A Novel Pectin Material: Extraction, Characterization and Gelling Properties
Abstract
:1. Introduction
2. Results and Discussion
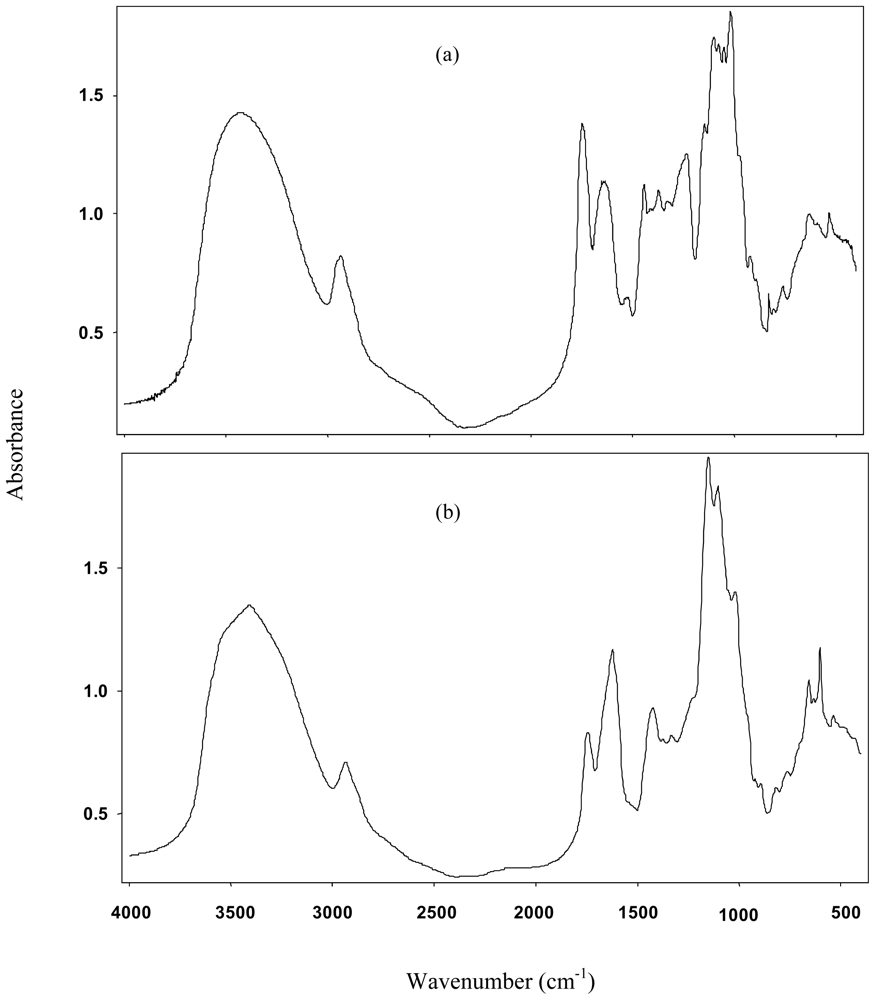
2.1. Pectin Extraction and Characterization
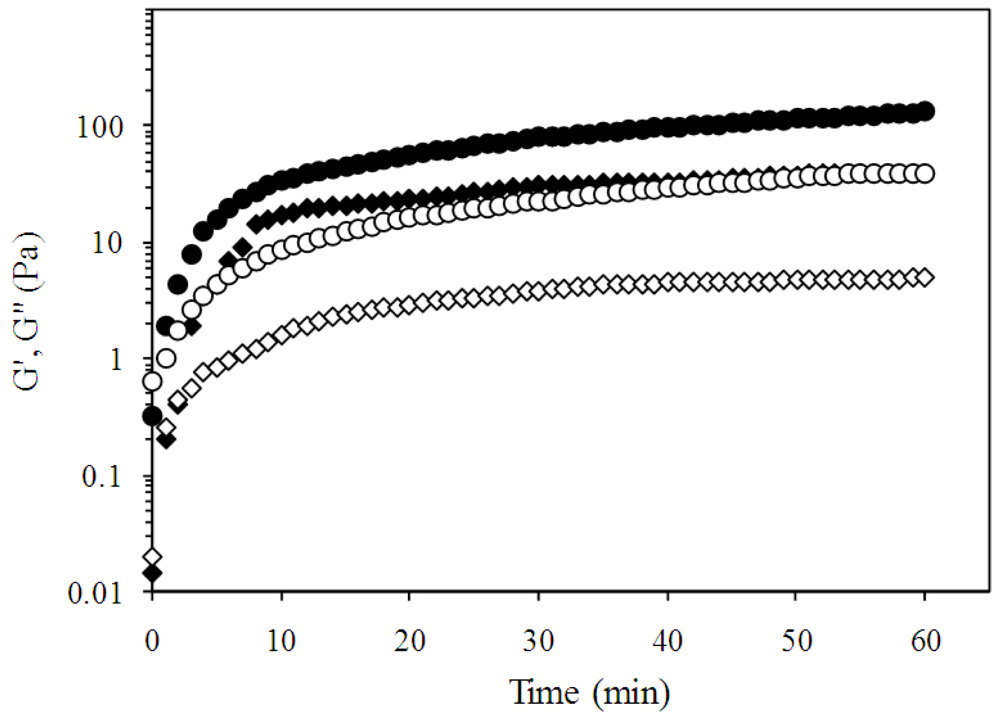
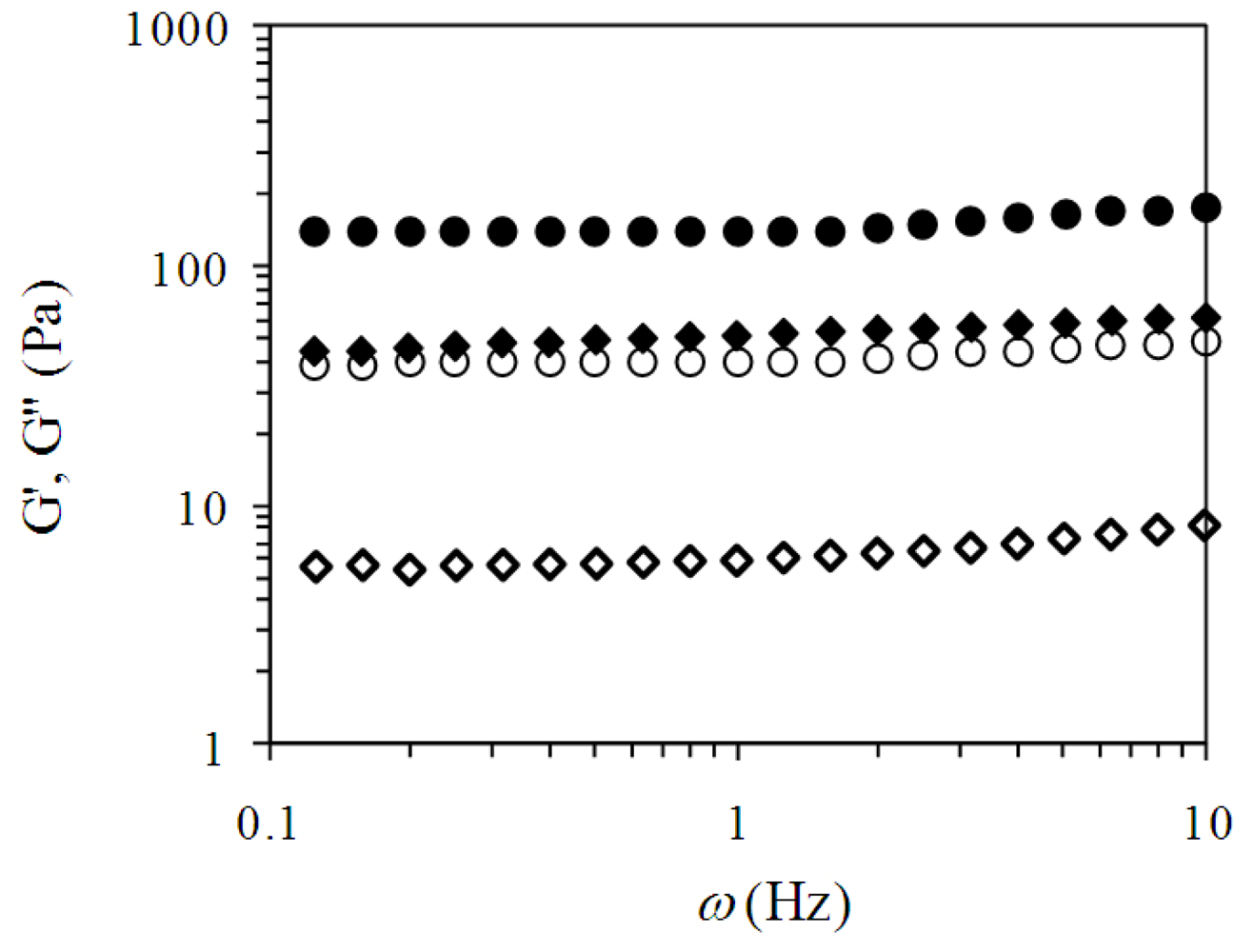
2.2. Pectin Gelation
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Pectin Extraction
3.2.2. Chemical Composition
3.2.3. Degree of Esterification (DE)
3.2.4. Intrinsic Viscosity
3.2.5. Viscosimetric Molecular Weight
3.2.6. Rheological Measurements
3.2.7. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Willats, WGT; Paul Knox, J; Mikkelsen, JD. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol 2006, 17, 97–104. [Google Scholar]
- Ström, A; Ribelles, P; Lundin, L; Norton, I; Morris, ER; Williams, AK. Influence of pectin fine structure on the mechanical properties of calcium-pectin and acid-pectin gels. Biomacromolecules 2007, 8, 2668–2674. [Google Scholar]
- Fu, JT; Rao, MA. Rheology and structure development during gelation of low-methoxyl pectin gels: The effect of sucrose. Food Hydrocolloid 2001, 15, 93–100. [Google Scholar]
- Ridley, BL; O’Neill, MA; Mohnen, D. Review Pectins: Structure, biosynthesis, and oligogalacturonide-related Signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar]
- Christodoulou, V; Bampidis, VA; Hucko, B; Ploumi, K; Iliadis, C; Robinson, PH; Mudrik, Z. Nutritional value of chickpeas in rations of lactating ewes and growing lambs. Anim. Feed Sci. Technol 2005, 118, 229–241. [Google Scholar]
- Maheri-Sis, N; Chamani, M; Sadeghi, AA; Mirza-Aghazadeh, A; Aghajanzadeh-Golshani, A. Nutritional evaluation of kabuli and desi type chickpeas (Cicer arietinum L.) for nutriments using in vitro gas production technique. Afr. J. Biotechnol 2008, 7, 2946–2951. [Google Scholar]
- Aisa, HA; Zhen, C; Yili, A; Bahang, R; Rakhmanberdyeva, RK; Sagdullaev, BT. Polysaccharides from two Cicer species cultivated in China. Chem. Nat. Compd 2006, 42, 349–350. [Google Scholar]
- Rascón-Chu, A; Martínez-López, AL; Carvajal-Millán, E; Ponce de León-Renova, N; Márquez-Escalante, J; Romo-Chacón, A. Pectin from low quality ‘Golden Delicious’ apples: Composition and gelling capability. Food Chem 2009, 116, 101–103. [Google Scholar]
- Mollea, C; Chiampo, F; Conti, R. Extraction and characterization of pectins from cocoa husks: A preliminary study. Food Chem 2008, 107, 1353–1356. [Google Scholar]
- Iglesias, MT; Lozano, JE. Extraction and characterization of sunflower pectin. J. Food Eng 2004, 62, 215–223. [Google Scholar]
- Yapo, BM; Koffi, KL. Yellow passion fruit rinds a potential source of low-methoxyl pectin. J. Agric. Food Chem 2006, 54, 2738–2744. [Google Scholar]
- Kurita, O; Fujiwara, T; Yamazaki, E. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydr. Polym 2008, 74, 725–730. [Google Scholar]
- Cardoso, SM; Coimbra, MA; Lopes da Silva, JA. Temperature dependence of the formation and melting of pectin-Ca2+ networks: A rheological study. Food Hydrocolloid 2003, 17, 801–807. [Google Scholar]
- Cernà, M; Barros, AS; Nunes, A; Rocha, SM; Delgadillo, I; Copìkovà, J; Coimbra, MA. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym 2003, 19, 793–801. [Google Scholar]
- Gnanasambandan, R; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem 2000, 68, 327–332. [Google Scholar]
- Cardoso, SM; Coimbra, MA; Lopes da Silva, JA. Calcium-mediated gelation of an olive pomace pectin extract. Carbohydr. Polym 2003, 52, 125–133. [Google Scholar]
- Durand, D; Bertrand, C; Clark, AH; Lips, A. Calcium-induced gelation of low methoxy pectin solutions-thermodynamic and rheological considerations. Int. J. Biol. Macromol 1990, 12, 14–18. [Google Scholar]
- Braccini, I; Pérez, S. Molecular basis of Ca-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar]
- Hwang, J; Pyun, YR; Kokini, JL. Sidechains of pectins: Some thoughts on their role in plant cell walls and foods. Food Hydrocolloid 1993, 7, 39–53. [Google Scholar]
- Doublier, JL; Cuvelier, G. Eliasson, AC, Ed.; Gums and hydrocolloids: Functional aspects. In Carbohydrates in Food; Marcel Dekker, Inc: New York, NY, USA, 1996; pp. 283–318. [Google Scholar]
- Ross-Murphy, SB. Chan, HWS, Ed.; Rheological methods. In Biophysical Methods in Food Research; Blackwell: Oxford, UK, 1984; pp. 138–199. [Google Scholar]
- Carvajal-Millán, E; Rascón-Chu, A; Márquez-Escalante, J; Ponce de León, N; Micard, V; Gardea, A. Maize bran gum: Extraction, characterization and functional properties. Carbohydr. Polym 2007, 6, 280–285. [Google Scholar]
- AACC, Approved Methods of the American Association of Cereal Chemists; The Association, University of Minnesota: St. Paul, MN, USA, 1998.
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Mead, DJ; Fouss, RM. Viscosities of solutions of polyvinyl chloride. J. Am. Chem. Soc 1942, 64, 277–282. [Google Scholar]
- Kraemer, EO. Molecular weight of celluloses and cellulose derivates. J. Ind. Eng. Chem 1938, 30, 1200–1203. [Google Scholar]

| Galacturonic acid | 67.0 ± 0.4 |
| Arabinose | 7.7 ± 0.3 |
| Galactose | 12.3 ± 0.5 |
| Glucose | 1.6 ± 0.2 |
| Xylose | 0.4 ± 0.1 |
| Mannose | 0.6 ± 0.1 |
| Rhamnose | 10.4 ± 0.7 |
| Protein | 0.02 ± 0.01 |
| Ash | 0.03 ± 0.01 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Urias-Orona, V.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Carvajal-Millán, E.; Gardea, A.A.; Ramírez-Wong, B. A Novel Pectin Material: Extraction, Characterization and Gelling Properties. Int. J. Mol. Sci. 2010, 11, 3686-3695. https://doi.org/10.3390/ijms11103686
Urias-Orona V, Rascón-Chu A, Lizardi-Mendoza J, Carvajal-Millán E, Gardea AA, Ramírez-Wong B. A Novel Pectin Material: Extraction, Characterization and Gelling Properties. International Journal of Molecular Sciences. 2010; 11(10):3686-3695. https://doi.org/10.3390/ijms11103686
Chicago/Turabian StyleUrias-Orona, Vania, Agustin Rascón-Chu, Jaime Lizardi-Mendoza, Elizabeth Carvajal-Millán, Alfonso A. Gardea, and Benjamín Ramírez-Wong. 2010. "A Novel Pectin Material: Extraction, Characterization and Gelling Properties" International Journal of Molecular Sciences 11, no. 10: 3686-3695. https://doi.org/10.3390/ijms11103686
APA StyleUrias-Orona, V., Rascón-Chu, A., Lizardi-Mendoza, J., Carvajal-Millán, E., Gardea, A. A., & Ramírez-Wong, B. (2010). A Novel Pectin Material: Extraction, Characterization and Gelling Properties. International Journal of Molecular Sciences, 11(10), 3686-3695. https://doi.org/10.3390/ijms11103686





