Gas-Phase Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide Over Co1.5PW12O40 Keggin-Type Heteropolyanion
Abstract
:1. Introduction
2. Results and Discussion
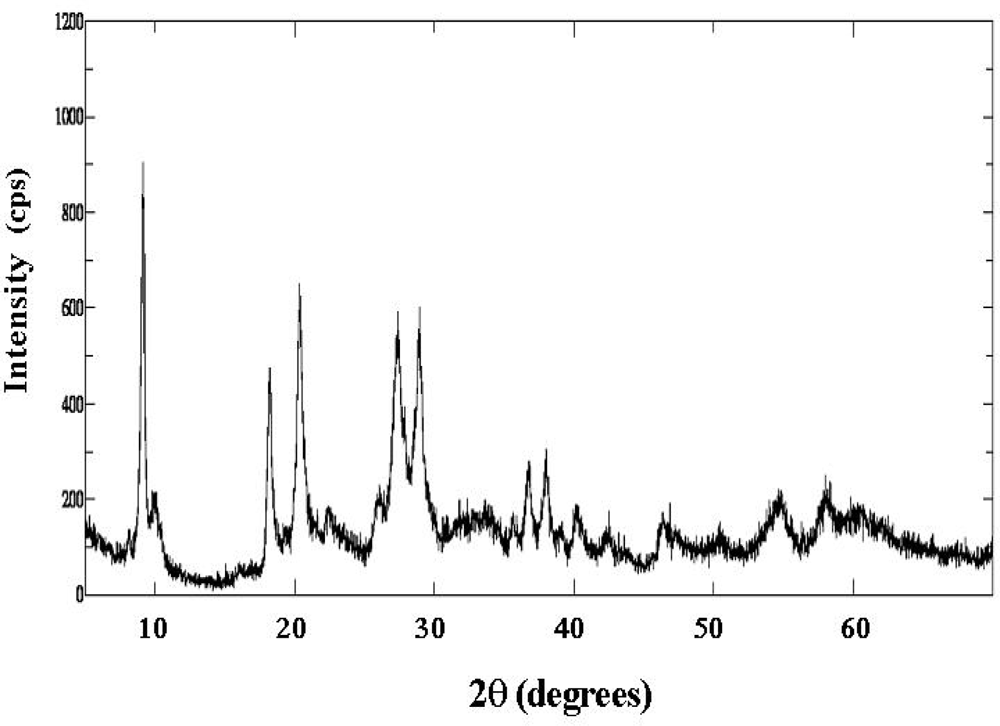
2.1. Characterization of Catalysts
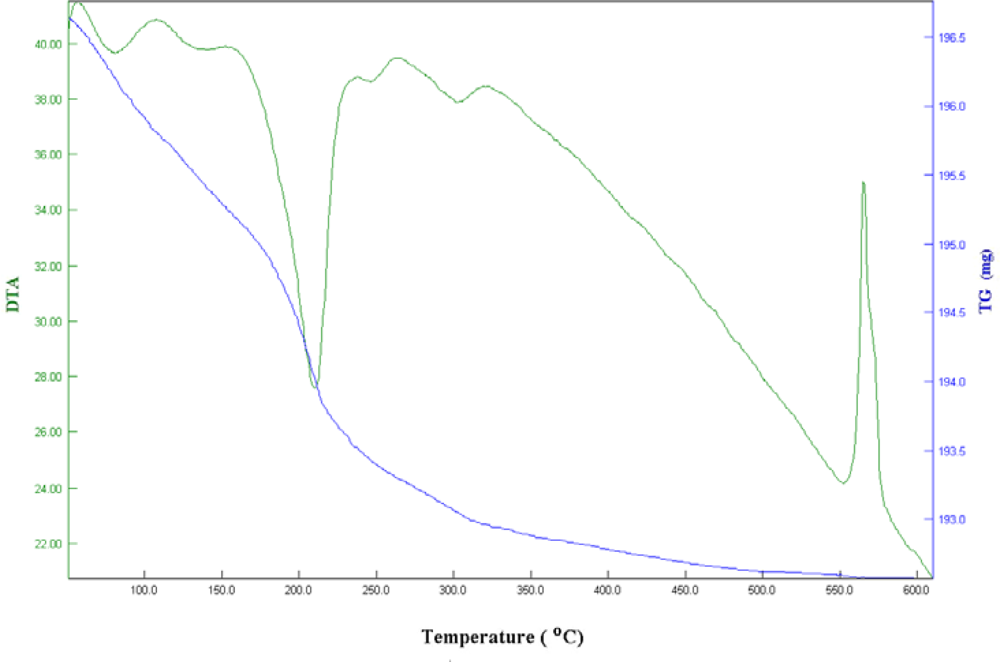
Thermogravimetric (TG) and Dfferential Thermal Analysis (DTA)
2.2. Catalytic Reaction
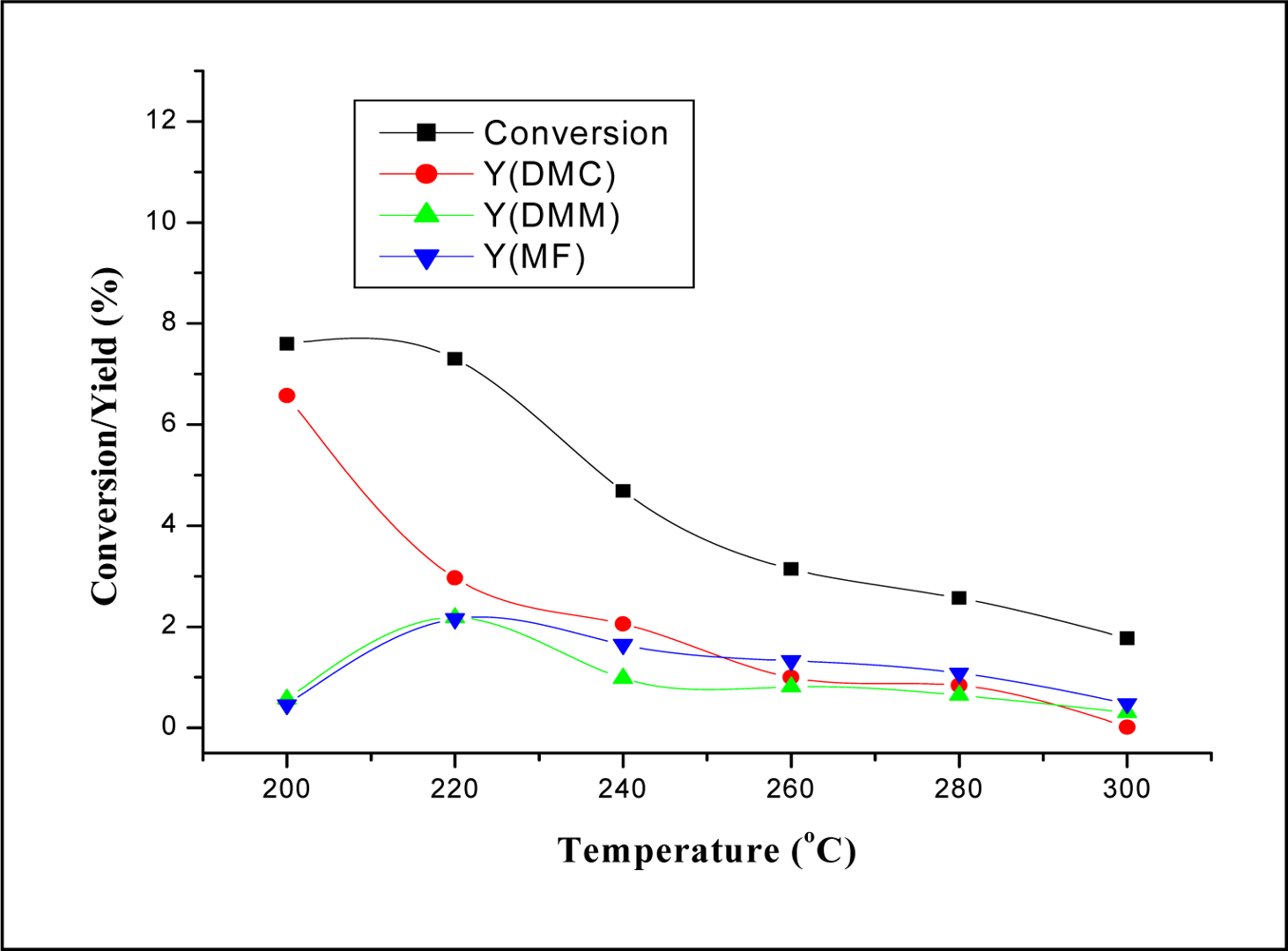
2.2.1. Effect of Reaction Temperature
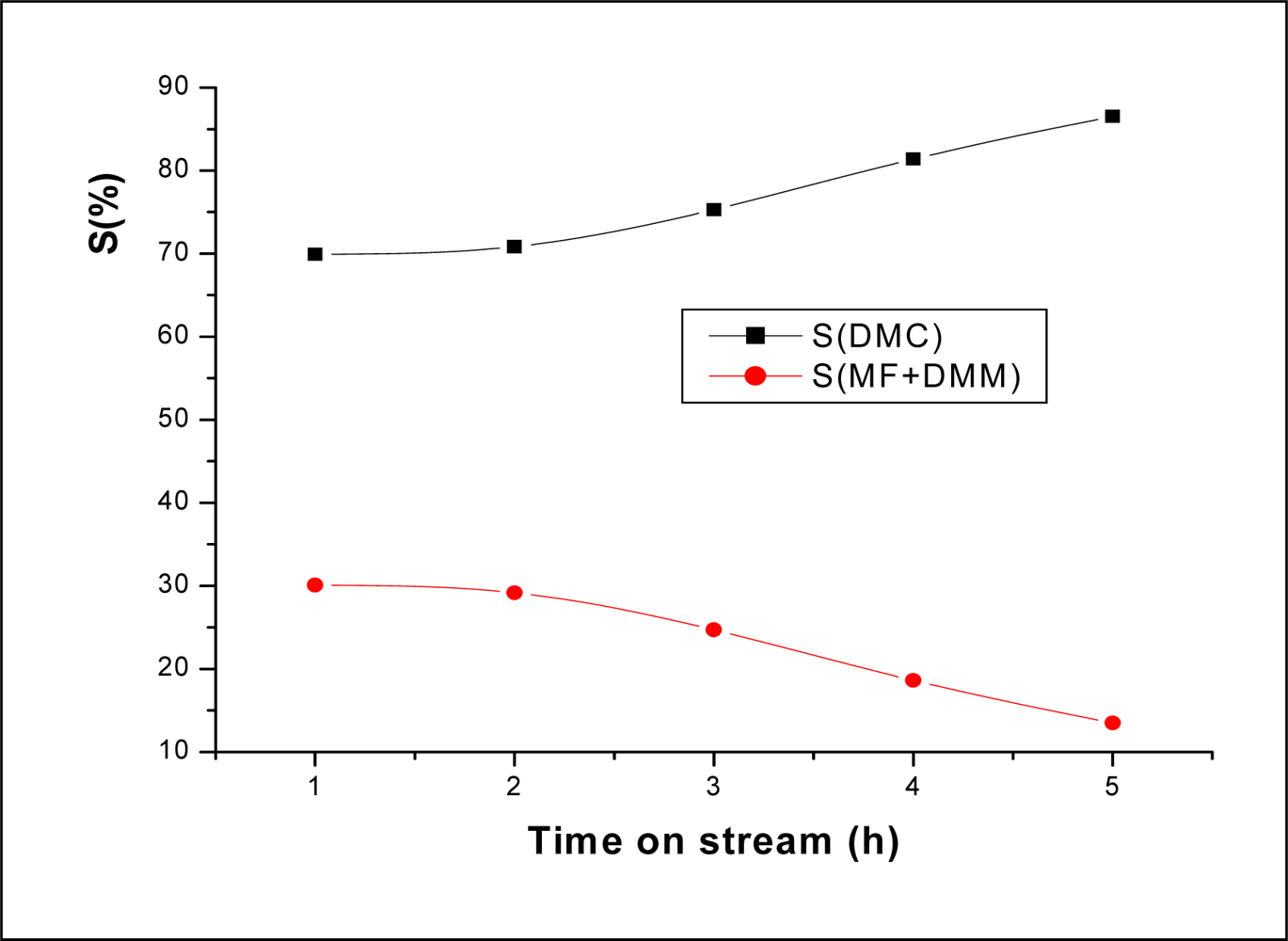
2.2.2. Effect of Time on Stream
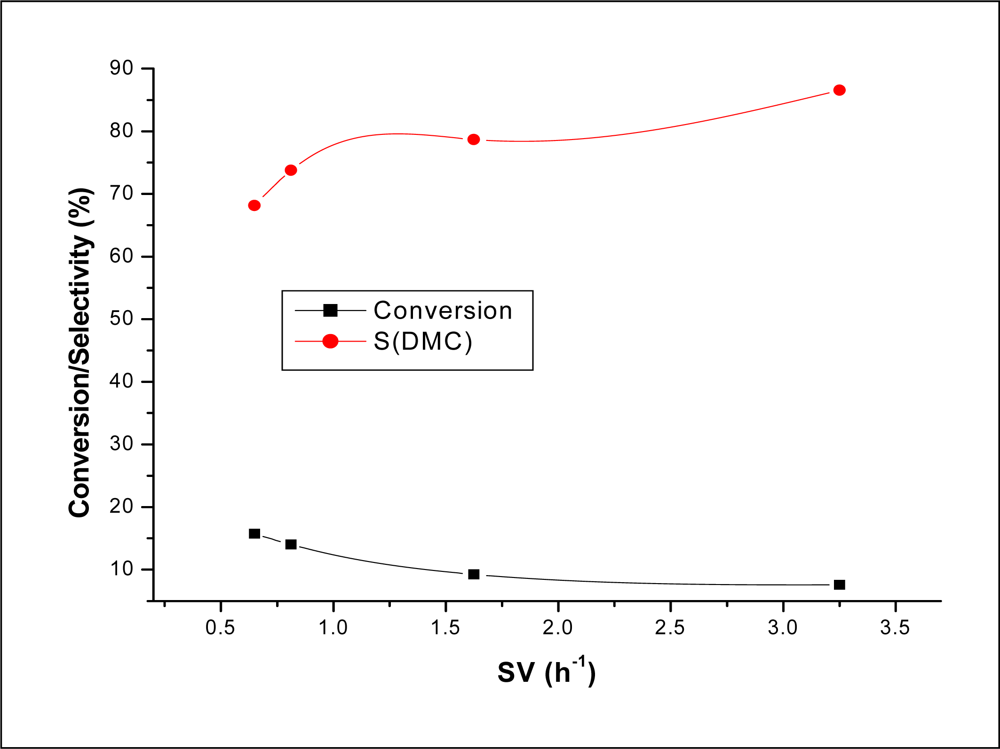
2.2.3. Effect of Space Velocity
3. Experimental Section
3.1. Catalyst Preparation
3.2. Physicochemical Techniques
3.3. Catalytic Tests
- No: number of moles of methanol introduced (mol/h)
- Ni: number of moles of the product i formed (mol/h)
- Nr: number of moles of reacted methanol (mol/h)
4. Conclusion
References
- Pacheco, MA; Marshall, CL. Review on Dimethyl carbonate manufacture and its characteristics as a fuel additive. Energy Fuels 1997, 11, 2–29. [Google Scholar]
- Guo, XC; Qin, ZF; Wang, GF; Wang, JG. Critical temperatures and pressures of reacting mixture in synthesis of dimethyl carbonate with methanol and carbon dioxide. Chin. Chem. Lett 2008, 19, 249–252. [Google Scholar]
- Ono, Y. Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block. Appl. Catal. A 1997, 155, 133–166. [Google Scholar]
- Tundo, P; Selva, M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res 2002, 35, 706–716. [Google Scholar]
- Richter, M; Fait, MJG; Eckelt, R; Schneider, M; Radnik, J; Heidemann, D; Fricke, R. Gas-phase carbonylation of methanol to dimethyl carbonate on chloride-free Cu-precipitated zeolite Y at normal pressure. J. Catal 2007, 245, 11–24. [Google Scholar]
- Delledonne, D; Rivetti, F; Romano, U. Developments in the production and application of dimethylcarbonate. Appl. Catal. A: Gen 2001, 221, 241–251. [Google Scholar]
- Shaikh, AG; Sivaram, S. Organic Carbonates. Chem. Rev 1996, 96, 951–976. [Google Scholar]
- Romano, U; Tesei, R; Mauri, MM; Rebora, P. Synthesis of Dimethyl Carbonate from Methanol, Carbon Monoxide, and Oxygen Catalyzed by Copper Compounds. Ind. Eng. Chem. Prod. Dev 1980, 19, 396–403. [Google Scholar]
- Choi, JC; Sakakura, T; Sako, T. Reaction of Dialkyltin Methoxide with Carbon Dioxide Relevant to the Mechanism of Catalytic Carbonate Synthesis. J. Am. Chem. Soc 1999, 121, 3793–3794. [Google Scholar]
- Wu, XL; Xiao, M; Meng, YZ; Lu, YX. Direct synthesis of dimethyl carbonate (DMC) using Cu-Ni/VSO as catalyst. J. Mol. Catal. A: Chem 2006, 249, 93–97. [Google Scholar]
- Wu, XL; Xiao, M; Meng, YZ; Lu, YX. Direct synthesis of dimethyl carbonate on H3PO4 modified V2O5. J. Mol. Catal. A: Chem 2005, 238, 158–162. [Google Scholar]
- Jessop, PG; Ikariya, T; Noyori, R. Homogeneous Catalysis in Supercritical Fluids. Science 1995, 269, 1065–1069. [Google Scholar]
- Tundo, P; Selva, M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res 2002, 35, 706–716. [Google Scholar]
- Omae, I. Aspects of carbon dioxide utilization. Catal. Today 2006, 115, 33–52. [Google Scholar]
- Bian, J; Xiao, M; Wang, SJ; Lu, YX; Meng, YZJ. Novel application of thermally expanded graphite as the support of catalysts for direct synthesis of DMC from CH3OH and CO2. J. Colloid. Interface Sci 2009, 334, 50–57. [Google Scholar]
- Bian, J; Xiao, M; Wang, S; Wang, X; Lu, Y; Meng, Y. Highly effective synthesis of dimethyl carbonate from methanol and carbon dioxide using a novel copper–nickel/graphite bimetallic nanocomposite catalyst. Chem. Eng. J 2009, 147, 287–296. [Google Scholar]
- Wu, XL; Xiao, M; Meng, YZ; Lu, YX. Direct synthesis of dimethyl carbonate on H3PO4 modified V2O5. J. Mol. Catal. A. Chem 2005, 238, 158–162. [Google Scholar]
- Rocchiccioli-Deltcheff, C; Fournier, M; Franck, R; Thouvenot, R. Vibrational nvestigations of polyoxometalates. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem 1983, 22, 207–216. [Google Scholar]
- Rocchiccioli-Deltcheff, C; Fournier, M. Catalysis by Polyoxometalates. Part 3,–Inflence of Vanadium (V) on the thermal Stability of 12-Metallophosphoric Acids from In Situ Infrared Studies. J. Chem. Soc. Faraday Trans 1991, 87, 3913–3920. [Google Scholar]
- Fournier, M; Feumi-Jantou, C; Rabia, C; Herve, G; Launay, S. Polyoxometalates Catalyst Materials: X-Ray Thermal Stability Study of Phosphorus-containing Heteropolyacids H3+xPM12-xVxO40.13–14H2O (M=Mo, W; x=0-1). J. Mater. Chem 1992, 2, 971–978. [Google Scholar]
- Gao, R; Chen, H; Le, Y; Dai, W-L; Fan, K. Highly active and selective Cs2.5H0.5PW12O40/SBA-15 composite material in the oxidation of cyclopentane-1,2-diol to glutaric acid by aqueous H2O2. Appl. Catal 2009, 352, 61–65. [Google Scholar]
- Fuchs, VM; Pizzio, LR; Blanco, MN. Synthesis and characterization of aluminum or copper tungstophosphate and tungstosilicate immobilized in a polymeric blend. Eur. Polym. J 2008, 44, 801–807. [Google Scholar]
- Cabello, CI; Egusquiza, MG; Botto, IL; Minelli, G. Heteropolyoxotungstates containing a wide catalytic target: reducibility and thermal stability. Mater. Chem. Phys 2004, 87, 264–274. [Google Scholar]
- Fu, Y; Zhu, H; Shen, J. Thermal decomposition of dimethoxymethane and dimethyl carbonate catalyzed by solid acids and bases. Thermochim. Acta 2005, 434, 88–92. [Google Scholar]
- Zhao, T; Han, Y; Sun, Y. Novel reaction route for dimethyl carbonate synthesis from CO2 and methanol. Fuel Proces. Tech 2000, 62, 187–194. [Google Scholar]
- Wang, XJ; Xiao, M; Wang, SJ; Lu, YX; Meng, YZ. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol using supported copper (Ni, V, O) catalyst with photo-assistance. J. Mol. Catal. A: Chem 2007, 278, 92–96. [Google Scholar]



© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aouissi, A.; Al-Othman, Z.A.; Al-Amro, A. Gas-Phase Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide Over Co1.5PW12O40 Keggin-Type Heteropolyanion. Int. J. Mol. Sci. 2010, 11, 1343-1351. https://doi.org/10.3390/ijms11041343
Aouissi A, Al-Othman ZA, Al-Amro A. Gas-Phase Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide Over Co1.5PW12O40 Keggin-Type Heteropolyanion. International Journal of Molecular Sciences. 2010; 11(4):1343-1351. https://doi.org/10.3390/ijms11041343
Chicago/Turabian StyleAouissi, Ahmed, Zeid Abdullah Al-Othman, and Amro Al-Amro. 2010. "Gas-Phase Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide Over Co1.5PW12O40 Keggin-Type Heteropolyanion" International Journal of Molecular Sciences 11, no. 4: 1343-1351. https://doi.org/10.3390/ijms11041343
APA StyleAouissi, A., Al-Othman, Z. A., & Al-Amro, A. (2010). Gas-Phase Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide Over Co1.5PW12O40 Keggin-Type Heteropolyanion. International Journal of Molecular Sciences, 11(4), 1343-1351. https://doi.org/10.3390/ijms11041343



