Physico-Chemical Properties and Phase Behaviour of Pyrrolidinium-Based Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
3. Modelling
4. Experimental Section
4.1. Materials
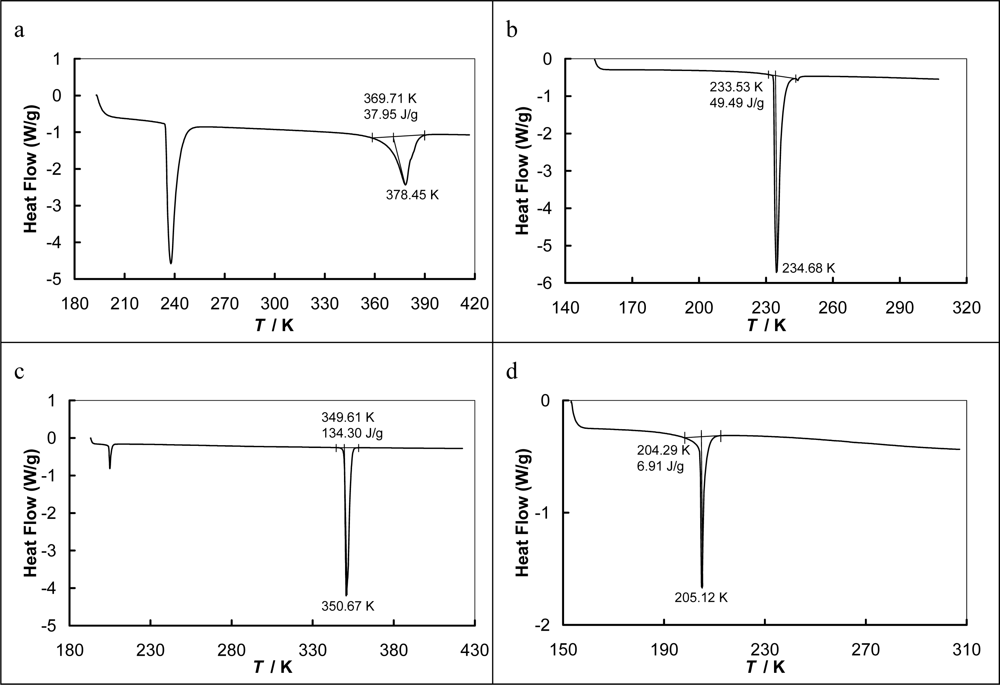
4.2. Differential Scanning Microcalorimetry
4.3. Apparatus and Experimental Procedure
5. Conclusions
Acknowledgments
References and Notes
- Lewandowski, A; Świderska, A. New composite solid electrolytes based on a polymer and ionic liquids. Solid State Ionics 2004, 169, 21–24. [Google Scholar]
- Kim, G-T; Appetecchi, GB; Alessandrini, F; Passerini, S. Solvent-free, PYR1A TFSI ionic liquid-based ternary polymer electrolyte systems I. Electrochemical characterization. J. Power Dources 2007, 171, 861–869. [Google Scholar]
- Lu, J; Yan, F; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci 2009, 34, 431–448. [Google Scholar]
- Pont, A-L; Marcilla, R; De Meatza, I; Grande, H; Mecerreyes, D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar]
- Bando, Y; Katayama, Y; Miura, T. Electrodeposition of palladium in a hydrophobic 1-n-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide room-temperature ionic liquid. Electrochim. Acta 2007, 53, 87–91. [Google Scholar]
- Azaceta, E; Tena-Zaera, R; Marcilla, R; Fantini, S; Echeberria, J; Pomposo, JA. Electrochemical deposition of ZnO in a room temperature ionic liquid: 1-Butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide. Electrochem. Commun 2009, 11, 2184–2186. [Google Scholar]
- Borisenko, N; Ispas, A; Zschippang, E; Liu, Q; Zein El Abedin, S; Bund, A; Endres, F. In situ STM and EQCM studies of tantalum electrodeposition from TaF5 in the air- and water-stable ionic liquid 1-butyl-1methylpyrrolidinium bis(trifluoromethylsulfonyl)amide. Electrochim. Acta 2009, 54, 1519–1528. [Google Scholar]
- Fujimori, T; Fujii, K; Kanzaki, R; Chiba, K; Yamamoto, H; Umebayashi, Y; Ishiguro, S. Conformational structure of room temperature ionic liquid N-butyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide-Raman spectroscopic study and DFT calculations. J Mol Liq 2007, 131–132, 216–224. [Google Scholar]
- Anouti, M; Jones, J; Boisset, A; Jacquemin, J; Caillon-Caravanier, M; Lemordant, D. Aggregation behavior in water of new imidazolium and pyrrolidinium alkycarboxylates protic ionic liquids. J. Coll. Interf. Sci 2009, 340, 104–111. [Google Scholar]
- Lombardo, M; Easwar, S; Pasi, F; Trombini, C; Dhavale, DD. Protonated arginine and lysine as catalysts for the direct asymmetric aldol reaction inionic liquids. Tetrahedron 2008, 64, 9203–9207. [Google Scholar]
- Poole, CF. Chromatographic and spectroscopic methods for the determination of solvent properties of room temperature ionic liquids. J. Chromatogr 2004, 1037, 49–82. [Google Scholar]
- Valderrama, JO; Rojas, RR. Critical properties of ionic liquids. Revisited. Ind. Eng. Chem. Res 2009, 48, 6890–6900. [Google Scholar]
- Earle, MJ; Esperanca, JMSS; Gilea, MA; Canongia Lopes, JN; Rebelo, LPN. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar]
- Kato, R; Gmehling, J. Systems with ionic liquids: Measurements of VLE and γ∞ data and prediction of their thermodynamic behavior using original UNIFAC, mod. UNIFAC(Do) and COSMO-RS(01). J. Chem. Thermodyn 2005, 37, 603–619. [Google Scholar]
- Nebig, S; Liebert, V; Gmehling, J. Measurements and prediction of activity coefficients at infinite dilution (γ∞), vapor-liquid equilibria (VLE) and excess enthalpies (HE) of binary systems with 1,1-dialkyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide using mod. UNIFAC (Dortmund). Fluid Phase Equilib 2009, 277, 61–67. [Google Scholar]
- Waliszewski, D; Stępniak, I; Piekarski, H; Lewandowski, A. Heat capacities of ionic liquids and their heats of solution in molecular liquids. Thermochim. Acta 2005, 433, 149–152. [Google Scholar]
- Gardas, RL; Coutinho, JAP. Extension of the Ye and Shreeve group contribution method for density estimation of ionic liquids in a wide range of temperatures and pressures. Fluid Phase Equilib 2008, 263, 26–32. [Google Scholar]
- Brennecke, JF; Maginn, EJ. Ionic liquids: Innovative fluids for chemical processing. AIChE J 2001, 47, 2384–2389. [Google Scholar]
- Holbrey, JD; López-Martin, I; Rothenberg, G; Seddon, KR; Sivero, G; Zheng, X. Desulfurisation of oils using ionic liquids: Selection of cationic and anionic components to enhance extraction efficiency. Green Chem 2008, 10, 87–92. [Google Scholar]
- Pereiro, AB; Rodríguez, A. An ionic liquid proposed as solvent in aromatic hydrocarbon separation by liquid extraction. AIChE J 2010, 56, 381–386. [Google Scholar]
- Westerholt, A; Liebert, V; Gmehling, J. Influence of ionic liquids on the separation factor of three standard separation problems. Fluid Phase Equilib 2009, 280, 56–60. [Google Scholar]
- Domańska, U; Redhi, GG; Marciniak, A. Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate using GLC. Fluid Phase Equilib 2009, 278, 97–102. [Google Scholar]
- Freire, MG; Neves, CMSS; Carvalho, PJ; Gardas, RL; Fernandes, AM; Marrucho, IM; Santos, LMNBF; Coutinho, JAP. Mutual solubilities of water and hydrophobic ionic liquids. J. Phys. Chem. B 2007, 111, 1382–1389. [Google Scholar]
- Freire, MG; Ventura, SPM; Santos, LMNBF; Marrucho, IM; Coutinho, JAP. Evaluation of COSMO-RS for the prediction of LLE and VLE of water and ionic liquids binary systems. Fluid Phase Equilib 2008, 268, 74–84. [Google Scholar]
- Marciniak, A; Karczemna, E. Influence of cation structure on binary liquid-liquid equilibria for systems containing ionic liquids based on trifluoromethanesulfonate anion with hydrocarbons. J Phys Chem B 2010. [Google Scholar]
- Domańska, U; Laskowska, M; Pobudkowska, A. Phase equilibria study of the binary sytems (1-butyl-3-methylimidazolium thiocyanate ionic liquid + organic solvent, or water). J. Phys. Chem. B 2009, 113, 6397–6404. [Google Scholar]
- Domańska, U; Laskowska, M; Marciniak, A. Phase equilibria of (1-ethyl-3-methylimidazolium ethylsufate + hydrocarbon, ketone, ether) binary systems. J. Chem. Eng. Data 2008, 53, 498–502. [Google Scholar]
- Domańska, U; Królikowski, M; Ślesińska, K. Phase equilibria study of the binary system (ionic liquid + thiophene): desulfurization process. J. Chem. Thermodyn 2009, 41, 1303–1311. [Google Scholar]
- Blesic, M; Canongia Lopes, JN; Pádua, AAH; Shimizu, K; Costa Gomes, MF; Rebelo, LPN. Phase equilibria in ionic liquid-aromatic compound mixtures, including benzene fluorination effects. J. Phys. Chem. B 2009, 113, 7631–7636. [Google Scholar]
- Barton, AFM. CRC Handbook of Solubility Parameters; CRC Press: Boca Raton, FL, USA, 1985; p. 64. [Google Scholar]
- Strechan, AA; Paulechka, YU; Kabo, AG; Blkhiin, AV; Kabo, GJ. 1-Butyl-3-methylimidazolium tosylate ionic liquid: Heat capacity, thermal stability, and phase equilibrium of its binary mixtures with water and caprolactam. J. Chem. Eng. Data 2007, 52, 1791–1799. [Google Scholar]
- Domańska, U; Królikowski, M. Phase equilibria study of the binary systems (1-butyl-3-methylimidazolium tosylate ionic liquid + water, or organic solvent). J. Chem. Thermodyn 2010, 42, 355–362. [Google Scholar]
- Domańska, U; Laskowska, M. Phase equilibria and volumetric properties of (1-ethyl-3-methylimidazolium ethylsulfate + alcohol or water) binary systems. J. Solution Chem 2008, 37, 1271–1287. [Google Scholar]
- Domańska, U; Laskowska, M. Phase equilibria and volumetric properties of (1-ethyl-3-methylimidazolium ethylsulfate + alcohol or water) binary systems. J. Solution Chem 2008, 37, 1271–1287. [Google Scholar]
- Domańska, U; Ługowska, K; Pernak, J. Phase equilibria of didecyldimethylammonium nitrate ionic liquid with water and organic solvents. J. Chem. Thermodyn 2007, 39, 729–736. [Google Scholar]
- Domańska, U. Thermophysical properties and thermodynamic phase behavior of ionic liquids. Thermochim. Acta 2006, 448, 19–30. [Google Scholar]
- Crosthwaite, JM; Aki, SNVK; Maginn, EJ; Brennecke, JF. Liquid phase behavior of imidazolium-based ionic liquids with alcohols. J. Phys. Chem. B 2004, 108, 5113–5119. [Google Scholar]
- Crosthwaite, JM; Aki, SNVK; Maginn, EJ; Brennecke, JF. Liquid phase behavior of imidazolium-based ionic liquids with alcohols: Effect of hydrogen bonding and non-polar interactions. Fluid Phase Equilib 2005, 228–229, 303–309. [Google Scholar]
- Wu, C-T; Marsh, KN; Deev, AV; Boxall, JA. Liquid-liquid equilibria of room-temperature ionic liquids and butan-1-ol. J. Chem. Eng. Data 2003, 48, 486–493. [Google Scholar]
- Sahandzhieva, K; Tuma, D; Breyer, S; Kamps, AP–S; Maurer, G. Liquid-liquid equilibrium in mixtures of the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate and an alcohol. J. Chem. Eng. Data 2006, 51, 1516–155. [Google Scholar]
- Heintz, A; Klasen, D; Lehmann, JK; Wertz, Ch. Excess molar volumes and liquid-liquid equilibria of the ionic liquid 1-methyl-3-octyl-imidazolium tetrafluoroborate mixed with butan-1-ol and pentan-1-ol. J. Solution Chem 2005, 34, 1135–1144. [Google Scholar]
- Domańska, U; Rękawek, A; Marciniak, A. Solubility of 1-alkyl-3-ethylimidazolium-based ionic liquids in water and 1-octanol. J. Chem. Eng. Data 2008, 53, 1126–1132. [Google Scholar]
- Prausnitz, JM; Lichtenthaler, RN; Azevedo, EG. Molecular Thermodynamics of Fluid-Phase Equilibria, 2nd ed; Prentice-hall Inc: Englewood Cliffs, NJ, USA, 1986. [Google Scholar]
- Wilson, GM. Vapor-liquid equilibrium. XI. A new expression for the excess free energy of mixing. J. Am. Chem. Soc 1964, 86, 127–130. [Google Scholar]
- Abrams, DS; Prausnitz, JM. Statistical thermodynamics of liquid mixtures: a new expression for the excess Gibbs energy of partly or completely miscible systems. AIChE J 1975, 21, 116–128. [Google Scholar]
- Renon, H; Prausnitz, JM. Local composition in thermodynamic excess functions for liquid mixtures. AIChE J 1968, 14, 135–144. [Google Scholar]
- Domańska, U. Vapour-liquid-solid equilibrium of eicosanoic acid in one and two component solvents. Fluid Phase Equilib 1986, 26, 201–220. [Google Scholar]
 ) [EMPYR][CF3SO3]; (
) [EMPYR][CF3SO3]; (  ) [PMPYR][CF3SO3]. Solid lines designated by the Wilson equation. Dotted lines represent an ideal solubility.
) [PMPYR][CF3SO3]. Solid lines designated by the Wilson equation. Dotted lines represent an ideal solubility.
 ) [EMPYR][CF3SO3]; (
) [EMPYR][CF3SO3]; (  ) [PMPYR][CF3SO3]. Solid lines designated by the Wilson equation. Dotted lines represent an ideal solubility.
) [PMPYR][CF3SO3]. Solid lines designated by the Wilson equation. Dotted lines represent an ideal solubility.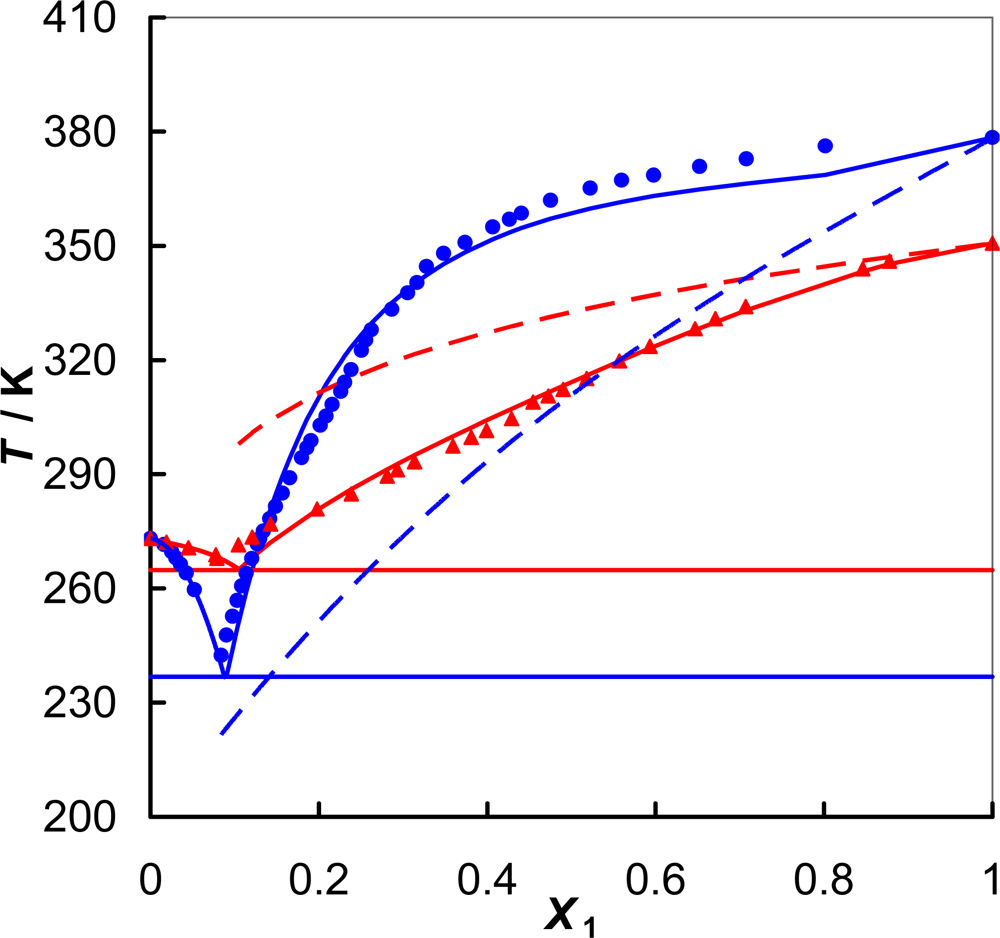
 ) 1-octanol; (
) 1-octanol; (  ) 1-hexanol; (
) 1-hexanol; (  ) 1-butanol. Solid lines designated by the Wilson equation. Dotted line represents an ideal solubility.
) 1-butanol. Solid lines designated by the Wilson equation. Dotted line represents an ideal solubility.
 ) 1-octanol; (
) 1-octanol; (  ) 1-hexanol; (
) 1-hexanol; (  ) 1-butanol. Solid lines designated by the Wilson equation. Dotted line represents an ideal solubility.
) 1-butanol. Solid lines designated by the Wilson equation. Dotted line represents an ideal solubility.
| Abbreviation | Name | Structure |
|---|---|---|
| [EMPYR][CF3SO3] | 1-ethyl-1-methylpyrrolidinium triflate | 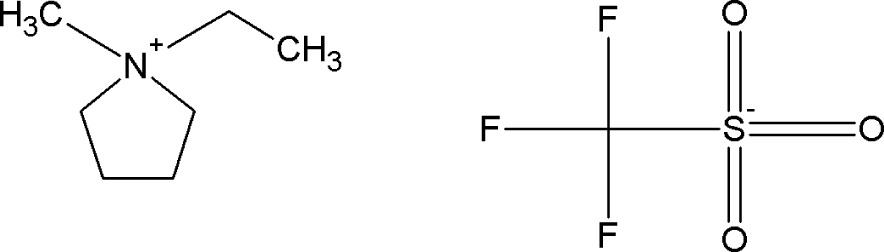 |
| [PMPYR][CF3SO3] | 1-propyl-1-methylpyrrolidinium triflate | 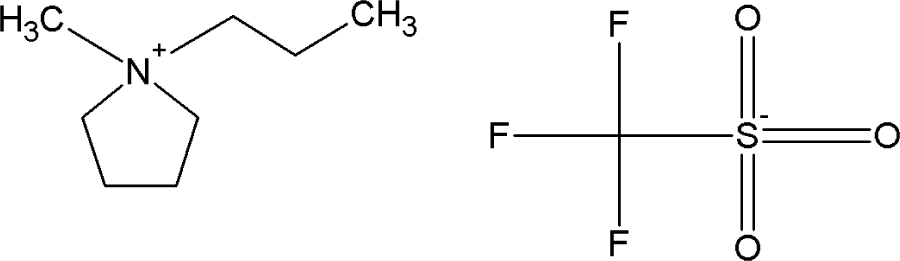 |
| Ionic liquid | M/g·mol−1 | Vm298.15/cm3·mol−1 | Tfus/K | ΔfusH/kJ·mol−1 | Ttr/K | ΔtrH/kJ·mol−1 | ΔfusCpa/J·mol−1·K−1 |
|---|---|---|---|---|---|---|---|
| [EMPYR][CF3SO3] | 263.26 | 239.6b | 378.5(SLE) 378.4(DSC) | 9.99 ± 0.05 | 234.15 | 13.03 ± 0.05 | 0.06c |
| [PMPYR][CF3SO3] | 277.29 | 255.7b | 350.7 | 37.24 ± 0.05 | 205.15 | 1.92 ± 0.05 | 106.2 |
| x1 | TSLE/K | γ1 | x1 | TSLE/K | γ1 |
|---|---|---|---|---|---|
| Water | |||||
| 0.0000 | 273.2 | 1.00 | 0.2087 | 305.3 | 2.24 |
| 0.0160 | 271.5 | 1.07 | 0.2159 | 308.4 | 2.25 |
| 0.0252 | 269.7 | 1.15 | 0.2258 | 311.8 | 2.25 |
| 0.0299 | 268.0 | 1.23 | 0.2308 | 314.2 | 2.26 |
| 0.0356 | 266.4 | 1.28 | 0.2384 | 317.5 | 2.28 |
| 0.0425 | 264.0 | 1.35 | 0.2506 | 322.6 | 2.30 |
| 0.0519 | 259.7 | 1.41 | 0.2555 | 325.3 | 2.33 |
| 0.0838 | 242.5 | 2.01 | 0.2623 | 328.0 | 2.34 |
| 0.0903 | 247.8 | 2.07 | 0.2866 | 333.4 | 2.27 |
| 0.0974 | 252.7 | 2.11 | 0.3055 | 337.7 | 2.23 |
| 0.1030 | 256.9 | 2.16 | 0.3166 | 340.4 | 2.21 |
| 0.1083 | 260.7 | 2.20 | 0.3278 | 344.6 | 2.23 |
| 0.1137 | 264.0 | 2.22 | 0.3480 | 348.1 | 2.18 |
| 0.1202 | 267.9 | 2.24 | 0.3736 | 350.9 | 2.09 |
| 0.1267 | 271.7 | 2.27 | 0.4064 | 355.0 | 1.99 |
| 0.1300 | 273.2 | 2.26 | 0.4262 | 357.0 | 1.94 |
| 0.1342 | 275.1 | 2.26 | 0.4405 | 358.6 | 1.90 |
| 0.1418 | 278.4 | 2.25 | 0.4751 | 362.0 | 1.82 |
| 0.1485 | 281.6 | 2.26 | 0.5224 | 365.2 | 1.71 |
| 0.1565 | 285.1 | 2.26 | 0.5595 | 367.3 | 1.62 |
| 0.1652 | 289.1 | 2.27 | 0.5977 | 368.6 | 1.54 |
| 0.1795 | 294.4 | 2.25 | 0.6521 | 370.9 | 1.44 |
| 0.1860 | 297.0 | 2.25 | 0.7078 | 372.9 | 1.35 |
| 0.1905 | 298.9 | 2.25 | 0.8015 | 376.3 | 1.22 |
| 0.2015 | 302.9 | 2.25 | 1.0000 | 378.5 | 1.00 |
| 1-Butanol | |||||
| 0.7388 | 351.5 | 1.06 | 0.8713 | 366.3 | 1.03 |
| 0.7620 | 353.7 | 1.05 | 0.8885 | 368.4 | 1.03 |
| 0.7839 | 355.9 | 1.04 | 0.9125 | 370.6 | 1.02 |
| 0.8135 | 359.0 | 1.03 | 0.9430 | 373.2 | 1.01 |
| 0.8368 | 361.6 | 1.03 | 0.9624 | 375.3 | 1.01 |
| 0.8529 | 363.8 | 1.03 | 1.0000 | 378.5 | 1.00 |
| x1 | TSLE/K | γ1 | x1 | TSLE/K | γ1 |
|---|---|---|---|---|---|
| Water | |||||
| 0.0000 | 273.2 | 1.00 | 0.3991 | 301.5 | 0.31 |
| 0.0189 | 272.2 | 0.97 | 0.4287 | 304.6 | 0.34 |
| 0.0451 | 270.7 | 0.95 | 0.4543 | 309.0 | 0.39 |
| 0.0775 | 268.8 | 0.90 | 0.4724 | 310.6 | 0.41 |
| 0.0788 | 267.9 | 0.87 | 0.4901 | 312.3 | 0.42 |
| 0.1044 | 271.5 | 0.23 | 0.5180 | 315.2 | 0.46 |
| 0.1209 | 273.5 | 0.22 | 0.5570 | 319.8 | 0.52 |
| 0.1427 | 277.0 | 0.23 | 0.5934 | 323.6 | 0.58 |
| 0.1978 | 280.9 | 0.21 | 0.6470 | 328.3 | 0.65 |
| 0.2385 | 284.9 | 0.22 | 0.6708 | 330.9 | 0.69 |
| 0.2813 | 289.5 | 0.24 | 0.7069 | 334.1 | 0.75 |
| 0.2932 | 291.2 | 0.25 | 0.8461 | 344.0 | 0.92 |
| 0.3138 | 293.3 | 0.26 | 0.8775 | 346.0 | 0.96 |
| 0.3591 | 297.4 | 0.28 | 1.0000 | 350.7 | 1.00 |
| 0.3810 | 299.7 | 0.30 | |||
| 1-Butanol | |||||
| 0.0698 | 295.6 | 1.32 | 0.4554 | 320.4 | 0.66 |
| 0.0896 | 296.8 | 1.10 | 0.4827 | 322.0 | 0.66 |
| 0.1041 | 297.7 | 0.99 | 0.5071 | 323.5 | 0.67 |
| 0.1119 | 298.3 | 0.95 | 0.5417 | 325.6 | 0.69 |
| 0.1309 | 299.4 | 0.86 | 0.5698 | 327.7 | 0.72 |
| 0.1528 | 300.8 | 0.79 | 0.5922 | 329.1 | 0.73 |
| 0.1735 | 302.1 | 0.74 | 0.6202 | 330.6 | 0.74 |
| 0.2050 | 304.0 | 0.69 | 0.6444 | 331.9 | 0.75 |
| 0.2384 | 306.2 | 0.66 | 0.6776 | 334.2 | 0.79 |
| 0.2681 | 307.9 | 0.63 | 0.7160 | 336.5 | 0.81 |
| 0.2897 | 309.4 | 0.63 | 0.7585 | 338.8 | 0.84 |
| 0.3117 | 310.9 | 0.63 | 0.7962 | 340.6 | 0.86 |
| 0.3330 | 312.2 | 0.62 | 0.8372 | 342.4 | 0.88 |
| 0.3535 | 313.4 | 0.62 | 0.8850 | 344.3 | 0.89 |
| 0.3716 | 314.8 | 0.63 | 0.9284 | 346.4 | 0.92 |
| 0.3961 | 316.4 | 0.63 | 0.9570 | 348.1 | 0.95 |
| 0.4206 | 318.0 | 0.64 | 1.0000 | 350.7 | 1.00 |
| 1-Hexanol | |||||
| 0.0863 | 310.4 | 2.21 | 0.5027 | 328.8 | 0.85 |
| 0.1164 | 312.2 | 1.78 | 0.5298 | 329.9 | 0.84 |
| 0.1457 | 313.8 | 1.53 | 0.5633 | 331.0 | 0.83 |
| 0.1736 | 315.2 | 1.37 | 0.5965 | 332.4 | 0.83 |
| 0.2026 | 316.6 | 1.25 | 0.6343 | 333.8 | 0.83 |
| 0.2344 | 318.0 | 1.15 | 0.6781 | 335.5 | 0.83 |
| 0.2595 | 319.1 | 1.09 | 0.7085 | 336.8 | 0.83 |
| 0.2886 | 320.2 | 1.03 | 0.7544 | 338.6 | 0.84 |
| 0.3196 | 321.3 | 0.97 | 0.7954 | 340.5 | 0.86 |
| 0.3586 | 322.8 | 0.92 | 0.8482 | 342.7 | 0.87 |
| 0.3796 | 323.7 | 0.91 | 0.8946 | 344.8 | 0.90 |
| 0.4207 | 325.2 | 0.87 | 0.9474 | 347.1 | 0.92 |
| 0.4519 | 326.4 | 0.85 | 1.0000 | 350.7 | 1.00 |
| 0.4800 | 327.7 | 0.85 | |||
| 1-Octanol | |||||
| 0.0253 | 318.1 | 10.67 | 0.3984 | 334.4 | 1.35 |
| 0.0344 | 320.0 | 8.53 | 0.4457 | 335.2 | 1.24 |
| 0.0456 | 321.7 | 6.93 | 0.5018 | 336.2 | 1.15 |
| 0.0557 | 323.2 | 6.05 | 0.5409 | 337.1 | 1.10 |
| 0.0676 | 324.3 | 5.23 | 0.5787 | 337.9 | 1.07 |
| 0.0867 | 325.5 | 4.29 | 0.6299 | 338.8 | 1.01 |
| 0.1175 | 327.2 | 3.40 | 0.6673 | 339.6 | 0.99 |
| 0.1580 | 328.9 | 2.71 | 0.7047 | 340.2 | 0.96 |
| 0.1666 | 329.3 | 2.62 | 0.7514 | 341.3 | 0.94 |
| 0.1980 | 330.2 | 2.28 | 0.7962 | 342.4 | 0.92 |
| 0.2396 | 331.1 | 1.96 | 0.8445 | 343.8 | 0.92 |
| 0.2809 | 332.1 | 1.74 | 0.8986 | 345.8 | 0.93 |
| 0.3151 | 332.8 | 1.60 | 0.9386 | 347.5 | 0.95 |
| 0.3582 | 333.7 | 1.46 | 1.0000 | 350.7 | 1.00 |
| 1-Decanol | |||||
| 0.0155 | 331.4 | 30.66 | 0.5131 | 342.4 | 1.43 |
| 0.0489 | 333.4 | 10.54 | 0.5470 | 342.4 | 1.34 |
| 0.0689 | 335.0 | 7.98 | 0.5580 | 342.7 | 1.33 |
| 0.0878 | 335.6 | 6.41 | 0.5915 | 342.8 | 1.26 |
| 0.1015 | 336.3 | 5.70 | 0.5998 | 342.7 | 1.24 |
| 0.1223 | 336.9 | 4.85 | 0.6407 | 343.1 | 1.18 |
| 0.1533 | 338.0 | 4.04 | 0.6798 | 343.1 | 1.11 |
| 0.1928 | 338.9 | 3.32 | 0.7310 | 343.5 | 1.05 |
| 0.2324 | 339.6 | 2.83 | 0.7648 | 343.8 | 1.01 |
| 0.2729 | 340.2 | 2.47 | 0.8094 | 344.5 | 0.98 |
| 0.3126 | 340.8 | 2.21 | 0.8554 | 345.3 | 0.96 |
| 0.3532 | 341.1 | 1.98 | 0.8848 | 346.2 | 0.96 |
| 0.3929 | 341.4 | 1.80 | 0.9445 | 347.8 | 0.95 |
| 0.4337 | 341.7 | 1.65 | 0.9822 | 350.2 | 1.00 |
| 0.4738 | 341.9 | 1.52 | 1.0000 | 350.7 | 1.00 |
| Parameters | RMSD | |||||
|---|---|---|---|---|---|---|
| Wilson | UNIQUAC | NRTL | Wilson | UNIQUAC | NRTL | |
| Solvent | λ12 – λ22 | u12 – u22 | g12 – g22 | σT/(K) | ||
| λ21 – λ11 | u21 – u11 | g21 – g11 | ||||
| J·mol−1 | J·mol−1 | J·mol−1 | ||||
| [EMPYR][CF3SO3] | ||||||
| Water | –6000.99 | - | 11108.24 | 5.68 | - | 1.77a |
| 14643.09 | - | –2407.88 | ||||
| 1-Butanol | 86142.98 | 6189.93 | 9151.76 | 2.52 | 0.82 | 0.77b |
| 2260.38 | –3213.90 | –4863.26 | ||||
| [PMPYR][CF3SO3] | ||||||
| Water | –11445.59 | - | 1729.43 | 2.53 | - | 2.04c |
| 7593.47 | - | –5366.87 | ||||
| 1-Butanol | –4777.45 | –2236.81 | –6688.84 | 5.62 | 5.01 | 2.45a |
| 3836.80 | 9586.35 | 16576.57 | ||||
| 1-Hexanol | 59160.06 | –1875.88 | –10141.14 | 3.50 | 3.01 | 1.97d |
| –186.86 | 4414.00 | 19911.02 | ||||
| 1-Octanol | 7114.00 | –1005.80 | –398.14 | 1.40 | 1.41 | 1.41c |
| 1263.48 | 2427.37 | 7364.46 | ||||
| 1-Decanol | 9729.67 | –797.71 | 2740.31 | 1.20 | 3.01 | 2.09e |
| 2622.93 | 2348.27 | 8010.29 | ||||
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Domańska, U. Physico-Chemical Properties and Phase Behaviour of Pyrrolidinium-Based Ionic Liquids. Int. J. Mol. Sci. 2010, 11, 1825-1841. https://doi.org/10.3390/ijms11041825
Domańska U. Physico-Chemical Properties and Phase Behaviour of Pyrrolidinium-Based Ionic Liquids. International Journal of Molecular Sciences. 2010; 11(4):1825-1841. https://doi.org/10.3390/ijms11041825
Chicago/Turabian StyleDomańska, Urszula. 2010. "Physico-Chemical Properties and Phase Behaviour of Pyrrolidinium-Based Ionic Liquids" International Journal of Molecular Sciences 11, no. 4: 1825-1841. https://doi.org/10.3390/ijms11041825
APA StyleDomańska, U. (2010). Physico-Chemical Properties and Phase Behaviour of Pyrrolidinium-Based Ionic Liquids. International Journal of Molecular Sciences, 11(4), 1825-1841. https://doi.org/10.3390/ijms11041825




