Impact of Deoxynivalenol on the Intestinal Microflora of Pigs
Abstract
:1. Introduction
2. Results
2.1. Zootechnical performance
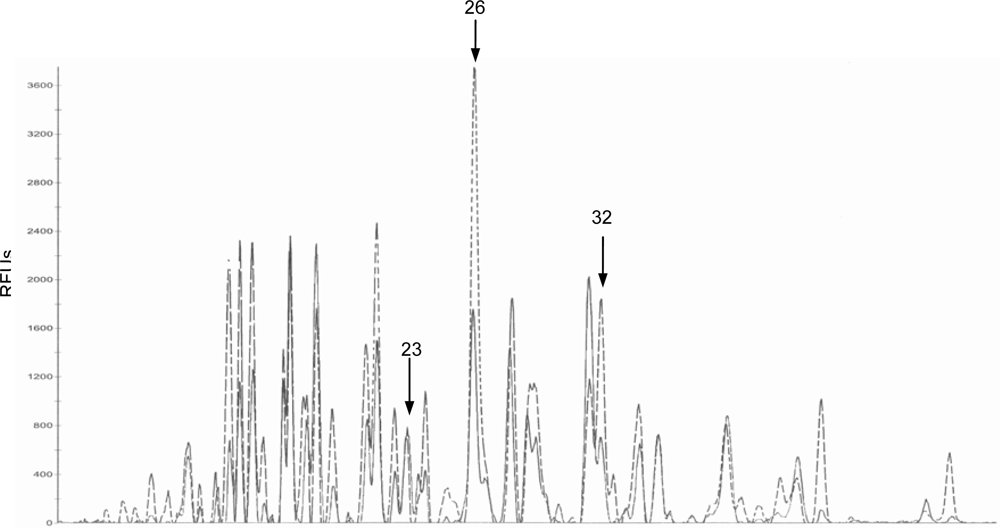
2.2. Bacteriological analysis
3. Discussion
4. Experimental Section
4.1. Animals, housing and experimental design
4.2. Experimental diet
4.3. Zootechnical performances and sample collection
4.4. Bacteriological counts
4.5. Capillary Single-Stranded Conformation Polymorphism (CE-SSCP) analysis
4.6. Data analysis
Bacteriological data
5. Conclusions
Acknowledgments
References and Notes
- CAST Report, Council for Agricultural Science and Technology. In Mycotoxins: Risks in plant, Animal, and Human Systems; Ames, IA, USA, 2003.
- Oswald, IP; Comera, C. Immunotoxicity of mycotoxins. Rev. Med. Vet 1998, 149, 585–590. [Google Scholar]
- .
- Smith, JE; Henderson, RS. Mycotoxins and Animal Foods, 1st Ed ed; CRC press: Boca Raton, FL, USA, 1991; p. 875. [Google Scholar]
- Rotter, BA; Prelusky, DB; Pestka, JJ. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar]
- Pestka, JJ; Smolinski, AT. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B. Crit. Rev 2005, 8, 39–69. [Google Scholar]
- Etienne, M; Wache, Y. Biological and physiological effects of deoxynivalenol (DON) in the pig. In Mycotoxins in Farm Animals, 1st Ed; Oswald, IP, Taranu, I, Eds.; Research Signpost: Kerala, India, 2008; pp. 113–130. [Google Scholar]
- Prelusky, DB; Hartin, KE; Trenholm, HL; Miller, JD. Pharmacokinetic fate of 14C-labeled deoxynivalenol in swine. Fundam. Appl. Toxicol 1988, 10, 276–286. [Google Scholar]
- Eriksen, GS; Pettersson, H; Lindberg, JE. Absorption, metabolism and excretion of 3-acetyl DON in pigs. Arch. Tierernahr 2003, 57, 335–345. [Google Scholar]
- Cote, LM; Beasley, VR; Bratich, PM; Swanson, SP; Shivaprasad, HL; Buck, WB. Sex-related reduced weight gains in growing swine fed diets containing deoxynivalenol. J. Anim. Sci 1985, 61, 942–950. [Google Scholar]
- Bouhet, S; Oswald, IP. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol 2005, 108, 199–209. [Google Scholar]
- Maresca, M; Mahfoud, R; Garmy, N; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr 2002, 132, 2723–2731. [Google Scholar]
- Awad, WA; Razzazi-Fazeli, E; Bohm, J; Zentek, J. Influence of deoxynivalenol on the D-glucose transport across the isolated epithelium of different intestinal segments of laying hens. J. Anim. Physiol. Anim. Nutr. (Berl.) 2007, 91, 175–180. [Google Scholar]
- Awad, WA; Razzazi-Fazeli, E; Bohm, J; Zentek, J. Effects of B-trichothecenes on luminal glucose transport across the isolated jejunal epithelium of broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl.) 2008, 92, 225–230. [Google Scholar]
- Van De Walle, J; Romier, B; Larondelle, Y; Schneider, YJ. Influence of deoxynivalenol on NF-kappaB activation and IL-8 secretion in human intestinal Caco-2 cells. Toxicol. Lett 2008, 177, 205–214. [Google Scholar]
- Pinton, P; Accensi, F; Beauchamp, E; Cossalter, AM; Callu, P; Grosjean, F; Oswald, IP. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett 2008, 177, 215–222. [Google Scholar]
- Maresca, M; Yahi, N; Younes-Sakr, L; Boyron, M; Caporiccio, B; Fantini, J. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: Stimulation of interleukin-8 secretion, potentiation of interleukin-1beta effect and increase in the transepithelial passage of commensal bacteria. Toxicol. Appl. Pharmacol 2008, 228, 84–92. [Google Scholar]
- Kollarczik, B; Gareis, M; Hanelt, M. In vitro transformation of the Fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Nat. Toxins 1994, 2, 105–110. [Google Scholar]
- Eriksen, GS; Pettersson, H; Johnsen, K; Lindberg, JE. Transformation of trichothecenes in ileal digesta and faeces from pigs. Arch. Tierernahr 2002, 56, 263–274. [Google Scholar]
- He, P; Young, LG; Forsberg, C. Microbial transformation of deoxynivalenol (vomitoxin). Appl. Environ. Microbiol 1992, 58, 3857–3863. [Google Scholar]
- Niderkorn, V; Boudra, H; Morgavi, DP. Binding of Fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Microbiol 2006, 101, 849–856. [Google Scholar]
- Young, JC; Zhou, T; Yu, H; Zhu, H; Gong, J. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol 2007, 45, 136–143. [Google Scholar]
- Tenk, I; Fodor, E; Szathmary, C. The effect of pure Fusarium toxins (T-2, F-2, DAS) on the microflora of the gut and on plasma glucocorticoid levels in rat and swine. Zentralbl. Bakteriol. Mikrobiol. Hyg. [A] 1982, 252, 384–393. [Google Scholar]
- Tanguy, M; Burel, C; Pinton, P; Guerre, P; Grosjean, F; Queguiner, M; Cariolet, R; Tardieu, D; Rault, JC; Oswald, IP; Fravalo, P. Effets des fumonisines sur la santé du porc: sensibilité aux salmonelles et statut immunitaire. Journées Rech. Porcine 2006, 38, 393–398. [Google Scholar]
- Becker, B; Bresch, H; Schillinger, U; Thiel, PG. The effect of fumonisin B1 on the growth of bacteria. World J. Microbiol. Biotechnol 1997, 13, 539–543. [Google Scholar]
- Hopwood, DE; Pethick, DW; Hampson, DJ. Increasing the viscosity of the intestinal contents stimulates proliferation of enterotoxigenic Escherichia coli and Brachyspira pilosicoli in weaner pigs. Br. J. Nutr 2002, 88, 523–532. [Google Scholar]
- Pluske, JR; Kim, JC; Hansen, CF; Mullan, BP; Payne, HG; Hampson, DJ; Callesen, J; Wilson, RH. Piglet growth before and after weaning in relation to a qualitative estimate of solid (creep) feed intake during lactation: A pilot study. Arch. Anim. Nutr 2007, 61, 469–480. [Google Scholar]
- Sneath, PH; Sokal, RR. Numerical taxonomy. Nature 1962, 193, 855–860. [Google Scholar]
- Marteau, P. Factor controlling the bacterial microflora. In Gut microflora: digestive physiology and pathology, 1st Ed; Rambaud, JC, Buts, JP, Cortier, G, Flourié, B, Eds.; J. Libbey Eurotext, DL: Paris, France, 2006; pp. 37–55. [Google Scholar]
- Rotter, BA; Thompson, BK; Lessard, M; Trenholm, HL; Tryphonas, H. Influence of low-level exposure to Fusarium mycotoxins on selected immunological and hematological parameters in young swine. Fundam. Appl. Toxicol 1994, 23, 117–124. [Google Scholar]
- Alm, H; Brussow, KP; Torner, H; Vanselow, J; Tomek, W; Danicke, S; Tiemann, U. Influence of Fusarium-toxin contaminated feed on initial quality and meiotic competence of gilt oocytes. Reprod. Toxicol 2006, 22, 44–50. [Google Scholar]
- Pollmann, DS; Koch, BA; Seitz, LM; Mohr, HE; Kennedy, GA. Deoxynivalenol-contaminated wheat in swine diets. J. Anim. Sci 1985, 60, 239–247. [Google Scholar]
- Bergsjo, B; Langseth, W; Nafstad, I; Jansen, JH; Larsen, HJ. The effects of naturally deoxynivalenol-contaminated oats on the clinical condition, blood parameters, performance and carcass composition of growing pigs. Vet. Res. Commun 1993, 17, 283–294. [Google Scholar]
- Prelusky, DB; Gerdes, RG; Underhill, KL; Rotter, BA; Jui, PY; Trenholm, HL. Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat. Toxins 1994, 2, 97–104. [Google Scholar]
- Swanson, SP; Helaszek, C; Buck, WB; Rood, HD, Jr; Haschek, WM. The role of intestinal microflora in the metabolism of trichothecene mycotoxins. Food. Chem. Toxicol 1988, 26, 823–829. [Google Scholar]
- Tzortzis, G; Goulas, AK; Gee, JM; Gibson, GR. A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. J. Nutr 2005, 135, 1726–1731. [Google Scholar]
- Mai, V; Morris, JGJ. Colonic bacterial flora: changing understandings in the molecular age. J. Nutr 2003, 134, 459–464. [Google Scholar]
- Stombeck, DR; Guilford, WG. Small animal gastroenterology, 2nd Ed ed; Sonegate Publishing: Davis, CA, USA, 1990; pp. 224–241. [Google Scholar]
- Jensen, TK; Boye, M; Moller, K; Leser, TD; Jorsal, SE. Association of Serpulina hyodysenteriae with the colonic mucosa in experimental swine dysentery studied by fluorescent in situ hybridization. Apmis 1998, 106, 1061–1068. [Google Scholar]
- Zoetendal, EG; Akkermans, AD; De Vos, WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol 1998, 64, 3854–3859. [Google Scholar]
- Peu, P; Brugere, H; Pourcher, AM; Kerouredan, M; Godon, JJ; Delgenes, JP; Dabert, P. Dynamics of a pig slurry microbial community during anaerobic storage and management. Appl. Environ. Microbiol 2006, 72, 3578–3585. [Google Scholar]
- Tanguy, M; Pissavin, C; Queguiner, M; Cariolet, R; Le Diguerher, G; Burel, C; Fravalo, P. Utilisation de la PCR-SSCP capillaire pour l’etude de la flore digestive de groupes de porcs EOPS. Journées Rech. Porcine 2007, 39, 369–376. [Google Scholar]
- Mul, AJ; Perry, FG. The role of fructo-oligosaccharides in animal nutrition. In Recent advances in Animal Nutrition, 2nd Ed; Garnsworthy, PC, Cole, DJA, Eds.; Nottingham University Press: Nottingham, UK, 1994; pp. 57–79. [Google Scholar]
- Simpson, JM; McCracken, VJ; Gaskins, HR; Mackie, RI. Denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA amplicons to monitor changes in fecal bacterial populations of weaning pigs after introduction of Lactobacillus reuteri strain MM53. Appl. Environ. Microbiol 2000, 66, 4705–4714. [Google Scholar]
- Gelsomino, A; Keijzer-Wolters, AC; Cacco, G; van Elsas, JD. Assessment of bacterial community structure in soil by polymerase chain reaction and denaturing gradient gel electrophoresis. J. Microbiol. Meth 1999, 38, 1–15. [Google Scholar]
- Tiedje, JM. Opening the black box of soil microbial diversity. Applied Soil Ecology 1999, 13, 109–122. [Google Scholar]
- Baker, GC; Cowan, DA. 16 S rDNA primers and the unbiased assessment of thermophile diversity. Biochem. Soc. Trans 2004, 32, 218–221. [Google Scholar]
- Klappenbach, JA; Saxman, PR; Cole, JR; Schmidt, TM. rrndb: the Ribosomal RNA Operon Copy Number Database. Nucleic Acids Res 2001, 29, 181–184. [Google Scholar]
- Loisel, P; Harmand, J; Zemb, O; Latrille, E; Lobry, C; Delgenes, JP; Godon, JJ. Denaturing gradient electrophoresis (DGE) and single-strand conformation polymorphism (SSCP) molecular fingerprintings revisited by simulation and used as a tool to measure microbial diversity. Environ. Microbiol 2006, 8, 720–731. [Google Scholar]
- Pryde, SE; Richardson, AJ; Stewart, CS; Flint, HJ. Molecular analysis of the microbial diversity present in the colonic wall, colonic lumen, and cecal lumen of a pig. Appl. Environ. Microbiol 1999, 65, 5372–5377. [Google Scholar]
- Hong, H; Pruden, A; Reardon, KF. Comparison of CE-SSCP and DGGE for monitoring a complex microbial community remediating mine drainage. J. Microbiol. Methods 2007, 69, 52–64. [Google Scholar]
- INRA, A. Table de composition et de valeur nutritive des matieres premières destinées aux animaux d’elevage; INRA: Paris, France, 2002. [Google Scholar]
- Delbes, C; Moletta, R; Godon, J. Bacterial and archaeal 16S rDNA and 16S rRNA dynamics during an acetate crisis in an anaerobic digestor ecosystem. FEMS Microbiol. Ecol 2001, 35, 19–26. [Google Scholar]
- SAS. SAS OnLineDoc version 9.1; Cary: NC, USA, 2004. [Google Scholar]
- Laird, NM; Ware, JH. Random-effects models for longitudinal data. Biometrics 1982, 38, 963–974. [Google Scholar]
- Wolfinger, RD. An example of using mixed models and PROC MIXED for longitudinal data. J. Biopharm. Stat 1997, 7, 481–500. [Google Scholar]
- Littell, RC; Henry, PR; Ammerman, CB. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci 1998, 76, 1216–1231. [Google Scholar]
- Forno, E; Onderdonk, AB; McCracken, J; Litonjua, AA; Laskey, D; Delaney, ML; Dubois, AM; Gold, DR; Ryan, LM; Weiss, ST; Celedon, JC. Diversity of the gut microbiota and eczema in early life. Clin. Mol. Allergy 2008, 6, 11. [Google Scholar]
| Parameters | Experimental Group | Experimental period (in days, d)
| ||||
|---|---|---|---|---|---|---|
| d-7 to d0(1) | d0 to d7 | d7 to d14 | d14 to d21 | d21 to d28 | ||
| Daily Weight Gain
| Control | 0.93 ± 0.05 (2) | 0.96 ± 0.04 | 1.02 ± 0.04 | 1.15 ± 0.07 | 1.00 ± 0.12 |
| DON
| 0.85 ± 0.04
| 0.42 ± 0.06 *** | 0.96 ± 0.06
| 1.00 ± 0.07
| 1.07 ± 0.06
| |
| Feed intake
| Control | 1.67 ± 0.05 (3) | 2.09 ± 0.09 | 2.59 ± 0.04 | 2.85 ± 0.01 | 3.05 ± 0.04 |
| DON
| 1.55 ± 0.04
| 1.12 ± 0.02
| 2.77 ± 0.12
| 2.86 ± 0.01
| 2.78 ± 0.06
| |
| Feed conversion ratio
| Control | 1.79 ± 0.07 (4) | 2.18 ± 0.07 | 2.54 ± 0.09 | 2.47 ± 0.02 | 3.06 ± 0.58 |
| DON
| 1.84 ± 0.02
| 2.68 ± 0.46
| 2.87 ± 0.11
| 2.88 ± 0.37
| 2.60 ± 0.07
| |
| Bacteria | Experimental group | Sampling days (d)
| |||
|---|---|---|---|---|---|
| d0(1) | d7 | d14 | d28 | ||
| Aerobic Mesophilic Bacteria (AMB)
| Control | 5.24 ± 0.25 a | 7.68 ± 0.22 b | 7.37 ± 0.13 b | 6.53 ± 0.14 c |
| DON
| 5.27 ± 0.08 a | 7.37 ± 0.32 b | 7.71 ± 0.35 b | 7.22 ± 0.10 b | |
| Anaerobic Sulfite-Reducing bacteria (ASR)
| Control | 9.52 ± 0.05 a | 9.31 ± 0.15 a | 9.02 ± 0.17 a | 7.83 ± 0.16 b |
| DON
| 9.05 ± 0.39 a | 8.66 ± 0.38 a | 8.45 ± 0.22 a | 7.27 ± 0.09 b | |
| Index(2) | Experimental group | Sampling days (d)
| |||
|---|---|---|---|---|---|
| d0(1) | d7 | d14 | d28 | ||
| Dominance (S)
| Control | 0.93 ± 0.01 a | 0.92 ± 0.01 a | 0.94 ± 0.01 a | 0.93 ± 0.02 a |
| DON
| 0.92 ± 0.01 a | 0.93 ± 0.01 a | 0.94 ± 0.01 a | 0.95 ± 0.01 a | |
| Richness (R)
| Control | 0.86 ± 0.04 a | 0.83 ± 0.03 a | 0.70 ± 0.02 b* | 0.84 ± 0.04 a |
| DON
| 0.84 ± 0.03 a | 0.80 ± 0.04 a | 0.87 ± 0.03 a | 0.83 ± 0.03 a | |
| Peak | Experimental group | Sampling days (d)
| |||
|---|---|---|---|---|---|
| d0(1) | d7 | d14 | d28 | ||
| P23
| Control | 3.56 ± 0.99 a, c | 3.43 ± 2.28 a, b | 1.20 ± 0.46 b* | 5.12 ± 1.52 c |
| DON
| 1.65 ± 0.32 a | 3.96 ± 1.12 b | 6.26 ± 1.41 b | 5.80 ± 1.29 b | |
| P26
| Control | 30.80 ± 9.21 a | 35.34 ± 4.83 a | 7.33 ± 2.65 b | 27.44 ± 6.20 a * |
| DON
| 21.21 ± 5.28 a, b | 26.27 ± 4.05 a | 2.60 ± 0.49 b | 10.90 ± 2.71 c | |
| P32
| Control | 17.18 ± 4.43 a | 19.38 ± 2.14 a | 4.27 ± 2.55 b | 16.28 ± 3.78 a ** |
| DON
| 11.43 ± 2.62 a | 15.12 ± 2.35 a | 8.35 ± 2.67 a | 5.21 ± 1.86 a | |
| Diet
| ||
|---|---|---|
| Control | Contaminated | |
| Energy | ||
| Net energy (MJ.kg–1) | 9.6 | 9.6 |
| Composition (%) | ||
| CleanWheat | 65 | 0 |
| Contaminated wheat | 0 | 65 |
| Soybean meal | 19.5 | 19.5 |
| Barley | 8.5 | 8.5 |
| Lucerne | 3 | 3 |
| Calcium phosphorus | 1.25 | 1.25 |
| Clay | 1 | 1 |
| Iodised salt | 0.3 | 0.3 |
| Limestone | 0.2 | 0.2 |
| Additives | 0.08 | 0.08 |
| Vitamins and mineral mixture1 | 1.07
| 1.07
|
| Nutrient component (%) | ||
| Dry matter | 87.0 | 87.2 |
| Mineral matter | 6.2 | 6.1 |
| Brut organic matter | 80.9 | 81.1 |
| Crude fat | 3.1 | 3.2 |
| Nitrogen extract | 18.2 | 18.3 |
| Cellulose | 2.8 | 3.2 |
| Amidon
| 43.4
| 42.7
|
| Mycotoxin (μg.kg–1)(2) | ||
| Deoxynivalenol (DON) | 65 | 2700 |
| 15-acetyl DON | nd (3) | 20 |
| Nivalenol | nd | 75 |
| Zearalenone | nd | 275 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Waché, Y.J.; Valat, C.; Postollec, G.; Bougeard, S.; Burel, C.; Oswald, I.P.; Fravalo, P. Impact of Deoxynivalenol on the Intestinal Microflora of Pigs. Int. J. Mol. Sci. 2009, 10, 1-17. https://doi.org/10.3390/ijms10010001
Waché YJ, Valat C, Postollec G, Bougeard S, Burel C, Oswald IP, Fravalo P. Impact of Deoxynivalenol on the Intestinal Microflora of Pigs. International Journal of Molecular Sciences. 2009; 10(1):1-17. https://doi.org/10.3390/ijms10010001
Chicago/Turabian StyleWaché, Yann J., Charlotte Valat, Gilbert Postollec, Stephanie Bougeard, Christine Burel, Isabelle P. Oswald, and Philippe Fravalo. 2009. "Impact of Deoxynivalenol on the Intestinal Microflora of Pigs" International Journal of Molecular Sciences 10, no. 1: 1-17. https://doi.org/10.3390/ijms10010001
APA StyleWaché, Y. J., Valat, C., Postollec, G., Bougeard, S., Burel, C., Oswald, I. P., & Fravalo, P. (2009). Impact of Deoxynivalenol on the Intestinal Microflora of Pigs. International Journal of Molecular Sciences, 10(1), 1-17. https://doi.org/10.3390/ijms10010001





