Dental Implant Systems
Abstract
:1. Introduction
2. Requirements for Successful Implant Systems
2.1. Safety Concerns
2.2. Compatibility
2.2.1. Biological Compatibility (Biocompatibility)
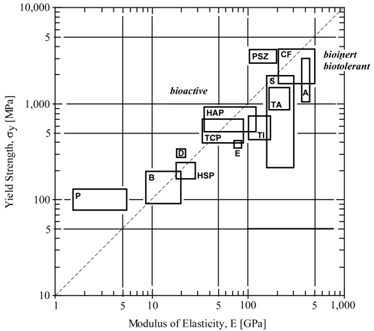
2.2.2. Mechanical Compatibility
2.2.3. Morphological Compatibility
2.3. MRI Safety and Image Compatibility
3. Surface Texturing
3.1. Sand-Blasting
3.2. Shot-Peening and Laser-Peening
3.3. Chemical, Electrochemical, and Thermal Modifications
3.4. Coating
3.4.1. Carbon, Glass, Ceramic Coating
3.4.2. Hydroxyapatite Coating
3.4.3. Ca-P Coating
3.4.4. Composite Coating
3.4.5. TiN Coating
3.4.6. Ti Coating
3.4.7. Titania Film Coating
3.5. Porosity Controlled Surface and Texturing
3.6. FoaMed Metal
4. Dental implants for Growing Patients
4.1. Special Considerations for Implant Therapy in Children
4.2. Clinical Studies on Use of Endosseous Implants
4.3. Alternative Treatments
5. Future Perspectives
5.1. Titanium Industry and New Materials R&D
5.2. Gradient Functional Material System
5.3. Coating
5.4. Fluoride Treatment
5.5. Laser Applications
5.6. Near-Net Shape (NNS) Forming
5.7. Tissue Engineering and Scaffold Structure and Materials
5.8. Application of Nanotechnology to Surface Modification
5.9. Bioengineering and Biomaterial-Integrated Implant System
5.10. Technology-Integrated Implant Systems
6. Conclusions
References
- Brånemark, PI; Zarb, GA; Albrektsson, T. Tissue-Integrated Prostheses; Osseointegration in Clinical Dentistry; Quintessence: Chicago, IL, USA, 1985; pp. 175–186. [Google Scholar]
- National Institutes of Health consensus development conference statement on dental implants. J. Dent. Educ 1988, 52, 686–691.
- Stillman, N; Douglass, CW. Developing market for dental implants. J. Am. Dent. Assoc 1993, 124, 51–56. [Google Scholar]
- Binon, PP. Implants and Components. Int. J. Oral Max. Implants 2000, 15, 76–94. [Google Scholar]
- Misch, CE. Contemporary Implant Dentistry; Misch, CE, Ed.; Mosby, Inc: St. Louis, MO, USA, 1999; Chapter 1, 3. [Google Scholar]
- Lemons, JE. Contemporary Implant Dentistry; Misch, CE, Ed.; Mosby, Inc: St. Louis, MO, USA, 1999; Chapter 20, 286–287. [Google Scholar]
- Giray, B; Akça, K; Ýplikçiođlu, H; Akça, E. Two-year follow-up of a patient with oligodontia treated with implant- and tooth-supported fixed partial dentures: A case report. Int. J. Oral Maxillofac. Implants 2003, 18, 905–911. [Google Scholar]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amsterdam, Holland, 2007; pp. 217–253. [Google Scholar]
- Oshida, Y; Tuna, EB. Advanced Biomaterials; Basu, B, Ed.; Wiley: Hoboken, NJ, USA, 2009; Chapter 5, 143–177. [Google Scholar]
- Anusavice, AJ. Phillip’s Science of Dental Materials, 11th ed; Anusavice, AJ, Ed.; Saunders: St. Louis MO, USA, 2003; Chapter 1, 3–19. [Google Scholar]
- Moore, BK; Oshida, Y. Materials science and technology in dentistry. In Encyclopedic Handbook of Biomaterilas and Bioengineering; Wise, DL, Ed.; Marcel Dekker: Boston, MA, USA, 1995; Chapter 48, 1325–1430. [Google Scholar]
- ISO/FDIS: 7405:2008, Dentistry – Evaluation of Biocompatibility of Medical Devices Used in Dentistry; BSI: London, UK, 2008.
- Long, M; Rack, HJ. Review: Titanium alloys in total joint replacement – a materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar]
- Brunski, JB; Puleo, DA; Nanci, A. Biomaterials and biomechanics of oral and maxillofacial implants: Current status and future developments. Int. J. Oral Maxillofac. Implants 2000, 15, 15–46. [Google Scholar]
- Bannon, BP; Mild, EE. Titanium Alloys in Surgical Implants; Luckey, HA, Kubli, F, Eds.; ASTM STP 796, American Society for Testing and Materials: West Conshohocken, PA, USA, 1983; pp. 7–15. [Google Scholar]
- Oshida, Y; Hashem, A; Nishihara, T; Yapchulay, MV. Fractal dimension analysis of mandibular bones: Toward a morphological compatibility of implants. J. BioMed. Mater Eng 1994, 4, 397–407. [Google Scholar]
- Oshida, Y. Requirements for successful biofunctional implants. In The 2nd Symposium International of Advanced Bio-Materials; Montreal, Canada, 2000; pp. 5–10. [Google Scholar]
- Brockhurst, PJ. Dental Materials: New teritories for materials science. Au. Inst. Metals 1980, 3, 200–210. [Google Scholar]
- Steinmann, SG. Evaluation of Biomaterials; Winter, GD, Leray, JL, de Groot, K, Eds.; John Wiley & Sons: New York, NY, USA, 1980; pp. 1–34. [Google Scholar]
- Kruger, J. Corrosion and Degradation of Implant Materials; Syrett, BC, Acharya, A, Eds.; ASTM STP 684, American Society for Testing and Materials: West Conshohocken, PA, USA, 1979; pp. 107–127. [Google Scholar]
- Greene, ND. Corrosion and Degradation of Implant Materials: Second Symposium; Farker, AC, Griffin, CD, Eds.; ASTM STP 859, American Society for Testing and Materials: West Conshohocken, PA, USA, 1983; pp. 5–10. [Google Scholar]
- Kubaschewski, O; Hopkins, BE. Oxidation of Metals and Alloys; Butterworths: London, UK, 1962; pp. 38–45. [Google Scholar]
- Kasemo, B. Biocompatibility of titanium implants: Surface science aspects. J. Pros Dent 1983, 49, 832–837. [Google Scholar]
- Tomashov, ND. Theory of Corrosion and Protection of Metals: The Science of Corrosion; The MacMillan: New York, NY, USA, 1966. [Google Scholar]
- Ashby, MF; Jones, DRH. Engineering Materials An Introduction to Their Properties and Applications; Pergamon Press: New York, NY, USA, 1980; pp. 194–200. [Google Scholar]
- Lautenschlager, EP; Monaghan, P. Titanium and titanium alloys as dental materials. Int. Dent. J 1993, 43, 245–253. [Google Scholar]
- Coddet, C; Chaze, AM; Beranger, G. Measurements of the adhesion of thermal oxide films: Application to the oxidation of titanium. J. Mater. Sci 1987, 22, 2969–2974. [Google Scholar]
- Meachim, G; Williams, DF. Changes in nonosseous tissue adjacent to titanium implants. J. BioMed. Mater. Res 1973, 7, 555–572. [Google Scholar]
- Ducheyne, P; Williams, G; Martens, M; Helsen, J. In vivo metal-ion release from porous titanium-silver material. J. BioMed. Mater. Res 1984, 18, 293–308. [Google Scholar]
- Sundgren, J-E; Bodö, P; Lundström, I. Auger electron spectroscopic studies of the interface between human tissue and implants of titanium and stainless steel. J. Colloid Interf. Sci 1986, 110, 9–20. [Google Scholar]
- Ducheyne, P; Healy, KE. Surface Characterization of Biomaterials; Ratner, BD, Ed.; Elsevier Science: Amsterdam, Holland, 1988; pp. 175–192. [Google Scholar]
- Fraker, AC; Ruff, AW. Titanium Science and Technology; Plenum Press: New York, NY, USA, 1973; Volume 4, pp. 2447–2457. [Google Scholar]
- Albreksson, T; Hansson, HA. An ultrastructural characterization of the interface between bone and sputtered titanium or stainless steel surfaces. Biomaterials 1986, 7, 201–205. [Google Scholar]
- Solar, RJ. Corrosion and Degradation of Implant Materials; Syrett, BD, Ed.; ASTM STP 684, American Society for Testing and Materials: West Conshohocken, PA, USA, 1979; pp. 259–273. [Google Scholar]
- Lim, YJ; Oshida, Y; Andres, CJ; Barco, MT. Surface characterizations of variously treated titanium materials. Int. J. Oral Maxillofac. Implants 2001, 16, 333–342. [Google Scholar]
- Gaydos, JM; Moore, MA; Garetto, LP; Oshida, Y; Kowolik, MJ. Bisphosphonate effect on neutrophil activation by titanium and hydroxyapatite implants. J Dent Res 2000, 79, 225. [Google Scholar]
- McQueen, D; Sundgren, JE; Ivarsson, B; Lundstrom, I; Ekenstam, CB; Svensson, A; Brånemark, PI; Albrektsson, T. Clinical Applications of Biomaterials; Lee, AJC, Ed.; Wiley: New York, NY, USA, 1982; pp. 179–185. [Google Scholar]
- Liedberg, B; Ivarsson, B; Lundstrom, I. Fourier transform infrared reflection absorption spectroscopy (FTIR-RAS) of fibrinogen adsorbed on metal and metal oxide surfaces. J. Biochem. Biophys. Method 1984, 9, 233–243. [Google Scholar]
- Hanawa, T. The Bone-Biomaterial Interface; Davies, JE, Ed.; Univ. of Toronto Press: Toronto, ON, Canada, 1991; pp. 49–61. [Google Scholar]
- Healy, KE; Ducheyne, P. Hydration and preferential molecular adsorption on titanium in vitro. Biomaterials 1992, 13, 553–561. [Google Scholar]
- Healy, KE; Ducheyne, P. The mechanisms of passive dissolution of titanium in a model physiological environment. J. BioMed. Mater. Res 1992, 26, 319–338. [Google Scholar]
- Brånemark, PI. Tissue-Integrated Protheses; Brånemark, PI, Ed.; Quintessence Pub.: Chicago, IL, USA, 1985; pp. 63–70. [Google Scholar]
- Kasemo, B; Lausmaa, J. Tissue-Integrated Protheses; Brånemark, PI, Ed.; Quintessence: Chicago, IL, USA, 1985; pp. 108–115. [Google Scholar]
- Skalak, R. Biomechanical considerations in osseointegrated prostheses. J. Prosthet Dent 1983, 49, 843–848. [Google Scholar]
- Hekimoglu, C; Anil, N; Cehreli, MC. Analysis of strain around endosseous implants opposing natural teeth or implants. J. Prosthet. Dent 2004, 92, 441–446. [Google Scholar]
- van Rossen, IP; Braak, LH; de Putter, C; de Groot, K. Stress-absorbing elements in dental implants. J. Prosthet. Dent 1990, 64, 198–205. [Google Scholar]
- Eriksson, C; Lausmaa, J; Nygren, H. Intercations between human whole blood and modified TiO2-surfaces: Influence of surface topography and oxide thickness on leukocyte adhesion and activation. Biomaterials 2001, 22, 1987–1996. [Google Scholar]
- Wen, X; Wang, X; Zhang, N. Microsurface of metallic biomaterials: A literature review. J. BioMed. Mater. Eng 1996, 6, 173–189. [Google Scholar]
- Keller, JC; Dougherty, WJ; Grotendorst, GR; Wrightman, JP. In vitro cell attachment to characterized cpTi surfaces. Adhesion 1989, 28, 115–133. [Google Scholar]
- Keller, JC; Wrightman, JP; Dougherty, WJ. Characterization of acid passivated cpTi surfaces. J Dent Res 1989, 68, 872, (Abstract). [Google Scholar]
- Keller, JC; Draughn, RA; Wrightman, JP; Dougherty, WJ. Characterization of sterilized CP titanium implant surfaces. Int. J. Oral Maxillofac. Implants 1990, 5, 360–369. [Google Scholar]
- Schroeder, A; van der Zypen, E; Stich, H; Sutter, F. The reactions of bone, connective tissue and epithelium to endosteal implants with titanium sprayed surfaces. J. Maxillofac. Surg 1981, 9, 15–25. [Google Scholar]
- Rich, A; Harris, AK. Anomalous preferences of cultured macrophages for hydrophobic and roughended substrata. J. Cell Sci 1981, 50, 1–7. [Google Scholar]
- Buser, D; Schenk, RK; Steinemann, S; Fiorellinni, JP; Fox, CH; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. BioMed. Mater. Res 1991, 25, 889–902. [Google Scholar]
- Buser, D; Nydegger, T; Oxland, T; Cochran, DL; Schenk, RK; Hirt, HP; Sneitivy, D; Nolte, LP. Interface shear strength of titanium implants with a sandblasted and acid-etched surface: A biomechanically study in the maxilla of miniature pigs. J. BioMed. Mater. Res 1999, 45, 75–83. [Google Scholar]
- Murray, DW; Rae, T; Rushton, N. The influence of the surface energy and roughness of implants on bone resorption. J. Bone JoInt. Surg 1989, 71B, 632–637. [Google Scholar]
- Cherhoudi, B; Gould, TR; Brunette, DM. Effects of a grooved epoxy substratum on epithelial behavior in vivo and in vitro. J. BioMed. Mater. Res 1988, 22, 459–477. [Google Scholar]
- von Recum, AF. Clinical Implant Materials; Heimke, G, Soltesz, U, Lee, AJC, Eds.; Elseveier Science Pub.: Amstrdam, The Netherlands, 1990; pp. 297–302. [Google Scholar]
- Deligianni, DD; Katsala, N; Ladas, S; Sotiropoulou, D; Amedee, J; Missirlis, YF. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar]
- Yang, Y; Cavin, R; Ong, LJ. Protein adsorption on titanium surfaces and their effect on osteoblast attachment. J. BioMed. Mater. Res. A 2003, 67, 344–349. [Google Scholar]
- Albrektsson, T. Direct bone anchorage of dental implants. J. Prosth. Dent 1983, 50, 255–261. [Google Scholar]
- Kasemo, B; Lausmaa, J. Surfaces science aspects on inorganic. Biomaterials CRC Crit Rev. Biocomp 1986, 2, 335–380. [Google Scholar]
- Schenk, RK; Buser, D. Osseointegration: A reality. Periodontology 1998, 17, 22–35. [Google Scholar]
- Masuda, T; Yliheikkaila, PK; Fleton, DA; Cooper, LF. Generalizations regarding the process and phenomenon of osseointegration. Part I. In vivo studies. Int. J. Oral Maxillofac. Implants 1998, 13, 17–29. [Google Scholar]
- Larsson, C; Esposito, M; Liao, H; Thomsen, P. Titanium in Medicine: Materials Science, Surface Science, Engineering, Biological Responsese, and Medical Applications; Brunette, DM, Tengvall, P, Textor, M, Thomsen, P, Eds.; Springer: New York, NY, USA, 2001; pp. 587–648. [Google Scholar]
- Oshida, Y; Sachdeva, R; Miyazaki, S. Microanalytical characterization and surface modification of NiTi orthodontic archwires. J. BioMed. Mater. Eng 1991, 2, 51–69. [Google Scholar]
- Zinger, O; Anselme, K; Denzer, A; Habersetzer, P; Wieland, M; Jeanfils, J; Hardouin, P; Landolt, D. Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 2004, 25, 2695–2711. [Google Scholar]
- Ratner, BD. Surface characterization of biomaterials by electron spectroscopy for chemical analysis. Ann. BioMed. Eng 1983, 11, 313–336. [Google Scholar]
- Baro, AM; Garcia, N; Miranda, R; Vázquez, L; Aparicio, C; Olivé, J; Lausmaa, J. Characterization of surface roughness in titanium dental implants measured with scanning tunneling microscopy at atmospheric pressure. Biomaterials 1986, 7, 463–466. [Google Scholar]
- Moroni, A; Caja, VL; Egger, EL; Trinchese, L; Chao, EY. Histomorphometry of hydroxyapatite coated and uncoated porous titanium bone implants. Biomaterials 1994, 15, 926–930. [Google Scholar]
- Keller, JC; Young, FA; Natiella, JR. Quantitative bone remolding resulting from the use of porous dental implants. J. BioMed. Mater. Res 1987, 21, 305–319. [Google Scholar]
- Pilliar, RM; Deporter, DA; Watson, PA; Valiquette, N. Dental implant design-effect on bone remodeling. J. BioMed. Mater. Res 1991, 25, 467–483. [Google Scholar]
- Gilbert, JL; Berkery, CA. Electrochemical reaction to mechanical disruption of titanium oxide films. J. Dent. Res 1995, 74, 92–96. [Google Scholar]
- Deporter, D; Watson, P; Pharoah, M; Levy, D; Todescan, R. Five-to six-year results of a prospective clinical trial using the ENDOPORE dental implant and a mandibular overdenture. Clin Oral Implants Res 1999, 10, 95–102. [Google Scholar]
- Palmer, RM; Plamer, PJ; Smith, BJ. A 5-year prospective study of astra single tooth implants. Clin Oral Implants Res 2000, 11, 179–182. [Google Scholar]
- Testori, T; Wiseman, L; Woolfe, S; Porter, SS. A prospective multicenter clinical study of the osseitite implant: Four-years interim report. Int. J. Oral Maxillofac. Implants 2001, 16, 193–200. [Google Scholar]
- Sul, Y-T. The significance of the surface properties of oxidized titanium to the bone response: Special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar]
- Ishizawa, H; Fujiino, M; Ogino, M. Mechanical and histological investigation of hydrothermally treated and untreated anodic titanium oxide films containing Ca and P. J. BioMed. Mater. Res 1995, 29, 1459–1468. [Google Scholar]
- Larsson, C; Emanuelsson, L; Thomsen, P; Ericson, L; Aronsson, B; Rodahl, M; Kasemo, B; Lausmaa, J. Bone response to surface-modified titanium implants: Studies on the tissue response after one year to machined and electropolished implants with different oxide thicknesses. J. Mater Sci.: Mater Med 1997, 8, 721–729. [Google Scholar]
- Skripitz, R; Aspenberg, P. Tensile bond between bone and titanium. Acta Orthop Scand 1998, 6, 2–6. [Google Scholar]
- Fini, M; Cigada, A; Rondelli, G; Chiesa, R; Giardino, R; Giavaresi, G; Aldini, N; Torricelli, P; Vicentini, B. In vitro and vivo behaviour of Ca and P-enriched anodized titanium. Biomaterials 1999, 20, 1587–1594. [Google Scholar]
- Henry, P; Tan, AE; Allan, BP. Removal torque comparison of TiUnite and turned implants in the Greyhound dog mandible. Appl. Osseointegr. Res 2000, 1, 15–17. [Google Scholar]
- Sul, Y-T; Johansson, CB; Jeong, Y; Röser, K; Wennerberg, A; Albreksson, T. Oxidized implants and their influence on the bone response. J. Mater Sci.: Mater Med 2001, 12, 1025–1031. [Google Scholar]
- Sul, Y-T; Johansson, CB; Jeong, Y; Wennerberg, A; Albrektson, T. Resonance frequency and removal torque analysis of implants with turned and anodized surface oxide. Clin Oral Implants Res 2002, 13, 252–259. [Google Scholar]
- Sul, Y-T; Johansson, CB; Albreksson, T. Oxidized titanium screws coated with calcium ions and their performance in rabbit bone. Int. J. Oral Maxillofac. Implants 2002, 17, 625–634. [Google Scholar]
- Oshida, Y. Surface science and technology – Titanium dental implant systems. J. Soc. Titanium Alloys Dent 2007, 5, 52–53. [Google Scholar]
- Elias, CN; Oshida, Y; Lima, JHC; Muller, CA. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant torque. J. Mech. Behav. Biomed. Mater 2008, 1, 234–242. [Google Scholar]
- Lausmaa, GJ. Chemical composition and morphology of titanium surface oxides. Mat. Res. Soc. Symp. Proc 1986, 55, 351–359. [Google Scholar]
- Albrektsson, T; Jacobsson, M. Bone-metal interface in osseointegration. J. Prosthet. Dent 1987, 57, 5–10. [Google Scholar]
- Lauterbur, PC. Image formation by induced local interactions: Example employing nuclear magnetic resonance. Nature 1973, 242, 190. [Google Scholar]
- Shellock, FG; Crues, JV. High field strength MR imaging and metallic biomedical implants: An ex vivo evaluation of deflection forces. Am. J. Roentgenol 1988, 151, 389–392. [Google Scholar]
- Shellock, FG; Morisoli, S; Kanal, E. MR Procedures and Biomedical Implants, Materials, and Devices: Update. Radiology 1993, 189, 587–599. [Google Scholar]
- Shellock, FG; Mink, JH; Curtin, S; Friesman, MJ. MR imaging and metallic implants for anterior cruciate ligament reconstruction: Assessment of ferromagnetism and artifact. J. Magn. Reson. Imaging 1992, 2, 225–228. [Google Scholar]
- Shellock, FG. Biomedical implants and devices: Assessment of magnetic field interactions with a 3.0 tesla MR system. J. Magn. Reson. Imaging 2002, 16, 721–732. [Google Scholar]
- Shellock, FG; Fieno, DS; Thompson, LJ; Talavage, TM; Berman, DS. Cardiac pacemaker: In vitro assessment at 1.5T. Am. Heart J 2006, 151, 436–443. [Google Scholar]
- Shellock, FG; Crues, J. High field strength MR imaging and metallic biomedical implants: An in vitro evaluation of deflection forces and temperature changes induced in large prostheses. Radiology 1987, 165, 150–152. [Google Scholar]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amstredam, The Netherlands, 2007; pp. 313–379. [Google Scholar]
- Kasemo, B; Lausmaa, J. Biomaterials and implant surfaces: A surface science approach. Int. J. Oral Maxillofac. Implants 1988, 3, 247–259. [Google Scholar]
- Baier, RE; Meyer, AE. Implant surface preparation. Int. J. Oral Maxillofac. Implants 1988, 3, 9–20. [Google Scholar]
- Reitz, WE. The eighth international conference on surface modification technology. J. Metal 1995, 47, 14–16. [Google Scholar]
- Smith, DC; Pilliar, RM; Chernecky, R. Dental implant materials. I. Some effects of preparative procesdures on surface topography. J. BioMed. Mater. Res 1991, 25, 1045–1068. [Google Scholar]
- Buddy, D; Ratner, B; Thomas, JL. Biomaterial surfaces. J. BioMed. Mater. Res 1987, 21, 59–89. [Google Scholar]
- Cooper, LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J. Prosthet. Dent 2000, 84, 522–534. [Google Scholar]
- Bachle, M; Kohal, RJ. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin Oral Implants Res 2004, 15, 683–692. [Google Scholar]
- Rompen, E; Domken, O; Degidi, M; Farias Pontes, AE; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin Oral Implants Res 2006, 17, S55–S67. [Google Scholar]
- Boyan, BD; Lossdorfer, S; Wang, L; Zhao, G; Lohmann, CH; Cochran, DL; Schwartz, Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur. Cell Mater 2003, 6, 22–27. [Google Scholar]
- Lossdorfer, S; Schwartz, Z; Wang, L; Lohmann, CH; Turner, JD; Wieland, M; Cochran, DL; Boyan, BD. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J. BioMed. Mater. Res. A 2004, 70, 361–369. [Google Scholar]
- Mustafa, K; Wennerberg, A; Wroblewski, J; Hultenby, K; Lopez, BS; Arvidson, K. Determining optimal surface roughness of TiO2 blasted titanium implant Material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin Oral Implants Res 2001, 12, 515–525. [Google Scholar]
- Castellani, R; de Ruijter, A; Renggli, H; Jansen, J. Response of rat bone marrow cells to differently roughened titanium discs. Clin Oral Implants Res 1999, 10, 369–378. [Google Scholar]
- Diniz, MG; Soares, GA; Coelho, MJ; Fernandes, MH. Surface topography modulates the osteogenesis in human bone marrow cell cultures grown on titanium samples prepared by a combination of mechanical and acid treatments. J. Mater. Sci. Mater. Med 2002, 13, 421–432. [Google Scholar]
- Anselme, K; Bigerelle, M; Noel, B; Dufresne, E; Judas, D; Iost, A; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res 2000, 49, 155–166. [Google Scholar]
- Sader, MS; Balduino, A; Soares, GA; Borojevic, R. Effect of three distinct treatments of titanium surface on osteoblast attachment, proliferation, and differentiation. Clin Oral Implants Res 2005, 16, 667–675. [Google Scholar]
- Citeau, A; Guicheux, J; Vinatier, C; Layrolle, P; Nguyen, TP; Pilet, P; Daculsi, G. In vitro biological effects of titanium rough surface obtained by calcium phosphate grid blasting. Biomaterials 2005, 26, 157–165. [Google Scholar]
- Oshida, Y; Daly, J. Fatigue damage evaluation of shot peened high strength aluminum alloy. In Surface Engineering; Meguid, SA, Ed.; Elsevier Applied: New York, NY, USA, 1990; pp. 404–416. [Google Scholar]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amstredam, The Netherlands, 2007; pp. 179–182. [Google Scholar]
- Piattelli, A; Scarano, A; Coriglano, M; Piattelli, M. Presence of multinucleated giant cells around machined, sandblasted and plasma-sprayed titanium implants: A histological and histochemical time-course study in rabbit. Biomaterials 1996, 17, 2053–2058. [Google Scholar]
- Kubo, M; Fijishima, A; Hotta, Y; Kunii, J; Shimakura, Y; Shimidzu, T; Kawawa, T; Miyazaki, T. Production method of resin-bonded titanium crown by CAD/CAM. Proc. Soc. Symp. Res 2006, 929, 42. [Google Scholar]
- Miyakawa, O; Watababe, K; Okawa, S; Shiokawa, N; Kobayashi, M; Tamura, H. Grinding of titanium, Part 1: Commercial and experimental wheels made of silicon carbide abrasives. Dent. Mater. J 1990, 9, 30–41. [Google Scholar]
- Miyakawa, O; Watanabe, K; Okawa, S; Nakano, S; Shiokawa, N; Kobayashi, M; Tamura, H. Grinding of titanium, Part 2: Commercial vitrified wheels made of alumina abrasives. Dent. Mater. J 1990, 9, 42–52. [Google Scholar]
- Miyakawa, O; Watanabe, K; Okawa, S; Kanatani, M; Nakano, S; Kobayashi, M. Surface contamination of titanium by abrading treatment. Dent. Mater. J 1996, 15, 11–21. [Google Scholar]
- Mandelbrot, BB. The Fractal Geometry of Nature; Freeman: New York, NY, USA, 1983; p. 34. [Google Scholar]
- Chesters, S; Wen, HY; Lundin, M; Kasper, G. Fractal-based characterization of surface texture. Appl. Surf. Sci 1989, 40, 185–192. [Google Scholar]
- Sayles, RS; Thomas, TR. Surface topography as a non-stationary random process. Nature 1978, 271, 431–434. [Google Scholar]
- Oshida, Y; Munoz, CA; Winkler, MM; Hashem, A; Ito, M. Fractal dimension analysis of aluminum oxide particle for sandblasting dental use. J. Biomed. Mater. Eng 1993, 3, 117–126. [Google Scholar]
- Miyakawa, O; Okawa, S; Kobayashi, M; Uematsu, K. Surface contamination of titanium by abrading treatment. Dent. Jpn 1998, 34, 90–96. [Google Scholar]
- Mustafa, K; Lopez, BS; Hultenby, K; Arvison, K. Attachment of HGF of titanium surfaces blasted with TiO2 particles. J Dent Res 1997, 76, 85. [Google Scholar]
- Johansson, CB; Albrektsson, T; Thomsen, P; Sonnerby, I; Lodding, A; Odelius, H. Tissue reactions to titanium-6aluminum-4vanadium alloy. Eur. J. Exp. Musculoskel. Res 1992, 1, 161–169. [Google Scholar]
- Wennerberg, A; Albrektsson, T; Johnsson, C; Andersson, B. Experimental study of turned and grit-blasted screw-shaped implants with special emphasis on effects of blasting material and surface topography. Biomaterials 1996, 17, 15–22. [Google Scholar]
- Wang, CS. Surface modification of titanium for enhancing titabium-pocelain bond strength; Master Degree ThesisIndiana University: Bloomington, IN, USA, 2004; pp. 97–115. [Google Scholar]
- Peutzfeld, A; Asmussen, E. Distortion of alloy by sandblasting. Am. J. Dent 1996, 9, 65–66. [Google Scholar]
- Peutzfeld, A; Asmussen, E. Distortion of alloys caused by sandblasting. J Dent Res 1996, 75, 259. [Google Scholar]
- Shot Peening Applications, 7th ed; Metal Improvement Company, Inc: Vernon, CA, USA, 1990.
- DeWald, AT; Rankin, JE; Hill, MR; Lee, MJ; Chen, H-L. Assessment of tensile residual stress mitigation in Alloy 22 welds due to laser peening. J. Eng. Mater. Technol. Trans. ASME 2004, 126, 81–89. [Google Scholar]
- Fairland, BP; Wilcox, BA; Gallagher, WJ; Williams, D. Laser shock-induced microstructural and mechanical property changes in 7075 aluminum. J. Appl. Phys 1972, 43, 3893–3895. [Google Scholar]
- Fairland, BP; Clauer, AH. Laser generation of high amplitude stress waves in materials. J. Appl. Phys 1979, 50, 1497–1502. [Google Scholar]
- Dane, CB; Hackel, LA; Daly, J; Harrison, J. High power laser for peening of metals enabling production technology. Mater. Manuf. Proc 2000, 15, 81–96. [Google Scholar]
- Cho, S-A; Jung, S-K. A removal torque of the laser-treated titanium implants in rabbit tibia. Biomaterials 2003, 24, 4859–4863. [Google Scholar]
- Gaggl, A; Schultes, G; Muller, WD; Karcher, H. Scanning electron microscopical analysis of laser-treated titanium implants surfaces–a comparative study. Biomaterials 2000, 21, 1067–1073. [Google Scholar]
- Hansson, S; Hansson, KN. The effect of limited lateral resolution in the measurement of implant surface roughness: A computer simulation. J. Biomed. Mater. Res. A 2005, 75, 472–477. [Google Scholar]
- Endo, K. Chemical modification of metallic implant surfaces with biofunctional proteins (Part 1) Molecular structure and biological activity of a modified NiTi alloy surface. Dent. Mater. J 1995, 14, 185–198. [Google Scholar]
- Browne, M; Gregson, PJ. Surface modification of titanium alloy implants. Biomaterials 1994, 15, 894–898. [Google Scholar]
- Krozer, A; Hall, J; Ericsson, I. Chemical treatment of machined titanium surfaces. Clin Oral Impl. Res 1999, 10, 204–211. [Google Scholar]
- Rupp, F; Scheideler, L; Rehbein, D; Axmann, D; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of aid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar]
- MacDonald, DE; Rapuano, BE; Deo, N; Stranick, M; Somasundaran, P; Boskey, AL. Thermal and chemical modification of titanium-aluminum-vanadium implant materials: Effects of surface properties, glycoprotein adsorption, and MG63 cell attachment. Biomaterials 2004, 25, 3135–3146. [Google Scholar]
- Li, L-H; Kong, Y-M; Kim, H-W; Kim, Y-W; Kim, H-E; Heo, S-J; Koak, J-Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 15, 1867–1875. [Google Scholar]
- Xie, Y; Liu, X; Huang, A; Ding, C; Chu, PK. Improvement of surface bioactivity on titanium by water and hydrogen plasma immersion ion implantation. Biomaterials 2005, 26, 6129–6135. [Google Scholar]
- Rohanizadeh, R; Al-Sadeq, M; LeGeros, RZ. Preparation of different forms of titanium oxide on titanium surface: Effects on apatite deposition. J. BioMed. Mater. Res. A 2004, 71, 343–352. [Google Scholar]
- Demri, B; Hage-Ali, M; Moritz, M; Muster, D. Surface characterization of C/Ti-6Al-4V coating treated with ion beam. Biomaterials 1997, 18, 305–310. [Google Scholar]
- Poon, RWY; Yeung, KWK; Liu, XY; Chu, PK; Chung, CY; Lu, WW; Cheung, KMC; Chan, D. Carbon plasma immersion ion implantation of nickel- titanium shape memory alloys. Biomaterials 2005, 26, 2265–2272. [Google Scholar]
- Schrooten, J; Helsen, JA. Adhesion of bioactive glass coating to Ti6Al4V oral implant. Biomaterials 2000, 21, 1461–1419. [Google Scholar]
- Saiz, E; Goldman, M; Gomez-Vega, JM; Tomsia, AP; Marshall, GW; Marshall, SJ. In vitro behavior of silicate glass coatings on Ti6Al4V. Biomaterials 2002, 23, 3749–3756. [Google Scholar]
- Lee, JH; Ryu, H-S; Lee, D-S; Hong, KS; Chang, B-S; Lee, C-K. Bio-mechanical and histomorphometric study on the bone-screw interface of bioactive ceramic-coated titanium screws. Biomaterials 2005, 26, 3249–3257. [Google Scholar]
- Chu, KT; Oshida, Y; Hancock, EB; Kowolik, MJ; Barco, TM; Zunt, SL. Hydroxyapatite/PMMA composites as bone cements. J. BioMed. Mat. Eng 2004, 14, 87–105. [Google Scholar]
- Liu, DM. Fabrication and characterization of porous hydroxyapatite granules. Biomatrials 1996, 17, 1955–1957. [Google Scholar]
- Luo, P; Nieh, TG. Preparing hydroxyapatite powders with controlled morphology. Biomaterials 1996, 17, 1959–1964. [Google Scholar]
- Porter, AE; Taakm, P; Hobbs, LW; Coathup, MJ; Blunn, GW; Spector, M. Bone bonding to hydroxyapatite and titanium surfaces on femoral stems retrieved from human subjects at autopsy. Biomaterials 2004, 25, 5199–5208. [Google Scholar]
- Souto, RM; Lemus, MM; Reis, RL. Electrochemical behavior of different preparations of plasma-sprayed hydroxyapatite coatings on Ti6Al4V substrate. J. BioMed. Mater. Res. A 2004, 70, 59–65. [Google Scholar]
- Filiaggi, MJ; Coombs, NA; Pilliar, RM. Characterization of the interface in the plasma-sprayed HA coating/Ti-6Al-4V implant system. J. BioMed. Mater. Res 1991, 25, 1211–1229. [Google Scholar]
- Yang, YC; Chang, E; Lee, ST. Mechanical properties and Young’s modulus of plasma-sprayed hydroxyapatite coating on Ti substrate in simulated body fluid. J. BioMed. Mater. Res. A 2003, 67, 886–899. [Google Scholar]
- Yang, YC; Ong, JL. Bond strength, compositional, and structural properties of hydroxyapatite coating on Ti, ZrO2-coated Ti, and TPS-coated Ti substrate. J. BioMed. Mater. Res. A 2003, 4, 509–516. [Google Scholar]
- Yang, YC; Kim, K-H; Agrawal, CM; Ong, JL. Interaction of hydroxyapatite-titanium at elevated temperature in vacuum environment. Biomaterials 2004, 25, 2927–2932. [Google Scholar]
- Brossa, F; Cigada, A; Chiesa, R; Paracchini, L; Consonni, C. Adhesion properties of plasma sprayed hydroxylapatite coating for orthopaedic prostheses. J. BioMed. Mater. Eng 1993, 3, 127–136. [Google Scholar]
- Lee, E-J; Lee, S-H; Kim, H-W; Kong, Y-M; Kim, H-E. Fluoridated apatite coatings on titanium obtained by electron-beam deposition. Biomaterials 2005, 26, 3843–3851. [Google Scholar]
- Kim, H-W; Kim, H-E; Knowles, LC. Fluor-hydroxyapatite sol–gel coating on titanium substrate for hard tissue implants. Biomaterials 2004, 25, 3351–3358. [Google Scholar]
- Filiaggi, MJ; Pilliar, RM; Coombs, NA. Post-plasma-spraying heat treatment of the HA coating/Ti-6Al-4V implant system. J. BioMed. Mater. Res 1993, 27, 191–198. [Google Scholar]
- Lynn, AK; DuQuesnay, DL. Hydroxyapatite-coated Ti-6Al-4V Part 2: The effect of post-deposition heat treatment at low temperatures. Biomaterials 2002, 23, 1947–1953. [Google Scholar]
- Yang, YC; Chang, E. Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti-6Al-4V substrate. Biomaterials 2001, 22, 1827–1836. [Google Scholar]
- Mano, T; Ueyama, Y; Ishikawa, K; Matsumura, T; Suzuki, K. Initial tissue response to a titanium implant coated with apatite at room temperature using a blast coating method. Biomaterials 2002, 23, 1931–1936. [Google Scholar]
- Moritz, N; Jokinen, M; Peltola, T; Areva, S; Yli-Urpo, A. Local induction of calcium phosphate formation on TiO2 coatings on titanium via surface treatment with a CO2 laser. J. BioMed. Mater. Res. A 2003, 65, 9–16. [Google Scholar]
- Han, Y; Xu, K. Photoexcited formation of bone apatite-like coatings on micro-arc oxidized titanium. J. BioMed. Mater. Res. A 2004, 71, 608–614. [Google Scholar]
- Kim, H-W; Kim, H-E; Salih, V; Knowles, JC. Sol-gel-modified titanium with hydroxyl-apatite thin films and effect on osteoblast-like cell responses. J. BioMed. Mater. Res. A 2005, 74, 294–305. [Google Scholar]
- Kim, H-W; Koh, Y-H; Li, L-H; Lee, S; Kim, H-E. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol-gel method. Biomaterials 2004, 25, 2533–2538. [Google Scholar]
- Ergun, C; Doremus, R; Lanford, W. Hydroxylapatite and titanium: Interfacial reactions. J. BioMed. Mater. Res. A 2003, 65, 336–343. [Google Scholar]
- Hayashi, K; Mashima, T; Uenoyama, K. The effect of hydroxyapatite coating on bony ingrowth into grooved titanium implants. Biomaterials 1999, 20, 111–119. [Google Scholar]
- Hayashi, K; Inadome, T; Tsumura, H; Nakashima, Y; Sugioka, Y. Effect of surface roughness of hydroxyapatite-coated titanium on the bone-implant interface shear strength. Biomaterials 1994, 15, 1187–1191. [Google Scholar]
- Moroni, A; Caja, VL; Egger, EL; Trinchese, L; Chao, EYS. Histomorphometry of hydroxyapatite coated and uncoated porous titanium bone implant. Biomaterials 1994, 15, 926–930. [Google Scholar]
- Thomas, KA; Cook, SD; Haddad, RJ; Kay, JF; Jarcho, M. Biologicla response to hydroxyapatite-coated titanium hips. J. Arthrop 1989, 4, 43–53. [Google Scholar]
- Jansen, JA; van de Waerden, JPCM; Wolke, JGC; de Groot, K. Histologic evaluation of the osseo adaptation to titanium and hydroxyapatite-coated titanium implants. J. BioMed. Mater. Res 1991, 25, 973–989. [Google Scholar]
- Gottlander, M; Albrektsson, T. Histomorphometric studies of hydroxylapatite-coated and uncoated cp titanium threaded implants in bone. Int. J. Oral Maxillofac. Implants 1991, 6, 399–404. [Google Scholar]
- Schreurs, BW; Huiskes, R; Buma, P; Slooff, TJJH. Biomechanicla and histological evaluation of a hydroxyapatite-coated titanium femoral stem fixed with an intramedullary morsellized bone grafting technique: An animal experiment on goats. Biomaterials 1996, 17, 1177–1186. [Google Scholar]
- Cabrini, M; Cigada, A; Rondelli, G; Vicentini, B. Effect of different surface finishing and of hydroxyapatite coatings on passive and corrosion current of Ti6Al4V alloy in simulated physiological solution. Biomaterials 1997, 18, 783–787. [Google Scholar]
- Cook, SD; Thomas, KA; Kay, J; Jarcho, M. Hydroxyapatite-coated titanium for orthopedic implant applications. Clin Orthop 1988, 186, 225–243. [Google Scholar]
- Yang, YC; Chang, E; Hwang, BH; Lee, SY. Biaxial residual stress states of plasma-sprayed hydroxyapatite coatings on titanium alloy substrate. Biomaterials 2000, 12, 1327–1337. [Google Scholar]
- Mimura, K; Watanabe, K; Okawa, S; Kobayashi, M; Miyakawa, O. Morphological and chemical characterizations of the interface of a hydroxyapatite-coated implant. Dent. Mater. J 2004, 23, 353–360. [Google Scholar]
- Jonášová, L; Müller, FA; Helebrant, A; Strnad, J; Greil, P. Hydroxyapatite formation on alkali-treated titanium with different content of Na+ in the surface layer. Biomaterials 2002, 23, 3095–3101. [Google Scholar]
- Jonášová, L; Müller, FA; Helebrant, A; Strnad, J; Greil, P. Biomimetic apatite formation on chemically treated titanium. Biomaterials 2004, 25, 1187–1194. [Google Scholar]
- Wang, CX; Wang, M; Zhou, X. Nucleation and growth of apatite on chemically treated titanium alloy: An electrochemical impedance spectroscopy study. Biomaterials 2003, 24, 3069–3077. [Google Scholar]
- Lu, X; Leng, Y. TEM study of calcium phosphate precipiotation on bioactive titanium surfaces. Biomaterials 2004, 25, 1779–1786. [Google Scholar]
- Hanawa, T; Ota, M. Calcium phosphate naturally formed on titanium in electrolyte solution. Biomaterials 1991, 12, 767–774. [Google Scholar]
- Barrère, F; van der Valk, CM; Dalmeijer, RAJ; van Blitterswijk, CA; de Groot, K; Layrolle, P. In vitro and in vivo degradation of biomimetic octacalcium phosphate and carbonate apatite coatings on titanium implants. J. BioMed. Mater. Res. A 2003, 64, 378–387. [Google Scholar]
- Barrère, F; Snel, MME; van Blitterswijk, CA; de Groot, K; Layrolle, P. Nano- scale study of the nucleation and growth of calcium phosphate coating on titanium implants. Biomaterials 2004, 25, 2901–2910. [Google Scholar]
- Li, SJ; Yang, R; Niinomi, M; Hao, YL; Cui, YY. Formation and growth ofcalcium phosphate on the surface of oxidized Ti-29Nb-13Ta-4.6Zr alloy. Biomaterials 2004, 25, 2525–2532. [Google Scholar]
- Bogdanski, D; Esenwien, SA; Prymak, O; Epple, M; Muhr, G; Köller, M. Inhibition of PMN apotosis after adherence to dip-coated calcium phosphate surfaces on a NiTi shape memory alloy. Biomaterials 2004, 25, 4627–4623. [Google Scholar]
- Kim, CS; Ducheyne, P. Compositional variations in the suface and interface of calcium phosphate ceramic coatings on Ti and Ti-6Al-4V due to sintering and immersion. Biomaterials 1991, 12, 461–469. [Google Scholar]
- Ducheyne, P; Bianco, PD; Kim, C. Bone tissue growth enhancement of calcium phosphate coatings on porous titanium alloys: Effect of shielding metal dissolution product. Biomaterials 1992, 13, 617–624. [Google Scholar]
- Krupa, D; Baszkiewicz, J; Kozubowski, JA; Barcz, A; Sobczak, JW; Biliński, A; Lewandowska-Szumieł, M; Rajchel, B. Effect of dual ion implantation of calcium and phosphorus on the properties of titanium. Biomaterials 2005, 26, 2847–2856. [Google Scholar]
- Jansen, JA; Hulshoff, JE; van Dijk, K. Biological evaluation of thin calcium-phosphate (Ca-P) coatings. J Dent Res 1997, 76, 282. [Google Scholar]
- Hayakawa, T; Yoshinari, M; Kiba, H; Yamamoto, H; Nemoto, K; Jansen, JA. Trabeular bone response to surface roughened and calcium phosphate (Ca-P) coated titanium implants. Biomaterials 2002, 23, 1025–1031. [Google Scholar]
- Zhang, Q; Leng, Y; Xin, R. A comparative study of electrochemical deposition and biomimetic deposition of calcium phosphate on porous titanium. Biomaterials 2005, 26, 2857–2865. [Google Scholar]
- Heimann, RB; Wirth, R. Formation and transformation of amorphous calcium phosphates on titanium alloy surfaces during atmospheric plasma spraying and their subsequent in vitro performance. Biomaterials 2006, 27, 823–831. [Google Scholar]
- Ban, S; Maruno, S; Iwata, H; Itoh, H. Calcium phosphate precipitation on the surface HA-G-Ti composite under physiologic conditions. J. BioMed. Mater. Res 1994, 28, 65–71. [Google Scholar]
- Lucas, LC; Ong, JL. Post deposition heat treatments of Ca-P coating. J Dent Res 1993, 71, 228. [Google Scholar]
- Gan, L; Wang, J; Pilliar, RM. Evaluating interface strength of calcium phosphate sol-gel-derived thin films to Ti6Al4 V substrate. Biomaterials 2005, 26, 189–196. [Google Scholar]
- Choi, J; Bogdanski, D; Köller, M; Esenwein, SA; Müller, D; Epple, M. Calcium phosphate coating of nickel-titanium shape-memory alloys, coating procedure and adherence of leukocytes and platelets. Biomaterials 2003, 24, 3689–3696. [Google Scholar]
- Fend, B; Weng, J; Yangm, BC; Qu, SX; Zhang, XD. Characterization of titanim surfaces with calcium and phosphate and osteoblast adhesion. Biomaterials 2004, 25, 3421–3428. [Google Scholar]
- Watanabe, K; Hashimoto, A; Nomura, S; Endo, MM; Okawa, S; Kanatani, M; Nakano, S; Kobayashi, M; Miyakawa, O. Surface properties of dental implants (Part 2) Surface analysis of the titanium implant extracted from a rat bone. J. Jpn Dent. Mater 2004, 23, 329–334. [Google Scholar]
- Kim, H-W; Lee, E-J; Jun, I-K; Kim, H-E. On the feasibility of phosphate glass and hydroxyapatite engineered coating on titanium. J. BioMed. Mater. Res. A 2005, 75, 656–667. [Google Scholar]
- Maxian, SH; Zawadsky, JP; Dunn, MG. Mechanical and histological evaluation of amorphous calcium phosphate and poorly crystallized hydroxyapatite coatings on titaniuim implants. J. BioMed. Mater. Res 1993, 27, 717–728. [Google Scholar]
- Khor, KA; Gu, YW; Pan, D; Cheang, P. Microstructure and mechanical properties of plasma sprayed HA/YSZ/Ti–6Al–4V composite coatings. Biomaterials 2004, 25, 4009–4017. [Google Scholar]
- Gu, YW; Khor, KA; Pan, D; Cheang, P. Activity of plasma sprayued yttria stabilized zirconia reinforced hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid. Biomaterials 2004, 25, 3177–3185. [Google Scholar]
- Chou, BY; Chang, E; Yao, SY; Chen, JM. Phase transformation during plasma spraying of hydroxyapatite-19wt%-zirconia composite coating. J Am CerAm Soc 2002, 85, 661–669. [Google Scholar]
- Kasuga, T; Yoshida, M; Ikushima, AJ; Tuchiya, M; Kusakari, H. Stability of zirconia-toughended bioactive glass-ceramics - invivo study using dogs. J Mater Sci: Mater Med 1993, 4, 36–39. [Google Scholar]
- Takagi, M; Mochida, M; Uchida, N; Saito, K; Uematsu, K. Filter cake forming and hot isostatic pressing for TZP-dispressed hydroxyapatite composite. J. Mater Sci.: Mater. Med 1992, 3, 199–203. [Google Scholar]
- Yamada, K; Imamura, K; Itoh, H; Iwata, H; Maruno, S. Bone bonding behavior of the hydroxyapatite containing glass-titanium composite prepared by the Cullet method. Biomaterials 2001, 22, 2207–2214. [Google Scholar]
- Suzuki, R; Muyco, J; McKittrick, J; Frangos, JA. Reactive oxygen species inhibited by titanium oxide coatings. J. BioMed. Mater. Res. A 2003, 66, 396–402. [Google Scholar]
- Li, H; Khor, KA; Cheang, P. Titanium dioxide reinforced bydroxyapatite coatings deposited by high velocity oxy-fuel (HVOF) spray. Biomaterials 2002, 23, 85–91. [Google Scholar]
- Lu, Y-P; Li, M-S; Li, S-T; Wang, Z-G; Zhu, R-F. Plasma-sprayed hydroxyapatite + titania composite bond coat for hydroxyapatite coating on titanium substrate. Biomaterials 2004, 25, 4393–4403. [Google Scholar]
- Ng, BS; Annergren, I; Soutar, AM; Khor, KA; Jarfors, AEW. Characterization of a douplex TiO2/CaP coatings on Ti6Al4V for hard tissue replacement. Biomaterials 2005, 26, 1087–1095. [Google Scholar]
- Knabe, C; Berger, G; Gildenhaar, R; Klar, F; Zreiqat, H. The modulation of osteogenesis in vitro by calcium titanium phosphate coatings. Biomaterials 2004, 25, 4911–4919. [Google Scholar]
- Shtansky, DV; Gloushankova, NA; Sheveiko, AN; Kharitonova, MA; Moizhess, TG; Levashov, EA; Rossi, F. Design, characterization and testing of Ti-based multi-component coatings for load-bearing medical applications. Biomaterials 2005, 26, 2909–2924. [Google Scholar]
- von Walter, M; Rüger, M; Ragoß, C; Steffens, GCM; Hollander, DA; Paar, O; Maier, HR; Jahnen-Dechent, W; Bosserhoff, AK; Erli, H-J. In vitro behavior of a porous TiO2/perlite composite and its surface modification with fibronectin. Biomaterials 2005, 26, 2813–2826. [Google Scholar]
- Rodrigues, CVM; Serricella, P; Linhares, ABR; Guerdes, RM; Borojevic, R; Rossi, MA; Duarte, MEL; Farina, M. Characteriation of a bovine collagen-hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials 2003, 24, 4987–4997. [Google Scholar]
- Cheng, X; Filiaggi, M; Roscoe, SG. Electrochemically assisted co-precipitation of protein with calcium phosphate coatings on titanium alloy. Biomaterials 2004, 25, 5395–5403. [Google Scholar]
- Redepenning, J; Venkataraman, G; Chen, J; Stafford, N. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J. BioMed. Mater. Res 2003, 66A, 411–416. [Google Scholar]
- Tamura, Y; Yokoyama, A; Watari, F; Kawasaki, T. Surface properties and biocompatibility of nitrided titanium for abrasion resistant implant materials. Dent. Mater. J 2002, 21, 355–372. [Google Scholar]
- Kokubo, T; Ito, S; Shigematsu, M; Sakka, S. Fatigue and life-time of bioactive glass-ceramic A-W containing apatite and wollastonite. J. Mater. Sci 1987, 22, 4067–4070. [Google Scholar]
- Cyster, LA; Parkerm, KG; Parker, TL; Grant, DM. The effect of surface chemistry and nanotopography of titanium nitride (TiN) filmson primary hippocampal neurons. Biomaterials 2004, 25, 97–107. [Google Scholar]
- Bull, SJ; Chalker, PR. The role of titanium interlayers in the adhesion of titanium nitride thin films. In Surface Modification Technologies; Sudarsahn, TS, Ed.; Cambridge University Press: Cambrodge, UK, 1992; pp. 205–215. [Google Scholar]
- Venugopalan, R; George, MA; Weimer, JJ; Lucas, LC. Surface topography, corrosion and microhardness of nitrogen-diffusion-hardened titanium alloy. Biomaterials 1999, 20, 1709–1716. [Google Scholar]
- Dion, I; Rouais, F; Trut, L; Baquency, CH; Monties, JR; Havlik, P. TiN coating: Surface characterization and haemocompatibility. Biomaterials 1993, 14, 1230–1235. [Google Scholar]
- Oshida, Y; Hashem, A. Titanium-procelain system. Part I: Oxidation kinetics of nitrided pure titanium, simulated to porcelain firing process. J. BioMed. Mater. Eng 1993, 3, 185–198. [Google Scholar]
- Bordji, K; Jouzeau, JM; Mainaird, D; Payan, E; Netter, P; Rie, KT; Stucky, T; Hage-Ali, M. Cytocompatibility of Ti-6Al-4V and Ti-5Al-2.5Fe alloys according to three surface treatments, using human fibroblasts and osteoblasts. Biomaterials 1996, 17, 929–940. [Google Scholar]
- Goldberg, JR; Gilbert, JL. The electrochemical and mechanical behavior of passivated and TiN/AlN-coated CoCrMo and Ti6Al4V alloys. Biomaterials 2004, 25, 851–864. [Google Scholar]
- Morton, PH; Bell, T; Weisheit, A; Kroll, J; Mordike, BL; Sagoo, K. Laser gas nitriding of titanium and titanium alloys. In Surface Modification Technologies V; Sudarshan, TS, Braza, JF, Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 593–609. [Google Scholar]
- Eberhardt, AW; Pandey, R; Williams, JM; Weimer, JJ; Ila, D; Zimmerrmann, RL. The role of residual stress and surface topography on hardness of ion implanted Ti-6Al-4V. Mater. Sci. Eng 1997, A229, 147–155. [Google Scholar]
- Shetty, RH. Mechanicl and corrosion properties of nitrogen diffusion hardened Ti-6Al-4V alloy. In Medical Applications of Titanium and Its Alloys: The Material and Biological Issues; Brown, SA, Lemons, JE, Eds.; ASTM STP 1272 American Society for Testing and Materials: West Conshohocken, PA, USA, 1996; pp. 240–251. [Google Scholar]
- Semlitsch, MF; Weber, H; Streicher, RM; Schon, R. Joint replacement components made of hot-forged ad surface-treated Ti-6Al-7Nb alloy. Biomaterials 1992, 13, 781–788. [Google Scholar]
- Thull, R. Semiconductive properties of passived titanium and titanium based hard coatings on metals for implants – an experimental approach. Med. Progr Technol 1990, 16, 225–234. [Google Scholar]
- Wisbey, A; Gregson, PJ; Tuke, M. Application of PVD TiN coating to Co-Cr-Mo-based surgical implants. Biomaterials 1987, 8, 477–480. [Google Scholar]
- Venugopalan, R; Weimer, JJ; George, MA; Lucas, LC. The effect of nitrogen diffusion hardening on the surface chemistry and scratch resistance of Ti-6Al-4V alloy. Biomaterials 2000, 21, 1669–1677. [Google Scholar]
- Oshida, Y; Fung, LW; Ishikbay, SC. Titanium-porcelain system. Part II: Bond strength of fired porcelain on nitrided pure titanium. J. BioMed. Mater. Eng 1997, 7, 13–34. [Google Scholar]
- Głuszek, J; Jedrokowiak, J; Markowski, J; Masalski, J. Galvanic couples of 316L steel with Ti and ion plated Ti and TiN coatings in Ringer’s solutions. Biomaterials 1990, 11, 330–335. [Google Scholar]
- Lee, B-H; Kim, JK; Kim, YD; Choi, K; Lee, KH. In vivo behavior and mechanical stability of surface-modified titanium implants by plasma spray coating and chemical treatments. J. BioMed. Mater. Res. A 2004, 69, 279–285. [Google Scholar]
- Sonoda, T; Kotake, S; Kakimi, H; Yamada, M; Naganuma, K; Kato, M; Saka, T; Shimizu, T; Katoh, K. On the dental application of titanium-base alloy. Part 7: Titanium coating by sputtering. News Govon. Ind. Res. Inst. (Osaka) 1991, 40, 291–299. [Google Scholar]
- Sonoda, T; Kotake, S; Kakimi, H; Yamada, M; Naganuma, M; Kato, M; Saka, T; Shimizu, T; Katoh, K. On the dental application of titanium-base alloy. Part 8: Pure titanium coating on the denture base of Ti-6Al-4V alloy by sputtering. Gov. Indust. Res. Inst 1991, 40, 300–307. [Google Scholar]
- Breme, J; Steinhäuser, E; Paulus, G. Commercially pure titanium Steinäuser plate-screw system for maxillofacial surgery. Biomaterials 1988, 9, 310–313. [Google Scholar]
- Rae, T. A study on the effects of particulate metals of orthopaedic interest on murine macrophages in vitro. J. Bone Jt Surg. Br 1975, 57, 444–450. [Google Scholar]
- Rae, T. The toxicity of metals used in orthopaedic prostheses. An experimental study using cultured human synovial fibroblasts. J Bone Jt Surg Br 1981, 63-B, 435–440. [Google Scholar]
- Brune, D; Evje, D; Melson, S. Corrosion of gold alloys and titanium in artificial saliva. Scand. J. Dent. Res 1982, 90, 168–171. [Google Scholar]
- Franchi, M; Bacchelli, B; Martini, D; DePasquale, V; Orsini, E; Ottani, V; Fini, M; Giavaresi, G; Giardino, R; Ruggeri, A. Early detachment of titanium particles from various different surfaces of endosseous dental implants. Biomaterials 2004, 25, 2239–2246. [Google Scholar]
- Smith, TS. Rationale for biological fixation of prosthetic devices. SAMPE J 1985, 21, 14–16. [Google Scholar]
- Engh, CA; Bobyn, JD. Biologic fixation of hip prosthesis: A review of the clinical status and current concepts. Adv. Orthop. Surg 1984, 18, 136–149. [Google Scholar]
- Smith, T. The effect of plasma-sprayed coatings on the fatigue of titanium alloy implants. J. Metal 1994, 46, 54–56. [Google Scholar]
- Head, WC; Mallory, TH; Emerson, RH. The promixal porous coating alternative for primary total hip arthroplasty. Orthopedics 1999, 22, 813–815. [Google Scholar]
- Bourne, RB; Rorabeck, CH; Burkart, BC; Kirk, PG. Ingrowth surfaces-plasma sprayed coating to titanium alloy hip replacements. Clin Orthop Relat. Res 1994, 298, 37–46. [Google Scholar]
- Reclaru, L; Lerf, R; Eschler, P-Y; Blatter, A; Meyer, J-M. Evaluation of corrosion on plasma sprayed and anodized titanium implants, both with and without bone cement. Biomaterials 2003, 24, 3027–3038. [Google Scholar]
- Xue, W; Liu, X; Zheng, XB; Ding, C. In vivo evaluation of plasma-sprayed titanium coating after alkali modification. Biomaterials 2005, 26, 3029–3037. [Google Scholar]
- Borsari, V; Giavaresi, G; Fini, M; Torricelli, P; Tschon, M; Chiesa, R; Chiusoli, L; Salito, A; Volpert, A; Giardino, R. Comparative in vitro study on a ultra-high roughness and dense titanium coating. Biomaterials 2005, 26, 4948–4955. [Google Scholar]
- Vercaigne, S; Wolke, JBC; Naert, I; Jansen, JA. The effect of titanium plasma-sprayed implants on trabecular bone healing in the goat. Biomaterials 1998, 19, 1093–1099. [Google Scholar]
- Ong, JL; Carnes, DL; Bessho, K. Evaluation of titanium plasma-sprayed and plasma-sprayed hydroxyapatite implants in vivo. Biomaterials 2004, 25, 4601–4606. [Google Scholar]
- Pan, J; Leygraf, C; Thierry, D; Ektessabi, AM. Corrosion resistance for biomaterial applications of TiO2 films deposited on titanium and stainless steel by ion-beam-assisted sputtering. J. BioMed. Mater. Res 1997, 35, 309–318. [Google Scholar]
- New Technology Japan. Ti-O oating in Ti-6Al-4V alloy by DC reactive sputtering method. JETR 1994, 22, 18. [Google Scholar]
- Głuszek, J; Masalski, J; Furman, P; Nitsch, K. Structural and electrochemical examinations of PACVD TiO2 films in Ringer solution. Biomaterials 1997, 18, 789–794. [Google Scholar]
- De Aza, PN; Guitian, F; De Aza, S. Reactivity of wollastonite-tricalcium phosphate bioeutectic ceramic in human parotid saliva. Biomaterials 2000, 21, 1735–1741. [Google Scholar]
- Siriphannon, P; Hayashi, S; Yasumori, A; Okada, K. Preparation and sintering of CaSiO3 from coprecipitated powder using NaOH as precipitant and its apatite formation in simulated body fluid solution. J. Mater. Res 1999, 4, 529–536. [Google Scholar]
- Lui, X; Ding, C. Plasma sprayed wollastonite/TiO2 composite coatings on titanium alloys. Biomaterials 2002, 23, 4065–4077. [Google Scholar]
- Haddow, DB; Kothari, S; James, PF; Short, RD; Hatton, PV; van Noort, R. Synthetic implant surfaces 1. The formation and characterization of sol-gel titania films. Biomaterials 1996, 17, 501–507. [Google Scholar]
- Sato, M; Slamovich, EB; Webster, TJ. Enhanced osteoblast adhesion on hydrothermally treated hydroxyapatite/titania/poly(lactide-co-glycolide) sol-gel titanium coatings. Biomaterials 2005, 26, 1349–1347. [Google Scholar]
- Wang, X-X; Hayakawa, S; Tsuru, K; Osaka, A. Bioactive titania gel layers formed by chemical treatment of Ti substrate with a H2O2/HCl solution. Biomaterials 2002, 23, 1353–1357. [Google Scholar]
- Manjubala, I; Kumar, TSS. Effect of TiO2-Ag2O additives on the formation of calcium phosphate based functionally graded bioceramics. Biomaterials 2000, 21, 1995–2002. [Google Scholar]
- Wheeler, KR; Karagianes, MT; Sump, KR. Porous titanium alloy for prosthesis attachment. In Titanium Alloys in Surgical Implants; Luckey, HA, Kubli, F, Eds.; ASTM STP 796, ASTM Internationla: West Conshohoden, PA, USA, 1983; pp. 241–54. [Google Scholar]
- Engelhard, G; Zaharias, R; Keller, JC. Effects of CP Ti surface roughness on osteoblast mineralization. J Dent Res 1995, 74, 189. [Google Scholar]
- Chung, FH; McAlarney, ME. Effects of variations in surface treatment on titanium topography. J Dent Res 1995, 74, 112. [Google Scholar]
- Petronis, S; Gretzer, C; Kasemo, B; Gold, J. Model porous surfaces for systematic studies of material-cell interactions. J. BioMed. Mater. Res. A 2003, 66, 707–721. [Google Scholar]
- Xiaoxiong, M; Shizhong, H. The states of bromide on titanium surface prior to pit initiation. J. Chin. Soc. Corr. Protec 1989, 9, 29–35. [Google Scholar]
- Ungersboeck, A; Pohler, OEM; Perren, SM. Evaluation of soft tissue reactions at the interface of titanium limited contact dynamic compression plate implants with different surface treatments: An experimental sheep study. Biomaterials 1996, 17, 797–806. [Google Scholar]
- Larsson, C; Thomsen, P; Aronsson, BO; Rodahl, M; Lausmaa, J; Kasemo, B; Ericson, LE. Bone response to surface-modified titanium implants: Studies on the early tissue response to machined and electropolished implants with different oxide thickness. Biomaterials 1996, 17, 605–616. [Google Scholar]
- Thelen, S; Barthelat, F; Brinson, LC. Mechanics considerations for microporous titanium as an orthopedic implant material. J. BioMed. Mater. Res. A 2004, 69, 601–610. [Google Scholar]
- Oshida, Y. Requirements for successful, biofunctional implants. In International Advanced Biomaterials; Yahia, LH, Ed.; Montréal, Canada, 2000; p. 5. [Google Scholar]
- Takemoto, M; Fujibayashi, S; Neo, M; Suzuki, J; Kokubo, T; Nakamura, T. Mechanical properties and osteoconductivity of porous bioactive titanium. Biomaterials 2005, 26, 6014–6023. [Google Scholar]
- Duocel Foam Metal: A New Basic Design Material; Energy Research & Generation, Inc.: Oakland, CA, USA, 1998.
- Adell, R; Lekholm, U; Rockler, B; Brånemark, P-I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg 1981, 10, 387–416. [Google Scholar]
- Adell, R. Clinical results of osseointegrated implants supporting fixed prostheses in edentulous jaws. J. Prosthet. Dent 1983, 50, 251–254. [Google Scholar]
- Björk, A; Skieller, V. Growth of the maxilla in three dimensions as revealed radiographically by the implant method. Br. J. Orthod 1977, 4, 53–64. [Google Scholar]
- Björk, A. Variations of the growth pattern of the human mandible: A longitudinal radiographic study by the implant method. J. Dent. Res 1963, 42, 400–411. [Google Scholar]
- Skieller, V; Björk, A; Linde-Hansen, T. Prediction of mandibular growth rotation evaluated from a longitudinal implant sample. Am. J. Orthod 1984, 86, 359–370. [Google Scholar]
- Enlow, DH. Facial Growth, 3rd ed; Saunders Publishers: Philedelphia, PA, USA, 1990; pp. 282–284. [Google Scholar]
- Kramer, F; Baethge, C; Tschernitschek, H. Implants in children ith ectodermal dysplasia: A case report and literature review. Clin Oral Impl. Res 2007, 18, 140–146. [Google Scholar]
- Oesterle, LJ. Implant considerations in the growing child. In Orthodontic Application sof Osseointegrated Implants; Huguchi, KW, Ed.; Quintessence Publishing Co.: Chicago, IL, USA, 2000; pp. 133–159. [Google Scholar]
- Bergendal, B; Bergendal, T; Hallonsten, A-L; Koch, G; Kurol, J; Kvint, S. A multidisciplinary approach to oral rehabilitation with osseointegrated implants in children and adolescents with multiple aplasia. Eur. J. Orthod 1996, 18, 119–129. [Google Scholar]
- Dietschi, D; Schatz, JP. Current restorative modalities for young patients with missing anterior teeth. Quintessence Int 1997, 28, 231–240. [Google Scholar]
- Thilander, B; Odman, J; Gröndahl, K; Friberg, B. Osseointagrated implants in adolescents. An alterative in replacing missing teeth? Eur. J. Orthod 1994, 16, 84–95. [Google Scholar]
- Iseri, H; Solow, B. Continued eruption of maxillary incisors and first molars in girls from 9 to 25 years, studied by the implant method. Eur. J. Orthod 1996, 18, 245–256. [Google Scholar]
- Perrott, DH. Restoration and maintenance of maxillary position. Atlas Oral Maxillofac. Surg Clin North Am 1993, 1, 31–55. [Google Scholar]
- Guckes, AD; Brahim, JS; McCarthy, GR; Rudy, SF; Cooper, LF. Using endosseous implants for patients with ectodermal dysplasia. J. Am. Dent. Assoc 1991, 122, 59–62. [Google Scholar]
- Cronin, R; Osterle, L; Ranly, D. Mandibular implants and the growing patient. Int. J. Oral Maxillofac. Implants 1994, 9, 55–62. [Google Scholar]
- Oesterle, LJ; Cronin, RJ, Jr; Ranly, DM. Maxillary implants and the growing patient. Int. J. Oral Maxillofac. Implants 1993, 8, 377–387. [Google Scholar]
- Odman, J; Gröndahl, K; Lekholm, U; Thilander, B. The effect of osseointegrated implants on the dento-alveolar development. A clinical and radiographic study in growing pigs. Eur. J. Orthod 1991, 13, 279–286. [Google Scholar]
- Thilander, B; Odman, J; Gröndahl, K; Lekholm, U. Aspects on osseointegrated implants on the dento-alveolar development. A clinical and radiographic study in growing opigs. Eur. J. Orthod 1992, 14, 99–109. [Google Scholar]
- Brugnolo, E; Mazzocco, C; Cordioll, G; Majzoub, Z. Clinical and radiographic findings following placement of single-tooth implants in young patinets – case reports. Int. J. Periodontics Restorative Dent 1996, 16, 421–433. [Google Scholar]
- Westwood, RM; Duncan, JM. Implants in adolescents: A literature review and case reports. Int. J. Oral Maxillofac. Implants 1996, 11, 750–755. [Google Scholar]
- Cronin, RJ; Oesterle, LJ. Implant use in growing patients. Treatment planning concerns. Dent. Clin North Am 1998, 42, 1–34. [Google Scholar]
- Sennerby, L; Odman, J; Lekholm, U; Thilander, B. Tissue reactions towards titanium implants inserted in growing jaws. A histological study in the pig. Clin Oral Implants Res 1993, 4, 65–75. [Google Scholar]
- Kawanami, M; Andreasen, JO; Borum, MK; Schou, S; Hjørting-Hansen, E; Kato, H. Infraposition of ankylosed permanent maxillary incisors after replantation related to age and sex. Endod Dent. Traumatol 1999, 15, 50–56. [Google Scholar]
- Björk, A. Prediction of mandibular growth rotation. Am. J. Orthod 1969, 55, 585–599. [Google Scholar]
- Oesterle, LJ; Cronin, RJ. Adult growth, aging, and the single-tooth implant. Int. J. Oral Maxillofac. Implants 2000, 15, 252–260. [Google Scholar]
- Kearns, G; Sharma, A; Perrott, D; Schmidt, B; Kaban, L; Vargervik, K. Placement of endosseous implants in children and adolescents with hereditary ectodermal dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 1999, 88, 5–10. [Google Scholar]
- Bergendal, B; Eckerdal, O; Hallonsten, A-L; Koch, G; Kurol, J; Kvint, S. Osseointegrated implants in the oral rehabilitation of a boy with ectodermal dysplasia: A case report. Int. Dent. J 1991, 41, 149–156. [Google Scholar]
- Bergendal, B. Prosthetic rehabilitation of a young patient with hypodidrotic ectodermal dysplasia and oligodontia: A case report of 20-year of treatment. Int. J. Prosthodont 2001, 14, 471–479. [Google Scholar]
- Kargul, B; Alcan, T; Kabalay, U; Atasu, M. Hypohidrotic ectodermal dysplasia: Dental, clinical, genetic and dermatoglyphic findings of three cases. J. Clin Pediatr. Dent 2001, 26, 5–12. [Google Scholar]
- Hickey, AJ; Vergo, TJ, Jr. Prosthetic treatments for patients with ectodermal dysplasia. J. Prosthet. Dent 2001, 86, 364–368. [Google Scholar]
- Behnoush, RB. Prosthodontic treatment with implant fixed prosthesis for a patient with ectodermal dysplasia: A clinical report. J. Prosthodont 2003, 12, 198–201. [Google Scholar]
- Kraut, RA. Dental implants for children: Creating smiles for children without teeth. Pract Periodontics Aesthet. Dent 1996, 8, 909–913. [Google Scholar]
- Dhanrajani, PJ; Jiffry, AO. Management of ectodermal dysplasia: A literatute review. Dent. Update 1998, 25, 73–75. [Google Scholar]
- Nunn, JH; Carter, NE; Gillgrass, TJ; Hobson, RS; Jepson, NJ; Meechan, JG; Nohl, FS. The interdisciplinary management of hypodontia: Background and role of paediatric dentistry. Br. Dent. J 2002, 194, 245–251. [Google Scholar]
- Akça, K; Iplikcioglu, H. One-year follow-up of an implant with early radiographic signs of loss of osseointegration: Case report. Clin Impl. Dent. Relat. Res 2002, 4, 43–46. [Google Scholar]
- Högberg, G; Lagerheim, B; Sennerstam, R. The 9-year crisis reflected at a rehabilitation center, at a child health care center and at a child and adolescent psychiatric center. Lakartidningen 1986, 83, 2038–2042. [Google Scholar]
- Nussbaum, B; Carrel, R. The behavior modification of a dentally disabled child. ASDC J. Dent. Child 1976, 43, 255–261. [Google Scholar]
- Guckes, AD; Roberts, MW; McCarthy, GR. Pattern of permanent teeth present in individuals with ectodermal dysplasia and severe hypodontia suggests treatment with dental implants. Pediatr. Dent 1998, 20, 278–280. [Google Scholar]
- Lederman, D. Oral medicine: It’s basic dentistry. NY State Dent. J 1993, 59, 35–37. [Google Scholar]
- Smith, RA; Vargervik, K; Kearns, G; Bosch, C; Koumjian, J. Placement of an endoesseous implant in a growing child with ectodermal dysplasia. Oral Surg. Oral Med. Oral Pathol 1993, 75, 669–673. [Google Scholar]
- Oesterle, LJ; Wood, LW. Raising the root: A look at orthodontic extrusion. J. Am. Dent. Assoc 1991, 122, 193–198. [Google Scholar]
- Mackie, IC; Quayle, AA. Alternative management of a crown root fractured tooth in a child. Br. Dent. J 1992, 173, 60–62. [Google Scholar]
- Mehrali, MC; Baraoidan, M; Cranin, AN. Use of endosseous implants in treatment of adolescent trauma patients. NY State Dent. J 1994, 60, 25–29. [Google Scholar]
- Guckes, AD; McCarthy, GR; Brahim, J. Use of endosseous implants in a 3-year old child with ectodermal dysplasia: Case report and 5 year follow-up. Pediatr Dent 1997, 19, 282–285. [Google Scholar]
- Ekstrand, K; Thomsson, M. Ectodermal dysplasia with partial anodontia: Prosthetic treatment with imaplnt fixed prosthesis. ASDC J. Dent. Child 1988, 55, 282–284. [Google Scholar]
- Escobar, V; Epker, BN. Alveolar bone growth in response to endosteal implants in two patients with ectodermal dysplasia. Int. J. Oral Maxillofac. Surg 1998, 27, 445–447. [Google Scholar]
- Alcan, T; Basa, S; Kargül, B. Grwoth analysis of a patient with ectodermal dysplasia treated with endosseous implants: 6-year follow-up. J. Oral Rehabil 2006, 33, 175–182. [Google Scholar]
- Balshi, TJ; Wolfinger, GJ. Treatment of congenital ectodermal dysplasia with zygomatic implants: A case report. Int. J. Oral Maxillofac. Implants 2002, 17, 277–281. [Google Scholar]
- Becktor, KB; Becktor, JP; Keller, EE. Growth analysis of a patient with ectodermal dysplasia treated with edosseous implants: A case report. Int. J. Oral Maxillofac. Implants 2001, 16, 864–874. [Google Scholar]
- Rad, AS; Siadat, H; Monzavi, A; Mangoli, AA. Full mouth rehabilitation of a hypohidrotic ectodermal dysplasia patient with dental implants: A clinical report. J. Prosthodont 2007, 16, 209–213. [Google Scholar]
- Isidof, F. Loss of osseointegration casued by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin Oral Implants Res 1996, 7, 143–152. [Google Scholar]
- Miyata, T; Kobayashi, Y; Araki, H; Motomura, Y; Shin, K. The influence of controlled occlucsal overload on peri-implant tissue: A histologoic study in monkeys. Int. J. Oral Maxillofac. Implants 1998, 13, 667–683. [Google Scholar]
- Quirynen, M; Naert, L; van Steenberghe, D. Fixture design and overload influence marginal bone loss and fixture success in the Brånemark system. Clin Oral Implants Res 1992, 3, 104–111. [Google Scholar]
- Celleti, R; Pamaeijer, CH; Brachetti, G; Donath, K; Persichetti, Z; Visani, I. Histologic evaluation of osseointagrated implants restored in nonaxial functional occlusion with pre-angled abutments. Int. J. Periodontics Restorative Dent 1995, 15, 563–573. [Google Scholar]
- Tonetti, M; Schmid, J. Pathogenesis of implant failures. Periodontology 1994, 4, 127–138. [Google Scholar]
- Loe, H; Theilade, E; Jensen, SB. Experiemntal gingivitis in man. J. Periodontol 1965, 36, 177–187. [Google Scholar]
- Quirynen, M; Listgarten, MA. The distribution of bacterial morphotypes around natural teeth and titanium implants ad modum Branemark. Clin Oral Implants Res 1990, 1, 8–12. [Google Scholar]
- Berglundh, T; Lindhe, J; Marinello, C; Ericsson, I; Liljenberg, B. Soft tissue reactions to de novo plaque formation at implants and teeth. Clin Oral Implants Res 1992, 3, 1–8. [Google Scholar]
- Bragger, U. Use of radiographs in evaluating success, stability and failure in implant dentistry. Periodontology 1998, 17, 77–88. [Google Scholar]
- Hammerie, CHF; Fourmousis, I; Winkler, JR; Weigel, C; Bragger, U; Lang, NP. Successful bone fill in late peri-implant defects using guided tissue regeneration. A short communication. J. Periodontol 1995, 66, 303–308. [Google Scholar]
- Jowanovic, SA. The management of peri-implant breakdown around functioning osseointegarted dental implants. J. Periodontol 1993, 64, 1176–1183. [Google Scholar]
- Persson, LG; Berglundh, T; Sennerby, L; Lindhe, J. Reoeesointagratio after treatment of peri-implantitis at different implant surfaces. An experimental study in dog. Clin Oral Implants Res 2001, 12, 595–603. [Google Scholar]
- Persson, LG; Ericsson, I; Berglundh, T; Lindhe, J. Guided bone regeneration in the treatment of peri-implantitis. Clin Oral Implants Res 1996, 7, 366–372. [Google Scholar]
- Bergendal, B; Ekman, A; Nilsson, P. Implant failure in young children with ectodermal dysplasia: A retrospective evaluation of use and outcome of dental implants treatment in children in Sweden. Int. J. Oral Maxillofac. Implants 2008, 23, 520–524. [Google Scholar]
- Rossi, E; Andreasen, JO. Maxillary bone growth and implant positioning in a young patient. A case report. Int. J. Periodontics Restorative Dent 2003, 23, 113–119. [Google Scholar]
- Rockman, RA; Hall, KB; Fiebiger, M. Magnetic retention of dental prostheses in a child with ectodermal dysplasia. J. Am. Dent. Assoc 2007, 138, 610–615. [Google Scholar]
- Dalkiz, M; Beydemirm, B. Pedodontic complete dentures. Turk. J. Med. Sci 2002, 32, 277–281. [Google Scholar]
- Hench, LL. Biomaterials: A forecast for the future. Biomaterials 1998, 19, 1419–1423. [Google Scholar]
- Froes, FH. The better characterization of titanium alloys. J. Metal 2005, 57, 41. [Google Scholar]
- Hartman, AD; Gerdeman, SJ; Hansen, JS. Producing low-cost titanium for automotive applications. J. Metal 1998, 50, 16–19. [Google Scholar]
- van Vuuren, DS; Engelbrecht, AD; Hadley, TD. Opportunities in the electro-winning of molten titanium from titanium dioxide. J. Metal 2005, 57, 53–55. [Google Scholar]
- Fuwa, A; Takaya, S. Producing titanium by reducing TiCl4-MgCl2 mixed salt with magnesium in the molten state. J. Metal 2005, 57, 56–60. [Google Scholar]
- Froes, FH; Mashl, SJ; Moxson, VS; Hebeisen, JC; Duz, VA. The tech-nologies of titanium powder metallurgy. J. Metal 2004, 56, 46–48. [Google Scholar]
- Lopez, MF; Gutierrez, A; Jimémez, JA. In vitro corrosion behavior of titanium alloys without vanadium. Electrochimia Acta 2002, 47, 1359–1364. [Google Scholar]
- Khan, MA; Williams, RL; Williams, DF. The corrosion behavior of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar]
- Katakura, N; Takada, Y; Iijima, K; Hosotani, M; Honma, H. Studies in the shape memory alloys for biomaterials (Part II) - Electrochemical behavior of Ti, Pd, Co and TiPd alloys. J. Jpn. Dent. Mater 1991, 10, 809–813. [Google Scholar]
- Sohmura, T; Kimura, HT. Shape recovery in Ti-V-Fe-Al alloy and its application to dental implant. Proceedings of the International Conference on Martensitic Transformation ICOMAT-86, Nara, Japan, 25–30 August, 1986; pp. 1065–1070.
- Takemoto, S; Hattori, M; Yoshinari, M; Kawada, E; Asami, K; Oda, Y. Corrosion behavior and surface characterization of Ti-20Cr alloy in a solution containing fluoride. Dent. Mater. J 2004, 23, 379–386. [Google Scholar]
- He, G; Eckert, J; Dai, QL; Sui, ML; Löser, W; Hagiwara, M; Ma, E. Nanostructured Ti-based multi-component alloys with potential for biomedical applications. Biomaterials 2003, 24, 5115–5120. [Google Scholar]
- Hattori, M; Takemoto, M; Yoshinari, M; Kawada, E; Oda, U. Alloying effect of Pd to Ti-Cu alloy. Proc 19th Meeting, Soc Titanium Alloys in Dentistry, Tokyo, Japan, June, 2005; p. 45.
- Koike, M; Okabe, T. Mechanical properties of Ti-Cu-Si alloys. Proc 19th Meeting, Soc Titanium Alloys in Dentistry, Tokyo, Japan, June, 2005; p. 44.
- Takahashi, S; Kikuchi, S; Okuno, O. Machinabilty of Ti-Zr alloy. Proc 19th Meeting, Soc Titanium Alloys in Dentistry, Tokyo, Japan, June, 2005; p. 58.
- Kikuchi, S; Takahashi, M; Satoh, H; Komatsu, S; Okabe, T; Okuno, O. Machinability of Ti-Hf alloy. Proc 19th Meeting, Soc Titanium Alloys in Dentistry, Tokyo, Japan, June, 2005; p. 46.
- Satoh, H; Kikuchi, S; Komatsu, S; Okuno, O; Okabe, T. Mechanical properties of Ti-Hf casts. Proc 19th Meeting, Soc Titanium Alloys in Dentistry, Tokyo, June, 2005; p. 57.
- Masumoto, T. Amorphous Ti-Cu system alloys. Japan Patent Application Laid-Open No7-54086 1995. [Google Scholar]
- Tregilgas, J. Amorphous hinge material. Adv. Mater. Proc 2005, 163, 46–49. [Google Scholar]
- Inoue, A. Synthesis and properties of Ti-based bulk amorphous alloys with a large supercooled liquid region. Mater Sci Forum 1999, 313/314, 307–314. [Google Scholar]
- Louzguine, DV; Inoue, A. Multicomponent metastable phase formed by crystallization of Ti-Ni-Cu-Sn-Zr amorphous alloy. J. Mater. Res 1999, 14, 4426–4430. [Google Scholar]
- Zhang, T; Inoue, K. Preparation of Ti-Cu-Ni-Si-B amorphous alloy with a large supercooled liquid region. Mater. Trans. JIM 1999, 40, 301–306. [Google Scholar]
- JETRO, Ti-O coating on Ti-6Al-4V alloy by DC reactive sputtering method, New Technology Japan. No. 94-06-001-01. 1994:22:18, 1994.
- Bogdanski, D; Köller, M; Müller, D; Muhr, G; Bram, M; Buchkremer, HP; Stöver, D; Choi, J; Epple, M. Easy assessment of the biocompatibility of Ni-Ti alloy by in vitro cell culture experiments on a functionally graded Ni-NiTi-Ti material. Biomaterials 2002, 23, 4549–4555. [Google Scholar]
- Kasuga, T; Mizuno, T; Watanabe, M; Nogami, M; Niinomi, M. Calcium Phosphate invert glass-ceramic coating joined by self-development of compositionally gradient layers on a titanium alloy. Biomaterials 2001, 22, 577–582. [Google Scholar]
- Okido, M; Kuroda, K; Ishikawa, M; Ichino, R; Takai, O. Hydroxyapatite coating on titanium solutions. Solid State Ionics 2002, 151, 47–52. [Google Scholar]
- van Dijk, K; Gupta, V; Yu, AK; Jansen, JA. Measurement and control of interface strength of RF mahnetron-sputtered Ca-PO coatings on Ti-6Al-4V substrates using a laser spallation technique. J. BioMed. Mater. Res 1998, 41, 624–632. [Google Scholar]
- Takamura, R. The bone response of titanium implant with calcium ion implantation. J Dent Res 1997, 76, 1177, (Abstract No. 1123). [Google Scholar]
- Ohtsu, N. Evaluation of degradability of CaTiO3 thin films in simulated body fluids. Mater Trans 2004, 45, 1778–1781. [Google Scholar]
- Dunn, DS; Raghavan, S; Volz, RG. Gentamicin sulfate attachment and release from anodized Ti-6Al-4V orthopedic materials. J. BioMed. Mater. Res 1993, 27, 895–900. [Google Scholar]
- Hill, J; Klenerman, L; Trustey, S; Blowers, B. Diffusion of antibiotics from acrylic bone-cement in vitro. J. Bone JoInt. Surg 1977, 59B, 197–199. [Google Scholar]
- Petty, W; Spanier, S; Shuster, JJ. Prevention of infection after total joint replacement. J. Bone JoInt. Surg 1988, 70A, 536–539. [Google Scholar]
- Dunn, DS; Raghavan, S; Volz, RG. Anodized layers on titanium and titanium alloy orthopedic materials for antimicrobial activity applications. Mater. Manuf. Proc 1992, 7, 123–137. [Google Scholar]
- Kato, M; Naganuma, K; Yamada, M; Sonoda, T; Kakimi, H; Kotake, S; Kawai, T. On the dental application of titanium-base alloy, Part 5: Anodic oxidation aiming at the clinical use of the material as a carrier for bone morphogenetic protein (BMP). Gov. Ind. Res. Inst 1991, 40, 269–280. [Google Scholar]
- Kato, M; Naganuma, K; Yamada, M; Sonoda, T; Kotake, S; Kakimi, H. On the dental application of titanium-base alloy, Part 6: Effect of the composition of electrolytic solutions in anodizing the material. Gov. Ind. Res. Inst 1991, 40, 282–290. [Google Scholar]
- Wassell, DTH; Embery, G. Adsorption of bovine serum albumin on to titanium powder. Biomaterials 1996, 17, 859–864. [Google Scholar]
- McAlarney, ME; Oshiro, MA. Possible role of C3 in competitive protein adsorption onto TiO2. J Dent Res 1994, 73, 401. [Google Scholar]
- Klinger, A; Steinberg, D; Kohavi, D; Sela, MN. Adsorption of human salivary albumin to titanium-oxide. J Dent Res 1996, 75, 273. [Google Scholar]
- Hayakawa, T; Yoshinari, M; Nemoto, K. Direct attachment of fibronectin to tresyl chloride-activated titanium. J. BioMed. Mater. Res. A 2003, 67, 684–688. [Google Scholar]
- Bierbaum, S; Beutner, R; Hanke, T; Scharnweber, D; Hempel, U; Worch, H. Modification of Ti6Al4V surfaces using collagen I, III, and fibronectin. I. Biochemical and morphological characteristics of the adsorbed matrix. J. BioMed. Mater. Res. A 2003, 67, 421–430. [Google Scholar]
- Bierbaum, S; Hempel, U; Geißler, U; Hanke, T; Scharnweber, D; Wenzel, K-W; Worch, H. Modification of Ti6AL4V surfaces using collagen I, III, and fibronectin. II. Influence on osteoblast responses. J. BioMed. Mater. Res. A 2003, 67, 431–438. [Google Scholar]
- Kim, H-W; Li, L-H; Lee, E-J; Lee, S-H; Kim, H-E. Fibrillar assembly and stability of collagen coating on titanium for improved osteoblast responses. J. BioMed. Mater. Res. A 2005, 75, 629–638. [Google Scholar]
- Wang, X-X; Xie, L; Wang, R. Biological fabrication of nacreous coating on titanium dental implant. Biomaterials 2005, 26, 6229–6232. [Google Scholar]
- Frosch, K-H; Drengk, A; Krause, P; Viereck, V; Miosge, N; Werner, C; Schild, D; Stürmer, EK; Stürmer, KM. Stem cell-coated titanium implants for the partial joint resurfacing of the knee. Biomaterials 2006, 27, 2542–2549. [Google Scholar]
- Bigi, A; Boanini, E; Bracci, B; Facchini, A; Panzavolta, S; Segatti, F; Sturba, L. Nanocrystalline hydroxyapatite coatings on titanium: A new fast biomimetic method. Biomaterials 2005, 26, 4085–4089. [Google Scholar]
- Ellingsen, JE; Birkeland, G. The effect on bone healing of fluoride conditioned titanium implants. J Dent Res 1996, 75, 400. [Google Scholar]
- Ellingsen, JE. Pre-treatment of titanium implants with fluoride improves their retention in bone. J. Mater. Sci.: Mater. Med 1995, 6, 749–758. [Google Scholar]
- Ellingsen, JE. Increasing biocompatibility by chemical modification of titanium surfaces. In Bioimplant interface; Ellingsen, JE, Lyndstadaas, SP, Eds.; CRC: Boa Raton, FL, USA, 2003; pp. 323–340. [Google Scholar]
- Mazumder, J. Laser welding. In Laser Materials Processing; Bass, M, Ed.; North-Holland Pub.: Amsterdam, Holland, 1983; pp. 115–200. [Google Scholar]
- Bass, M; Jau, B; Wallace, R. Shaping materials with lasers. In Laser Materials Processing; Bass, M, Ed.; North-Holland Pub: Amsterdam, Holland, 1983; pp. 290–336. [Google Scholar]
- Dahotre, NB. Laser surface engineering. Adv. Mater. Proc 2002, 160, 35–39. [Google Scholar]
- Kopel, A; Reitza, W. Laser surface treatment. Adv. Mater. Proc 1999, 157, 39–41. [Google Scholar]
- Singh, J. The constitution and microstructure of laser- surface-modified metals. J. Metal 1992, 44, 8–14. [Google Scholar]
- Draper, CW; Poate, JM. Laser surface alloying. Int. Metal. Rev 1985, 30, 85–108. [Google Scholar]
- Galerie, A; Fasasi, A; Pons, M; Caillet, M. Improved high temperature oxidation resistance of Ti-6Al-4V by superficial laser alloying. In Surface Modification Technologies V; Sudarshan, TS, Braza, JF, Eds.; Cambridge University Press: Cambrodge, UK, 1992; pp. 401–411. [Google Scholar]
- Hollander, DA; von Walter, M; Wirtz, T; Sellei, R; Schmidt-Rohlfing, B; Paar, O; Erli, H-J. Structural, mechanical and in vitro characterization of individually structured Ti–6Al–4V produced by direct laser forming. Biomaterials 2006, 7, 955–963. [Google Scholar]
- Weigl, P; Kasenbacher, A; Werelius, K. Dental applications. In Femtosecond Technology for Technical and Medical Applications; Dausinger, F, Lichtner, F, Lubatschowski, H, Eds.; Springer: New York, NY, USA, 2004; Volume 96, pp. 167–187. [Google Scholar]
- Gamaly, EG; Rode, AV; Luther-Davis, B; Tikhonchuk, VT. Ablation of solids by femtosecond lasers: Ablation mechanism and ablation thresholds for metals and dielectrics. Phys. Plasmas 2002, 9, 949–953. [Google Scholar]
- Frentzen, M; Winkelstraeter, C; van Benthem, H; Koort, HJ. Bearbeitung der Schmelzorberflachen mit gepulster Laserstrahlung. Dtsch. Zahnaertzl. Z 1994, 49, 166–168. [Google Scholar]
- Femtosecond Lasers, Technology Application Programme, Missile Defense Agency: Dexter MI, USA, 1999; Available at: www.mdatechnology.net/techsearch.asp?articleid=191 (Accessed on 26 March 2010).
- Ringeisen, BR; Chrisey, DB; Piqué, A; Krizman, D; Bronks, M; Spargo, B. Direct write technology and a tool to rapidly prototype patterns of biological and electronic systems. Nanotechnology 2001, 1, 414–417. [Google Scholar]
- Finke, S; Feenstra, K. Solid freedom fabrication by extrusion and deposition of semi-solid alloys. J. Mater Sci 2002, 37, 3101–3106. [Google Scholar]
- Klug, KL; Ucok, I; Gungor, MN; Gulu, M; Kramer, LS; Tack, WT; Nastac, L; Martin, NR; Dong, H. The near-net-shape manufacturing of affordable titanium components for the M777 lightweight howitzer. J. Metal 2004, 56, 35–42. [Google Scholar]
- MacArthur, BD; Oreffo, ROC. Bridging the gap. Nature 2005, 433, 19. [Google Scholar]
- Boyan, BD; Hummert, TW; Dean, DD; Schwartz, Z. Role of materials surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar]
- Baier, RE. Conditioning surfaces to suit the biomedical environment: Recent Progress. J. Biomech. Eng 1982, 104, 257–271. [Google Scholar]
- Taira, M; Araki, Y. Scaffolds for tissue engineering – Kinds and clinical application. J. Dental Eng 2005, 152, 27–30. [Google Scholar]
- Li, H; Chang, J. Fabrication and characterization of bioactive wollastonite/PHBV composite scaffolds. Biomaterials 2004, 25, 5473–5480. [Google Scholar]
- Kawase, T; Sirringhaus, H; Friend, RH; Shimoda, T. Inkjet printed via-hole interconnection and resistors for all-polymer transistor circuits. Adv. Mater 2001, 13, 1601–1605. [Google Scholar]
- Burgess, DS. InkJet Technique precludes microvessels and mirolenses. Technology World 2005, 5, 213. [Google Scholar]
- Lee, M; Dunn, JCY; Wu, BM. Scaffold fabrication by indirect three-dimensional printing. Biomaterials 2005, 26, 4281–4289. [Google Scholar]
- Walboomers, XF; Jansen, JA. Bone tissue induction, using a COLLOSS®-filled titanium fibre mesh-scaffolding material. Biomaterials 2005, 26, 4779–4785. [Google Scholar]
- Hohman, MM; Shin, M; Rutledge, G; Brenner, MP. Electrospinning and electrorically fored jets. Phys. Fluids 2001, 13, 2201–2220. [Google Scholar]
- Li, M; Mondrinos, MJ; Gandhi, MR; Ko, FK; Weiss, AS; Lelkes, PI. Electropun protein fibers as matrices for tissue engineering. Biomaterials 2005, 26, 5999–6008. [Google Scholar]
- Buttafoco, L; Kolkman, NG; Engbers-Buijtenhuijs, P; Poot, AA; Dijkstra, PJ; Vermes, I; Feijen, J. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials 2006, 27, 724–734. [Google Scholar]
- Liu, X; Won, Y; Ma, PX. Surface modification of interconnected porous scaffolds. J. BioMed. Mater. Res. A 2005, 74, 84–91. [Google Scholar]
- MacDonald, RA; Laurenzi, BF; Viswanathan, G; Ajayan, PM; Stegemann, JP. Collagen-carbon nanotube composite materials as scaffolds in tissue engineering. J. BioMed. Mater. Res. A 2005, 74, 489–496. [Google Scholar]
- Funakoshi, T; Majima, T; Iwasaki, N; Yamane, S; Masuko, T; Minami, A; Harada, K; Tamura, H; Tokura, S; Nishimura, S-I. Novel chitosan-based hyaluronan hybrid polymer fibers as a scaffold in ligament tissue engineering. J. BioMed. Mater. Res. A 2005, 7, 338–346. [Google Scholar]
- Ni, S; Chang, J; Chou, L. A novel bioactive porous CaSiO3 scaffold for bone tissue engineering. J. BioMed. Mater. Res. A 2006, 76, 196–205. [Google Scholar]
- Wu, L; Jing, D; Ding, J. A “room-temperature” injection molding/particulate leaching approach for fabrication of biodegradable three-dimensional porous scaffolds. Biomaterials 2006, 27, 185–191. [Google Scholar]
- Gutwein, LG; Webster, TJ. Increased viable osteoblast density in the presence of nanophase compared to conventional alumina and titania particles. Biomaterials 2004, 25, 4175–4183. [Google Scholar]
- Webster, TJ; Ejiofor, JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar]
- Price, RL; Ellison, K; Haberstroh, KM; Webster, TJ. Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J. BioMed. Mater. Res. A 2004, 70, 129–138. [Google Scholar]
- Khang, D; Lu, J; Yao, C; Haberstroh, KM; Webster, TJ. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials 2008, 29, 970–983. [Google Scholar]
- Taylor, J. Nanobiotechnology. Available at: http://www.dti.gov.uk (Accessed on 26 March 2010).
- Frosch, K-H; Barvencik, F; Viereck, V; Lohmann, CH; Dresing, K; Breme, J; Brunner, E; Stürmer, KM. Growth behavior, matrix production, and gene expression of human osteoblasts in defined cylindrical titanium channels. J. BioMed. Mater. Res. A 2004, 68, 325–334. [Google Scholar]
- Macak, JM; Tsuchiya, H; Taveira, L; Ghicov, A; Schmuki, P. Self-organized nanotubular oxide layers on Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F solutions. J. BioMed. Mater. Res. A 2005, 75, 928–933. [Google Scholar]
- Oh, S; Finõnes, RR; Daraio, C; Chen, L-H; Jin, S. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 2005, 26, 4938–4943. [Google Scholar]
- Webster, TJ; Schadler, LS; Siegel, RW; Bizios, R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng 2001, 7, 91–301. [Google Scholar]
- Kobayashi, S; Hamasaki, N; Suzuki, M; Kimura, M; Shirai, H; Hanabusa, K. Preparation of helical transition-metal oxide tubes using organogelators as structure-directing agents. J. Am. Chem. Soc 2002, 124, 6550–6551. [Google Scholar]
- Michailowski, A; AlMawlawi, D; Cheng, G; Moskovits, M. Highly regular anatase nanotubule arrays fabricated in porous anodic templates. Chem. Phys. Lett 2001, 349, 1–5. [Google Scholar]
- Gong, D; Grimes, CA; Varghese, OK; Chen, Z; Dickey, EC. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res 2001, 16, 3331–3334. [Google Scholar]
- Oh, S; Daraio, C; Chen, LH; Pisanic, TR; Fiñones, RR; Jin, S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. BioMed. Mater. Res. A 2006, 78, 97–103. [Google Scholar]
- Popat, KC; Leoni, L; Grimes, CA; Desai, TA. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar]
- Park, J; Bauer, S; von der Mark, K; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett 2007, 7, 1686–1691. [Google Scholar]
- Oh, S; Brammer, KS; Li, YS; Teng, D; Engler, AJ; Chien, S; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar]
- Köse, GT; Ber, S; Korkusuz, F; Hasirci, V. Poly(3-hydroxybutyric acid-co-3- hydroxyvaleric acid) based tissue engineering matrices. J. Mater. Sci.: Mater. Med 2003, 14, 121–126. [Google Scholar]
- Bauer, S; Park, J; Mark, KV; Schmuki, P. Improved attachment of mesenchymal stem cells on super-hydrophobic TiO2 nanotubes. Acta Biomater 2008, 4, 1576–1582. [Google Scholar]
- Goto, K; Tamura, J; Shinzato, S; Fujibayashi, S; Hashimoto, M; Kawashita, M; Kokubo, T; Nakamura, T. Bioactive bone cements containing nano-sized titania particles for use as bone substitutes. Biomaterials 2005, 26, 6496–6505. [Google Scholar]
- Sato, M; Sambito, MA; Aslani, A; Kalkhoran, NM; Slamovich, EB; Webster, TJ. Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials 2006, 27, 2358–2369. [Google Scholar]
- Tyas, MJ. Minimum intervention dentistry – the cutting edge? Available at: http://www.mdhs.unimelb.edue.au (Accessed on 26 March 2010).
- .
- Bulard, RA; Vance, JB. Multi-clinic evaluation using mini-dental implants for long-term denture stabilization: A preliminary biometric evaluation. Compend. Contin. Educ. Dent 2005, 26, 892–897. [Google Scholar]
- Lazzarra, RJ. Immediate implant placement into extraction sites: Surgical and restorative advantages. Int. J. Periodontics Restorative Dent 1989, 9, 333–343. [Google Scholar]
- Parel, SK; Triplett, RG. Immediate fixture placement: A treatment planning alternative. Int. J. Oral Maxillofac. Implants 1990, 5, 337–345. [Google Scholar]
- Werbitt, MJ; Goldberg, PV. The immediate implant. Bone preservation and bone regeneration. Int. J. Periodontics Restorative Dent 1992, 12, 207–217. [Google Scholar]
- Gruder, U; Polizzi, G; Goebe, R; Hatano, N; Henry, P; Jackson, WJ; Kawamura, K; Koehler, S; Renouard, F; Rosenberg, R; Triplett, G; Werbitt, MJ; Lithner, B. A 3-year prospective multicenter follow-up report on the immediate and delayed-immediate placement of implants. Int. J. Oral Maxillofac. Implants 1999, 14, 210–216. [Google Scholar]
- Kalpakjian, S; Schmid, S. Manufacturing Engineering and Technology; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2006; pp. 854–855. [Google Scholar]
- Peckitt, NS. Stereoscopic lithography: Customized titanium implants on orofacial reconstruction. Br. J. Oral Maxillofac. Surg 1999, 37, 353–369. [Google Scholar]
- Nishimura, RD; Chang, TL; Perri, GR; Beumer, J. Restoration of partially edentulous patients using customized implant abutmens. Pract. Period Aesthet. Dent 1999, 11, 669–676. [Google Scholar]
- De Putter, M; de Lange, GL; de Groot, K. Permucosla dental implants of dense hydroxyapatite: Fixation in alveolar bone. In Proc Int Congress on Tissue Integration in Oral and Maxillofacial Reconstruction; Excerta Media: Amsterdam, Holland, 1986; pp. 389–394. [Google Scholar]
- Albrektsson, T; Wennerberg, A. Oral implant surfaces: Part 1 –review focusing on topographic and chemical properties of different surfaces and in vitro responses to them. Int. J. Prosthodont 2004, 17, 536–543. [Google Scholar]
- Ellingsen, JE. A study on the mechanism of protein adsorption to TiO2. Biomaterials 1991, 12, 593–596. [Google Scholar]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amsterdam, Holland, 2007; pp. 406–411. [Google Scholar]
- Masuda, T; Yliheikkaila, PK; Fleton, DA; Cooper, LF. Generalizations regarding the process and phenomenon of osseointegration. Part I. In vivo studies. Int. J. Oral Maxillofac. Implants 1998, 13, 17–29. [Google Scholar]
- Leven, RM; Virdi, AS; Sumner, DR. Patterns of gene expression in rat bone marrow stromal cells cultured on titanium alloy discs of different roughness. J. BioMed. Mater. Res. A 2004, 70, 391–401. [Google Scholar]
- Jasty, M; Bragdon, CR; Haire, T; Mulroy, RO; Harris, WH. Comparison of bone ingrowth into cobalt chrome sphere and titanium fiber mesh porous coated cementless canine acetabular components. J BioMed Mater Res 1993, 27, 639–644. [Google Scholar]
- Simske, SJ; Sachdeva, R. Cranial bone apposition and ingrowth in a porous nickel-titanium implant. J. BioMed. Mater. Res 1995, 29, 527–533. [Google Scholar]
- Chang, Y-S; Oka, M; Kobayashi, M; Gu, H-O; Li, Z-L; Nakamura, T; Ikada, Y. Significance of interstitial bone ingrowth under load-bearing conditions: A comparison between solid and porous implant materials. Biomaterials 1996, 17, 1141–1148. [Google Scholar]
- Aziz-Kerrzo, M; Conroy, KG; Fenelon, AM; Farrell, AT; Breslin, CB. Electrochemical studies on the stability and corrosion resistance of titanium-based implant materials. Biomaterials 2001, 22, 1531–1539. [Google Scholar]
- Cook, SD; Thongpreda, N; Anderson, RC; Haddad, RJ. The effect of post-sintering heat treatments on the fatigue properties of porous coated Ti-6Al-4V alloy. J. BioMed. Mater. Res 1988, 22, 287–302. [Google Scholar]
- Kohn, DH; Ducheyne, P. A parametric study of the factors affecting the fatigue strength of porous coated Ti-6Al-4V implant alloy. J. BioMed. Mater. Res 1990, 24, 1483–1501. [Google Scholar]
- Wisbey, A; Gregson, PJ; Peter, LM; Tuke, M. Effect of surface treatment on the dissolution of titanium-based implant materials. Biomaterials 1991, 12, 470–473. [Google Scholar]
- Sutherland, DS; Forshaw, PD; Allen, GC; Brown, IT; Williams, KR. Surface analsyis of titanium implants. Biomaterials 1993, 14, 893–899. [Google Scholar]
- Lausmaa, GJ; Kasemo, B. Surface spectroscopic characterization of titanium implant materials. Appl. Surf. Sci 1990, 44, 133–146. [Google Scholar]
- Kubaschewski, O; Hopkins, BE. Oxidation of Metals and Alloys; Geissman, TA, Ed.; Butterworths: London, UK, 1962; p. 24. [Google Scholar]
- Tummler, H; Thull, R. Model of the metal/tissue connection of implants made of titanium or tantalum. In Biological and Biochemical Performance of Biomaterials; Elsevier: Amsterdam, Holland, 1986; pp. 403–408. [Google Scholar]
- Eliades, T. Passive film growth on titanium alloys: Physiochemical and biologic considerations. Int. J. Oral Maxillofac. Implants 1997, 12, 621–627. [Google Scholar]
- Drath, DB; Karnovsky, ML. Superoxide production by phagocytic leukocytes. J. Exp Med 1975, 144, 257–261. [Google Scholar]
- Root, BK; Metcalf, JA. H2O2 release from human granulocytes during phagocytosis: Relationship to superoxide anion formation and cellular catabolism of H2O2. Studies with normal and cytochalasin B-treated cells. J. Clin Invest 1977, 60, 1266–1279. [Google Scholar]
- Jain, R; von Recum, AF. Fibroblast attachment to smooth and microtextured PET and thin cp-Ti films. J. BioMed. Mater. Res. A 2004, 68, 296–304. [Google Scholar]
- Clem, WC; Konovalov, VK; Chowdhury, S; Vohra, YK; Catledge, SA; Bellis, SL. Mesenchymal stem cell adhesion and spreading on microwave plasma-nitrided titanium alloy. J. BioMed. Mater. Res. A 2006, 76, 279–287. [Google Scholar]
- McQueen, D; Sundgren, JE; Ivarsson, B; Lundstrom, I; Af Ekenstam, CB; Svensson, A; Brånemark, PI; Albrektsson, T. Auger electron spectroscopic studies of titanium implants. In Clinical Applications of Biomaterials; Lee, AJC, Ed.; Wiley: New York, NY, USA, 1982; pp. 179–185. [Google Scholar]
- Parsegian, VA. Molecular forces governing tight contact between cellular surfaces and substrates. J. Prosthet. Dent 1983, 49, 838–842. [Google Scholar]
- Uo, M; Watari, F; Yokoyama, A; Matsuno, H; Kawasaki, T. Tissue reaction around metal implant observed by X-ray scanning analytical microscopy. Biomaterials 2001, 22, 677–685. [Google Scholar]
- Lu, Y-P; Li, M-S; Li, S-T; Wang, Z-G; Zhu, R-F. Plasma-sprayed hydroxyapatite + titania composite bond coat for hydroxyapatite coating on titanium substrate. Biomaterials 2004, 25, 4393–4403. [Google Scholar]
- Spriano, S; Bosetti, M; Bronzoni, M; Vernè, E; Maina, G; Bergo, V; Cannas, M. Surface properties and cell response of low metal ion release Ti-6Al-7Nb alloy after multi-step chemical and thermal treatments. Biomaterials 2005, 26, 1219–1229. [Google Scholar]
- JETRO. Titanium surface reforming technology. New Technology Japan No. 92-02-100-08. 1992:19:18, 1992.
- Klinger, A; Steinberg, D; Kohavi, D; Sela, MN. Adsorption of human salivary Albumin to titanium-oxide. J Dent Res 1996, 75, 273. [Google Scholar]
- Pham, MT; Reuther, H; Maitz, MF. Native extracellular matrix coating on Ti surfaces. J. Biomed. Mater. Res. A 2003, 66, 310–316. [Google Scholar]
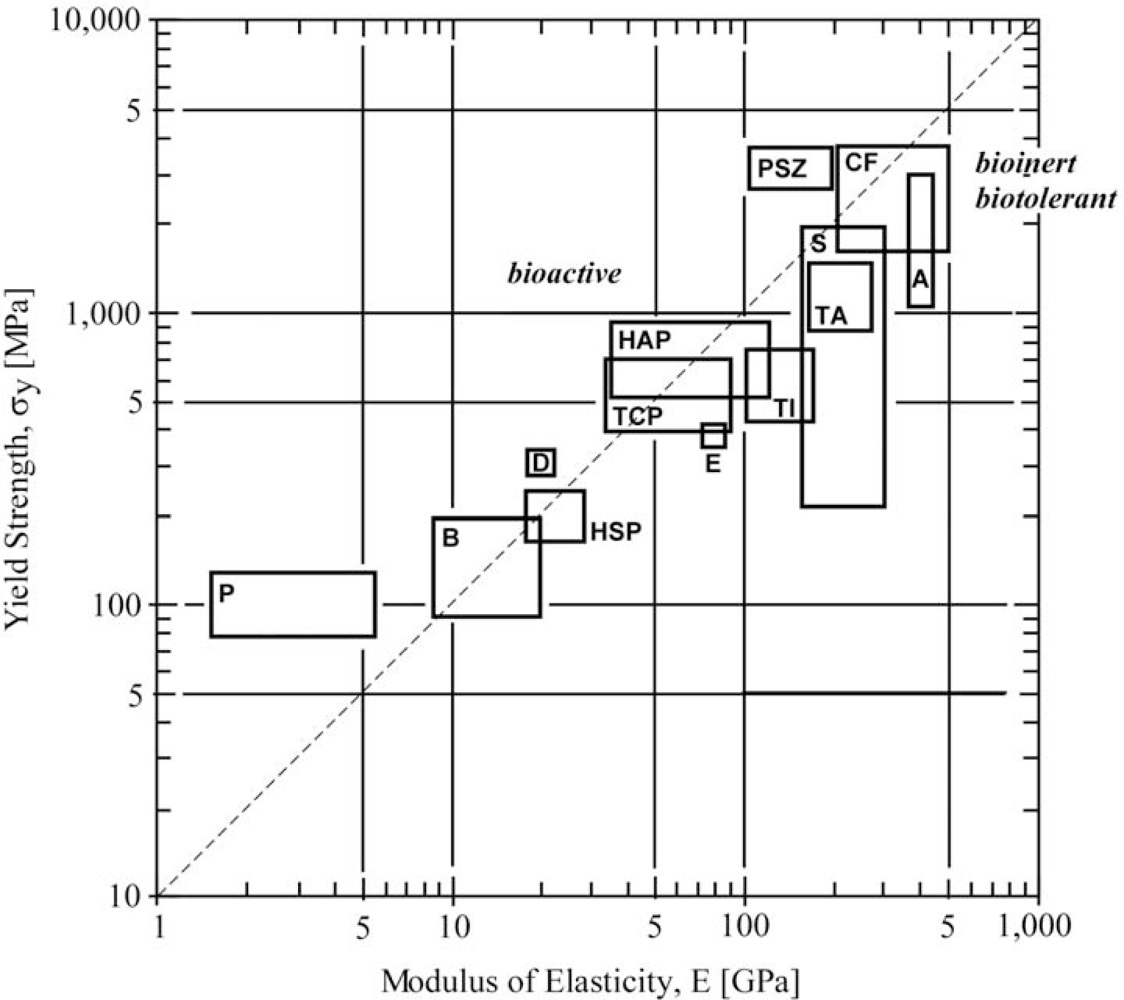
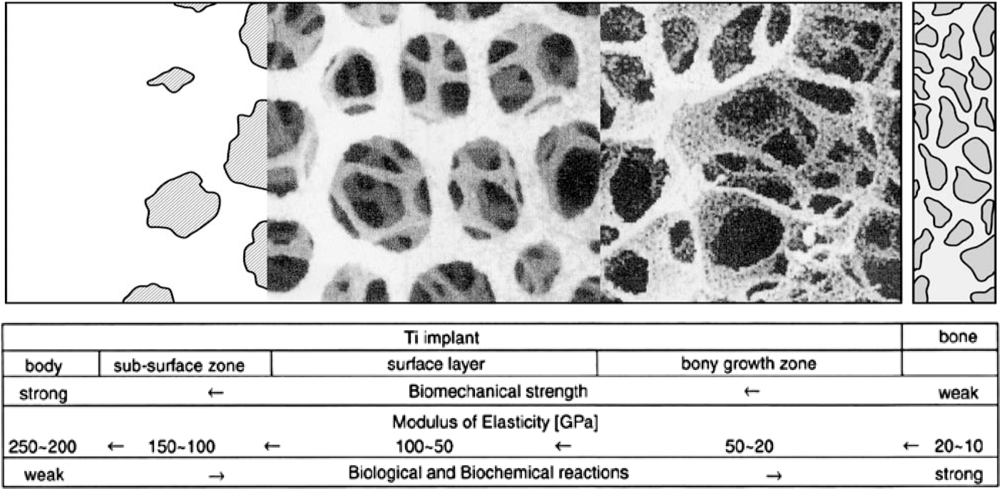
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Oshida, Y.; Tuna, E.B.; Aktören, O.; Gençay, K. Dental Implant Systems. Int. J. Mol. Sci. 2010, 11, 1580-1678. https://doi.org/10.3390/ijms11041580
Oshida Y, Tuna EB, Aktören O, Gençay K. Dental Implant Systems. International Journal of Molecular Sciences. 2010; 11(4):1580-1678. https://doi.org/10.3390/ijms11041580
Chicago/Turabian StyleOshida, Yoshiki, Elif B. Tuna, Oya Aktören, and Koray Gençay. 2010. "Dental Implant Systems" International Journal of Molecular Sciences 11, no. 4: 1580-1678. https://doi.org/10.3390/ijms11041580
APA StyleOshida, Y., Tuna, E. B., Aktören, O., & Gençay, K. (2010). Dental Implant Systems. International Journal of Molecular Sciences, 11(4), 1580-1678. https://doi.org/10.3390/ijms11041580