 1993, 58, 3245–3251. [Google Scholar]
- Verboom, W; Bodewes, PJ; van Essen, G; Timmerman, P; van Hummel, GJ; Harkema, S; Reinhoudt, DN. A Novel Approach to Inherently Chiral Calix[4]arenes by Direct Introduction of a Substituent at the meta Position. Tetrahedron 1995, 51, 499–512. [Google Scholar]
- Troisi, F; Pierro, T; Gaeta, C; Carratù, M; Neri, P. Appending Aromatic Moieties at the para- and meta-Position of Calixarene Phenol Rings via p-Bromodienone Route. Tetrahedron Lett 2009, 50, 4416–4419. [Google Scholar]
- Herbert, SA; Arnott, GE. An Asymmetric Ortholithiation Approach to Inherently Chiral Calix[4]arenes. Org. Lett 2009, 11, 4986–4989. [Google Scholar]
- Xu, Z-X; Zhang, C; Zheng, Q-Y; Chen, C-F; Huang, Z-T. A New Approach to Enantiopure Inherently Chiral Calix[4]arenes: Determination of Their Absolute Configurations. Org. Lett 2007, 9, 4447–4450. [Google Scholar]
- Mastalerz, M; Hüggenberg, W; Dyker, G. Photochemistry of Styrylcalix[4]arenes. Eur J Org Chem 2006, 3977–3987. [Google Scholar]
- Miao, R; Zheng, Q-Y; Chen, C-F; Huang, Z-T. Efficient Syntheses and Resolutions of Inherently Chiral Calix[4]quinolines in the Cone and Partial-Cone Conformation. J. Org. Chem 2005, 70, 7662–7671. [Google Scholar]
- Shinkai, S; Arimura, T; Kawabata, H; Murakami, H; Araki, K; Iwamoto, K; Matsuda, T. Synthesis and Optical Resolution of an Asymmetrically Substituted Calix[4]arene. J Chem Soc, Chem Commun 1990, 1734–1736. [Google Scholar]
- Caccamese, S; Notti, A; Pappalardo, S; Parisi, MF; Principato, G. Inherently Chiral α-Picolyloxy-p-tert-Butylcalix[5]arene Crown Ethers: Synthesis, Structure Proof, and Enantioselective HPLC Resolution. Tetrahedron 1999, 55, 5505–5514. [Google Scholar]
- Otsuka, H; Shinkai, S. Definitive Evidence for Inhibition of Calix[6]arene Ring Inversion Obtained from a 1,3-Xylenyl-Bridged Chiral Calix[6]arene. J. Am. Chem. Soc 1996, 118, 4271–4275. [Google Scholar]
- Caccamese, S; Principato, G; Geraci, C; Neri, P. Resolution of Inherently Chiral 1,4-2,5- Calix[8]bis-Crown-4 Derivatives by Enantioselective HPLC. Tetrahedron: Asymmetry 1997, 8, 1169–1173. [Google Scholar]
- Cao, Y-D; Luo, J; Zheng, Q-Y; Chen, C-F; Huang, Z-T. Preparation of Both Antipodes of Enantiopure Inherently Chiral Calix[4]crowns. J. Org. Chem 2004, 69, 206–208. [Google Scholar]
- Luo, J; Zheng, Q-Y; Chen, C-F; Huang, Z-T. Synthesis and Optical Resolution of a Series of Inherently Chiral Calix[4]crowns with Cone and Partial Cone Conformations. Chem. Eur. J 2005, 11, 5917–5928. [Google Scholar]
- Xu, B; Carroll, PJ; Swager, TM. Chiral Metallocalix[4]arenes: Resolution by Diastereomeric Tungsten(VI) Alkoxides. Angew. Chem. Int. Ed. Engl 1996, 35, 2094–2097. [Google Scholar]
- Li, S-Y; Zheng, Q-Y; Chen, C-F; Huang, Z-T. Preparation of Enantiopure Inherently Chiral Calix[5]arenes. Tetrahedron: Asymmetry 2005, 16, 641–645. [Google Scholar]
- Boyko, VI; Shivanyuk, A; Pyrozhenko, VV; Zubatyuk, RI; Shishkinb, OV; Kalchenkoa, VI. A Stereoselective Synthesis of Asymmetrically Substituted Calix[4]arenecarbamates. Tetrahedron Lett 2006, 47, 7775–7778. [Google Scholar]
- Yakovenko, AV; Boyko, VI; Danylyuk, O; Suwinska, K; Lipkowski, J; Kalchenko, VI. Diastereoselective Lower Rim (1S)-Camphorsulfonylation as the Shortest Way to the Inherently Chiral Calix[4]arene. Org. Lett 2007, 9, 1183–1185. [Google Scholar]
- Xu, Z-X; Zhang, C; Yang, Y; Chen, C-F; Huang, Z-T. Effective Nonenzymatic Kinetic Resolution of Racemic m-Nitro-Substituted Inherently Chiral Aminocalix[4]arenes. Org. Lett 2008, 10, 477–479. [Google Scholar]
- Xu, Z-X; Zhang, C; Huang, Z-T; Chen, C-F. Efficient Synthesis and Resolution of meta-Substituted Inherently Chiral Aminocalix[4]arene Derivatives. Chin. Sci. Bull 2010, 55, 2859–2869. [Google Scholar]
- Jin, T; Monde, K. Synthesis and Optical Resolution of a Fluorescent Chiral Calix[4]arene with Two Pyrene Moieties Forming an Intramolecular Excimer. Chem Commun 1998, 1357–1358. [Google Scholar]
- Luo, J; Zheng, Q-Y; Chen, C-F; Huang, Z-T. Facile Synthesis and Optical Resolution of Inherently Chiral Fluorescent Calix[4]crowns: Enantioselective Recognition towards Chiral Leucinol. Tetrahedron 2005, 61, 8517–8528. [Google Scholar]
- Shirakawa, S; Moriyama, A; Shimizu, S. Synthesis, Optical Resolution and Enantiomeric Recognition Ability of Novel, Inherently Chiral Calix [4]arenes: Trial Application to Asymmetric Reactions as Organocatalysts. Eur J Org Chem 2008, 5957–5964. [Google Scholar]
- Kliachyna, MA; Yesypenko, OA; Pirozhenko, VV; Shishkina, SV; Shishkin, OV; Boyko, VI; Kalchenko, VI. Synthesis, Optical Resolution and Absolute Configuration of Inherently Chiral Calixarene Carboxylic Acids. Tetrahedron 2009, 65, 7085–7091. [Google Scholar]
- Dieleman, C; Steyer, S; Jeunesse, C; Matt, D. Diphosphines Based on an Inherently Chiral Calix[4]arene Scaffold: Synthesis and Use in Enantioselective Catalysis. J Chem Soc, Dalton Trans 2001, 2508–2517. [Google Scholar]
- Xu, Z-X; Huang, Z-T; Chen, C-F. Synthesis and Structures of Novel Enantiopure Inherently Chiral Calix[4]arene-Derived Salphen Ligands and their Transition-Metal Complexes. Tetrahedron Lett 2009, 50, 5430–5433. [Google Scholar]
- Xu, Z-X; Li, G-K; Chen, C-F; Huang, Z-T. Inherently Chiral Calix[4]arene-Based Bifunctional Organocatalysts for Enantioselective Aldol Reactions. Tetrahedron 2008, 64, 8668–8675. [Google Scholar]
- Miao, R; Xu, Z-X; Huang, Z-T; Chen, C-F. Enantiopure Inherently Chiral Calix[4]arene Derivatives Containing Quinolin-2-yl-methanol moiety: Synthesis and Application in the Catalytic Asymmetric Addition of Diethylzinc to Benzaldehyde. Sci. China Ser. B Chem 2009, 52, 505–512. [Google Scholar]
- Shirakawa, S; Kimura, T; Murata, S; Shimizu, S. Synthesis and Resolution of a Multifunctional Inherently Chiral Calix[4]arene with an ABCD Substitution Pattern at the Wide Rim: The Effect of a Multifunctional Structure in the Organocatalyst on Enantioselectivity in Asymmetric Reactions. J. Org. Chem 2009, 74, 1288–1296. [Google Scholar]
- Shirakawa, S; Shimizu, S. Synthesis of an Inherently Chiral Calix[4]arene Amino Acid and its Derivatives: Their Application to Asymmetric Reactions as Organocatalysts. Eur. J. Org. Chem 2009, 12, 1916–1924. [Google Scholar]
- Shirakawa, S; Shimizu, S. Improved Design of Inherently Chiral Calix[4]arenes as Organocatalysts. New J. Chem 2010, 34, 1217–1222. [Google Scholar]







© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, S.-Y.; Xu, Y.-W.; Liu, J.-M.; Su, C.-Y. Inherently Chiral Calixarenes: Synthesis, Optical Resolution, Chiral Recognition and Asymmetric Catalysis. Int. J. Mol. Sci. 2011, 12, 429-455. https://doi.org/10.3390/ijms12010429
Li S-Y, Xu Y-W, Liu J-M, Su C-Y. Inherently Chiral Calixarenes: Synthesis, Optical Resolution, Chiral Recognition and Asymmetric Catalysis. International Journal of Molecular Sciences. 2011; 12(1):429-455. https://doi.org/10.3390/ijms12010429
Chicago/Turabian StyleLi, Shao-Yong, Yao-Wei Xu, Jun-Min Liu, and Cheng-Yong Su. 2011. "Inherently Chiral Calixarenes: Synthesis, Optical Resolution, Chiral Recognition and Asymmetric Catalysis" International Journal of Molecular Sciences 12, no. 1: 429-455. https://doi.org/10.3390/ijms12010429
APA StyleLi, S.-Y., Xu, Y.-W., Liu, J.-M., & Su, C.-Y. (2011). Inherently Chiral Calixarenes: Synthesis, Optical Resolution, Chiral Recognition and Asymmetric Catalysis. International Journal of Molecular Sciences, 12(1), 429-455. https://doi.org/10.3390/ijms12010429




