Changes in the Polyphenolic Profile and Antioxidant Activity of Wheat Bread after Incorporating Quinoa Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical, Reagents, and Quinoa Samples
2.2. Bread-Making Process
2.3. Extractable and Hydrolyzable Polyphenols Fraction Extraction
2.4. UPLC-MS/MS Analysis
2.5. Antioxidant Activity Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. Quantification of the EPF and HPF Phenolic Compounds in Raw Materials
3.2. Quantification of the EPF and HPF Phenolic Compounds in Bread
3.3. Impact of the Bread-Making Process on Phenolic Content and Individual Compounds
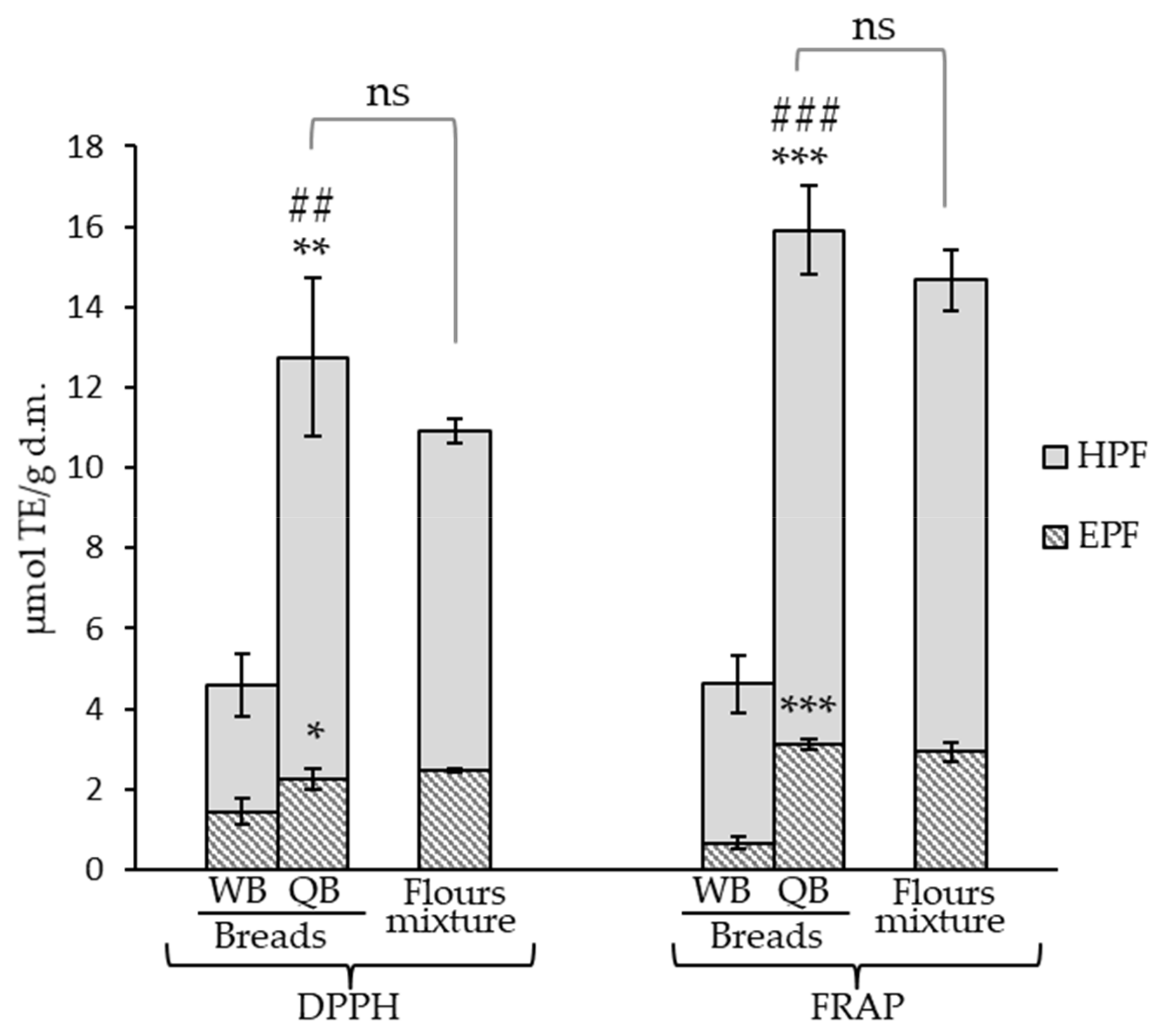
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals--just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Langhans, W. Food components in health promotion and disease prevention. J. Agric. Food Chem. 2018, 66, 2287–2294. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewettinck, K.; Van Bockstaele, F.; Kühne, B.; Van De Walle, D.; Courtens, T.M.; Dellynck, X. Review: Nutritional value of bread: Influence of processing, food interaction and consumer perception. J. Cereal Sci. 2008, 10, 1–15. [Google Scholar] [CrossRef]
- Dziki, D.; Rozylo, R.; Gawlik-Dziki, U.; Swieca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767–1600782. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Simnadis, T.G.; Tapsell, L.C.; Beck, E.J. Physiological effects associated with quinoa consumption and implications for research involving humans: A review. Plant Foods Hum. Nutr. 2015, 70, 238–249. [Google Scholar] [CrossRef]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeón, M.E.; Raskin, I. Innovations in health value and functional food development of quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, F. Formulation and quality attributes of quinoa food products. Food Bioprocess Technol. 2016, 9, 49–68. [Google Scholar] [CrossRef]
- Iglesias-Puig, E.; Monedero, V.; Haros, M. Bread with whole quinoa flour and bifidobacterial phytases increases dietary mineral intake and bioavailability. LWT Food Sci. Technol. 2015, 60, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Ballester-Sánchez, J.; Millán-Linares, M.C.; Fernández-Espinar, M.T.; Haros, C.M. Development of healthy, nutritious bakery products by incorporation of quinoa. Foods 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballester-Sánchez, J.; Yalcin, E.; Fernández-Espinar, M.T.; Haros, M. Rheological and thermal properties of royal quinoa and wheat flour blends for breadmaking. Eur. Food Res. Technol. 2019, 245, 1571–1582. [Google Scholar] [CrossRef]
- Álvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Brend, Y.; Galili, L.; Badani, H.; Hovav, R.; Galili, S. Total phenolic content and antioxidant activity of red and yellow quinoa (Chenopodium quinoa Willd.) seeds as affected by baking and cooking conditions. Food Nutr. Sci. 2012, 3, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Chlopicka, J.; Pasko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT Food Sci. Technol. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Ballester-Sánchez, J.; Gil, J.V.; Haros, C.M.; Fernández-Espinar, M.T. Effect of incorporating white, red or black quinoa flours on free and bound polyphenol content, antioxidant activity and colour of bread. Plant Foods Hum. Nutr. 2019, 74, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of food antioxidants in modulating gut microbial communities: Novel understandings in intestinal oxidative stress damage and their impact on host health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Contreras, M.d.M.; Xu, D.; Xing, C.; Wang, L.; Yang, D. Different distribution of free and bound phenolic compounds affects the oxidative stability of tea seed oil: A novel perspective on lipid antioxidation. LWT 2020, 129, 109389. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Macromolecular antioxidants or non-extractable polyphenols in fruit and vegetables: Intake in four European countries. Food Res. Int. 2015, 74, 315–325. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power: The FRAP assay”. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Huang, F.; Xue, K.; Liu, L. Characterization of saponins and phenolic compounds: Antioxidant activity and inhibitory effects on α-glucosidase in different varieties of colored quinoa (Chenopodium quinoa Willd). Biosci. Biotechnol. Biochem. 2019, 83, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Stikić, R.I.; Milinčić, D.D.; Kostić, A.Ž.; Jovanović, Z.B.; Gašić, U.M.; Tešić, Ž.L.; Djordjević, N.Z.; Savić, S.K.; Czekus, B.G.; Pešić, M.B. Polyphenolic profiles, antioxidant, and in vitro anticancer activities of the seeds of Puno and Titicaca quinoa cultivars. Cereal Chem. 2020, 97, 626–633. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Biondi, S.; Mandrioli, R.; Marincich, L.; Ruiz, K.B. Free and conjugated phenolic profiles and antioxidant activity in quinoa seeds and their relationship with genotype and environment. Plants 2021, 10, 1046. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. Genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, P.; Bomzan, D.P.; Rao, B.S.; Sreerama, Y.N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2016, 199, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutíerrez, A.; Caboni, M.F. Simultaneous determination of phenolic compounds and saponins in Quinoa (Chenopodium quinoa Willd) by a liquid chromatography-diode array detection-electrospray ionization-time of flight mass spectrometry methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F.; Marconi, E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. J. Agric. Food Chem. 2012, 60, 4620–4627. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Xihong, L.; Chen, P.X.; Zhang, H.; Ronghua, L.; Tsao, R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food. Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Kurtys, E.; Eisel, U.L.M.; Hageman, R.J.J.; Verkuyl, J.M.; Broersen, L.M.; Dierckx, R.; de Vries, E.F.J. Anti-inflammatory effects of rice bran components. Nutr. Rev. 2018, 76, 372–379. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Pandi, A.; Kalappan, V.M. Pharmacological and therapeutic applications of sinapic acid—An updated review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Kim, D.K.; Jeon, H.; Cha, D.S. 4-Hydroxybenzoic acid-mediated lifespan extension in Caenorhabditis elegans. J. Funct. Foods 2014, 7, 630–640. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Leváková, L.; Lacko-Bartošová, M. Phenolic acids and antioxidant activity of wheat species: A review. Agriculture 2017, 63, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.F.; Wong, W.T. Design and optimization of quercetin-based functional foods. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Alazzouni, A.S.; Dkhil, M.A.; Gadelmawla, M.H.A.; Gabri, M.S.; Farag, A.H.; Hassan, B.N. Ferulic acid as anticarcinogenic agent against 1,2-dimethylhydrazine induced colon cancer in rats. J. King Saud Univ. Sci. 2021, 33, 101354. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J. Food Sci. 2011, 76, H7–H10. [Google Scholar] [CrossRef]
- Sung, J.-H.; Gim, S.-A.; Koh, P.-O. Ferulic acid attenuates the cerebral ischemic injury-induced decrease in peroxiredoxin-2 and thioredoxin expression. Neurosci. Lett. 2014, 30, 88–92. [Google Scholar] [CrossRef]
- Russo, G.I.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary consumption of phenolic acids and prostate cancer: A case-control study in Sicily, southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; Galván D′Alessandro, L.; Manrique, G.D. Effect of roasting conditions on the antioxidant compounds of quinoa seeds. Int. J. Food Sci. Technol. 2016, 51, 1018–1025. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Deng, J.; Ouyang, D.; Wang, D.; Liang, Y.; Chen, Y.; Sun, Y. Effect of thermal processing on free and bound phenolic compounds and antioxidant activities of hawthorn. Food Chem. 2020, 332, 127429. [Google Scholar] [CrossRef]
- Duodu, G. Effects of processing on phenolic phytochemicals in cereals and legumes. Cereal Foods World 2014, 59, 64–70. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Vogrincic, M.; Timoracka, M.; Melichacova, S.; Vollmannova, A.; Kreft, I. Degradation of rutin and polyphenols during the preparation of tartary buckwheat bread. J. Agric. Food Chem. 2010, 58, 4883–4887. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Polyphenol composition and “in vitro” antiradical activity of single and multigrain breads. J. Cereal Sci. 2011, 53, 90–96. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Sivam, A.S.; Cooney, J.; Zhou, J.; Perera, C.O.; Waterhouse, G.I.N. Effects of added fruit polyphenols and pectin on the properties of finished breads revealed by HPLC/LC-MS and Size-Exclusion HPLC. Food Res. Int 2011, 44, 3047–3056. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Martins, N.; Barros, L. Phenolic compounds and its bioavailability: In vitro bioactive compounds or health promoters. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Elsevier Inc.: Cambridge, MA, USA, 2017; Volume 82, pp. 1–44. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Swieca, M.; Seczyk, T.; Rozylo, R.; Szymanowska, U. Bread enriched with Chenopodium quinoa leaves powder—The procedures for assessing the fortification efficiency. LWT Food Sci. Technol. 2015, 62, 1226–1234. [Google Scholar] [CrossRef]
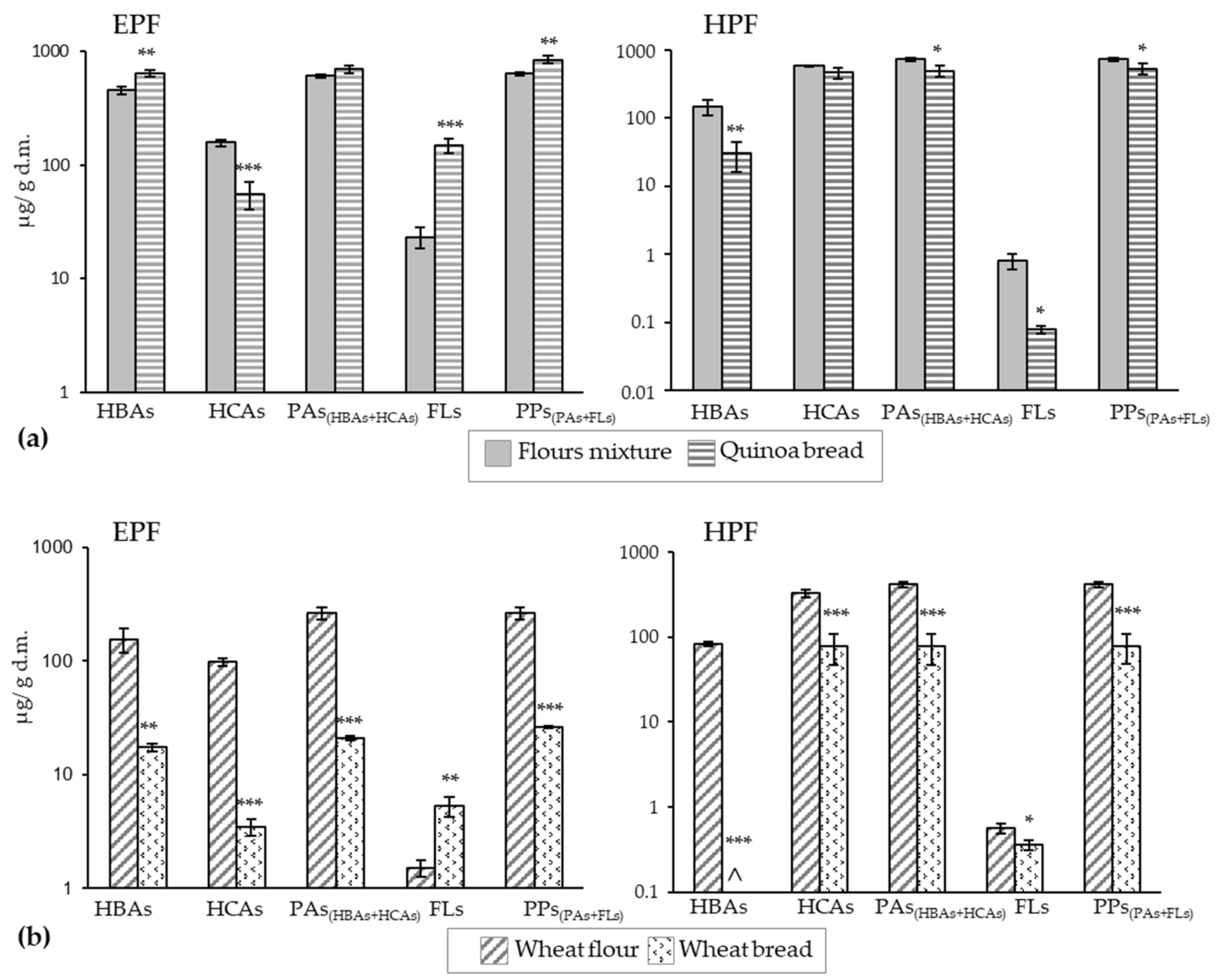
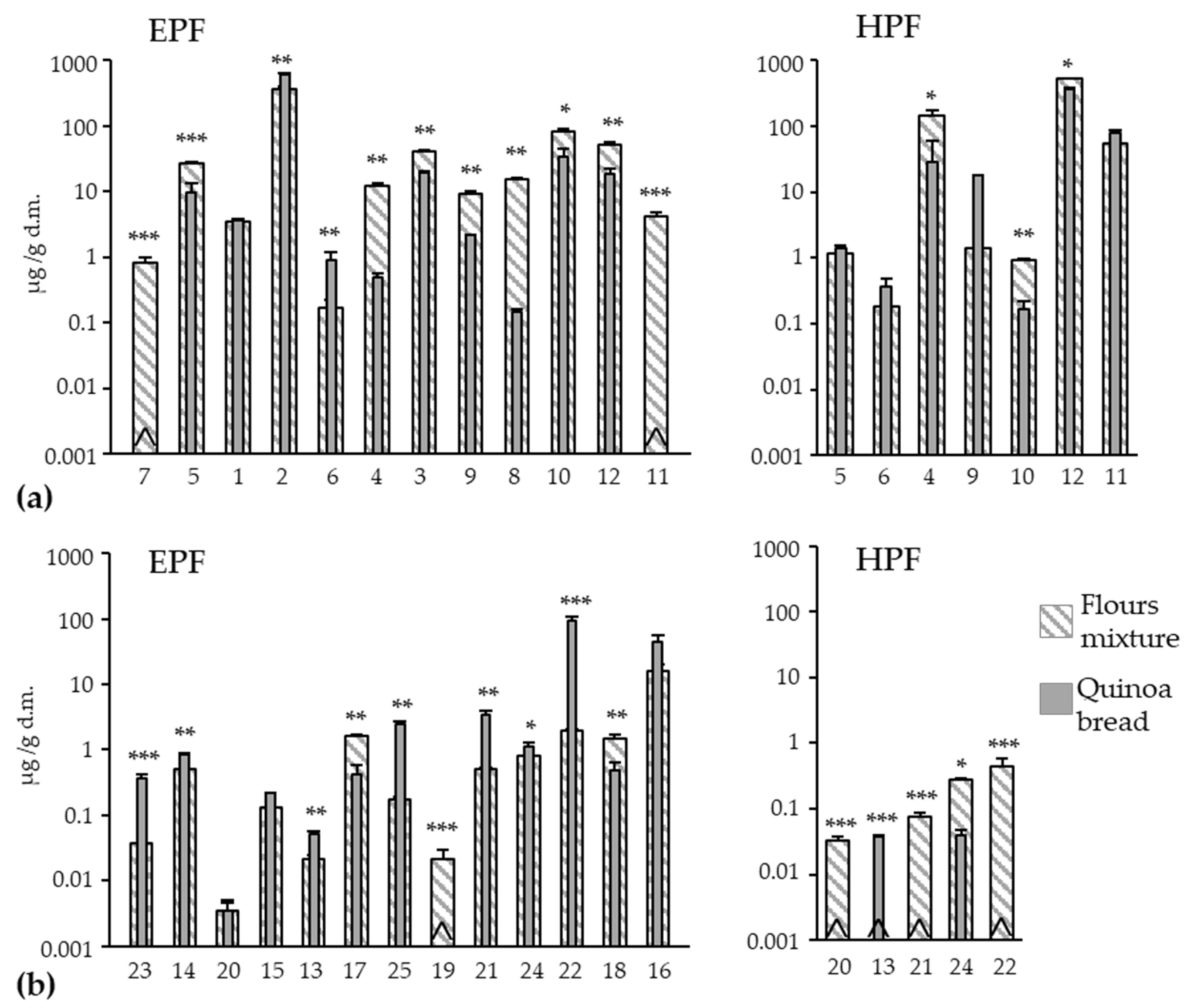
| Peak No. | Name | Molecular Formula | Ms [M−H]− (m/z) | Rt (min) | MS Fragments | Type of Extract (Sample) |
|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | ||||||
| 1 | Gallic acid | C7H6O5 | 169.01 | 2.06 | 123, 106 | EPF (WF, QF, WB, QB) |
| 2 | p-HBA a | C7H6O3 | 137.02 | 3.62 | 121 | EPF (WF, QF, WB, QB) |
| 3 | Vanillic acid | C8H8O4 | 167.03 | 4.00 | 151, 122 107 | EPF (WF, QF, WB, QB) |
| 4 | Syringic acid | C9H10O5 | 197.04 | 4.07 | 181, 166, 122 | EPF (WF, QF, WB, QB); HPF (WF, QF, ---, QB) |
| 5 | 2,4-DHBA a | C7H6O4 | 153.01 | 4.13 | 137, 108 | EPF (WF, QF, WB, QB); HPF (WF, QF, ---, QB) |
| 6 | Rosmarinic acid | C18H16O8 | 359.08 | 5.51 | 197, 179, 161 | EPF (WF, QF, WB, QB); HPF (---, QF, ---, QB) |
| 7 | Benzoic acid | C7H6O2 | 121.03 | 5.83 | 77 | EPF (WF, ---, ---, ---) |
| Hydroxycinnamic acids | ||||||
| 8 | Chlorogenic acid | C16H18O9 | 353.09 | 3.34 | 191, 179 | EPF (WF, ---, WB, QB) |
| 9 | Caffeic acid | C9H8O4 | 179.03 | 3.98 | 135, 118, 107 | EPF (WF, QF, WB, QB); HPF (WF, ---, WB, QB) |
| 10 | p-Coumaric acid | C9H8O3 | 163.04 | 4.85 | 118, 96, 92 | EPF (WF, QF, ---, QB); HPF (WF, QF, WB, QB) |
| 11 | Sinapic acid | C11H12O5 | 223.06 | 5.02 | 207, 193, 149 | EPF (WF, QF, ---, ---); HPF (---, QF, WB, QB) |
| 12 | Ferulic acid | C10H10O4 | 193.05 | 5.08 | 178, 133, 116 | EPF (WF, QF, ---, QB); HPF (WF, QF, WB, QB) |
| Flavonoids | ||||||
| 13 | Epigallocatechin | C15H14O7 | 305.07 | 3.20 | 287, 179, 121 | EPF (WF, QF, WB, QB); HPF (---, ---, ---, QB) |
| 14 | Catechin | C15H14O6 | 289.07 | 3.55 | 257, 203, 123 | EPF (WF, QF, WB, QB) |
| 15 | Epicatechin | C15H14O6 | 289.07 | 3.97 | 242, 203, 179 | EPF (WF, QF, ---, QB) |
| 16 | Rutin | C27H30O16 | 609.15 | 4.51 | 300, 271, 255 | EPF (WF, QF, ---, QB) |
| 17 | Hyperoside | C21H20O12 | 463.08 | 4.71 | 301 | EPF (---, QF, ---, QB) |
| 18 | Q3G a | C21H20O12 | 463.08 | 4.75 | 301 | EPF (---, QF, ---, QB) |
| 19 | K3G a | C21H20O11 | 447.09 | 5.17 | 311, 285, 255 | EPF (WF, ---, ---, ---) |
| 20 | Daidzein | C15H10O4 | 253.05 | 6.44 | 224, 192, 132 | EPF (WF, QF, WB, QB); HPF (WF, ---, ---, ---) |
| 21 | Luteolin | C15H10O6 | 285.04 | 6.70 | 203, 175, 151 | EPF (WF, QF, WB, QB); HPF (---, QF, ---, ---) |
| 22 | Quercetin | C15H10O7 | 301.03 | 6.77 | 255, 239 | EPF (WF, QF, WB, QB); HPF (---, QF, ---, ---) |
| 23 | Apigenin | C15H10O5 | 269.05 | 7.50 | 209, 151, 117 | EPF (WF, QF, WB, QB) |
| 24 | Naringenin | C15H12O5 | 271.06 | 7.52 | 177, 151, 119 | EPF (WF, QF, WB, QB); HPF (WF, QF, WB, QB) |
| 25 | Kaempferol | C15H10O6 | 285.04 | 7.67 | 203, 185, 151 | EPF (---, QF, WB, QB); HPF (---, ---, WB, ---) |
| Compound | FLOURS | BREAD | ||||||
|---|---|---|---|---|---|---|---|---|
| EPF | HPF | EPF | HPF | |||||
| Wheat | Quinoa | Wheat | Quinoa | Wheat | Quinoa | Wheat | Quinoa | |
| HYDROXYBENZOIC ACIDS | ||||||||
| Benzoic | 1.10 ± 0.21 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2,4-DHBA | 31.8 ± 2.1 | 13.8 ± 1.9 ** | 0.71 ± 0.21 | 1.31 ± 0.15 | 2.06 ± 0.89 | 9.56 ± 3.6 | n.d. | 1.43 ± 0.05 |
| Gallic | 3.49 ± 0.65 | 3.18 ± 0.71 | n.d. | n.d. | 1.58 ± 0.05 | 3.62 ± 0.23 * | n.d. | n.d. |
| p-HBA | 49.1 ± 7.8 | 1282 ± 56 *** | n.d. | n.d. | 12.3 ± 2.0 | 611 ± 38 *** | n.d. | n.d. |
| Rosmarinic | 0.04 ± 0.00 | 0.56 ± 0.20 | n.d. | 0.74 ± 0.51 | 0.18 ± 0.07 | 0.99 ± 0.25 * | n.d. | 0.36 ± 0.27 |
| Syringic | 16.0 ± 1.8 | 0.09 ± 0.04 ** | 82.24 ± 5.6 | 168 ± 43 * | 0.33 ± 0.04 | 0.50 ± 0.07 | n.d. | 28 ± 14 |
| Vanillic | 45.7 ± 2.2 | 25.4 ± 3.3 ** | n.d. | n.d. | 1.25 ± 0.25 | 19.1 ± 1.7 *** | n.d. | n.d. |
| Sum of HBAs a | 147.2 ± 8.5 | 1325 ± 56 *** | 82.9 ±5.6 | 170 ± 43 * | 17.7 ± 2.2 | 645 ± 38 *** | 30 ± 14 | |
| HYDROXYCINNAMIC ACIDS | ||||||||
| Caffeic | 11.8 ± 1.1 | 2.17 ± 0.20 *** | 1.90 ± 0.01 | n.d. | 0.37 ± 0.10 | 2.17 ± 0.06 *** | 24.2 ± 9.2 | 18.1 ± 7.2 |
| Chlorogenic | 20.9 ± 1.0 | n.d. | n.d. | n.d. | 3.01 ± 0.37 | 0.15 ± 0.01 ** | n.d. | n.d. |
| p-Coumaric | 2.1 ± 0.4 | 322 ± 36 *** | 1.66 ± 0.31 | 0.70 ± 0.09 * | n.d. | 35 ± 11 | 0.34 ± 0.13 | 0.25 ± 0.05 |
| Ferulic | 57.6 ± 5.6 | 35.5 ± 8.3 * | 324 ± 34 | 587 ± 32 * | n.d. | 18.8 ± 3.7 | 48 ± 17 | 367 ± 49 ** |
| Sinapic | 5.58 ± 0.95 | 0.09 ± 0.03 ** | n.d. | 223 ± 46 | n.d. | n.d. | 5.8 ± 2.2 | 78 ± 15 * |
| Sum of HCAs a | 98.0 ± 5.9 | 359 ± 37 ** | 328 ± 35 | 811 ± 56 *** | 3.38 ± 0.38 | 56 ± 12 ** | 78 ± 21 | 463 ± 52 ** |
| Sum of Pas a | 245 ± 10 | 1685 ± 67 *** | 411 ± 35 | 982 ± 71 *** | 21.1 ± 2.2 | 700 ± 40 *** | 78 ± 20A | 493 ± 54 ** |
| Compound | FLOURS | BREAD | ||||||
|---|---|---|---|---|---|---|---|---|
| EPF | HPF | EPF | HPF | |||||
| Wheat | Quinoa | Wheat | Quinoa | Wheat | Quinoa | Wheat | Quinoa | |
| Apigenin | 0.019 ± 0.003 | 0.095 ± 0.001 *** | n.d. | n.d. | 0.87 ± 0.12 | 0.367 ± 0.046 ** | n.d. | n.d. |
| Catechin | 0.594 ± 0.014 | 0.240 ± 0.045 *** | n.d. | n.d. | 0.0344 ± 0.016 | 0.833 ± 0.050 *** | n.d. | n.d. |
| Daidzein | 0.004 ± 0.001 | 0.001 ±0.000 | 0.04 ± 0.005 | n.d. | 0.005 ± 0.001 | 0.004 ± 0.002 | n.d. | n.d. |
| Epicatechin | 0.051 ± 0.032 | 0.38 ± 0.12 | n.d. | n.d. | n.d. | 0.220 ± 0.003 | n.d. | n.d. |
| EGC a | 0.013 ± 0.009 | 0.045 ± 0.010 | n.d. | n.d. | 0.065 ± 0.017 | 0.052 ± 0.005 | n.d. | 0.038 ± 0.002 |
| Hyperoside | n.d. | 6.60 ± 0.34 | n.d. | n.d. | n.d. | 0.43 ± 0.15 | n.d. | n.d. |
| Kaempferol | n.d. | 0.704 ± 0.063 | n.d. | n.d. | 0.189 ± 0.032 | 2.471 ± 0.28 *** | 0.267 ± 0.044 | n.d. |
| K3G a | 0.134 ± 0.021 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Luteolin | 0.093 ± 0.001 | 1.757 ± 0.089 *** | n.d. | 0.310 ± 0.040 | 2.60 ± 0.52 | 3.46 ± 0.56 | n.d. | n.d. |
| Naringenin | 0.053 ± 0.007 | 3.095 ± 0.036 *** | 0.264 ± 0.027 | 0.275 ± 0.024 | 0.065 ± 0.008 | 1.15 ± 0.12 *** | 0.094 ± 0.000 | 0.040 ± 0.009 * |
| Quercetin | 0.034 ± 0.007 | 7.69 ± 0.54 *** | n.d. | 1.42 ± 0.17 | 1.48 ± 0.31 | 95 ± 13 *** | n.d. | n.d. |
| Q3G a | n.d. | 6.01 ± 0.69 | n.d. | n.d. | n.d. | 0.48 ±0.17 | n.d. | n.d. |
| Rutin | 0.67 ± 0.21 | 62 ± 16 ** | n.d. | n.d. | n.d. | 45 ± 14 | n.d. | n.d. |
| Sum of FLs a | 1.55 ± 0.21 | 89 ± 16 ** | 0.31 ± 0.03 | 1.99 ± 0.18 ** | 5.30 ± 0.62 | 149 ± 18 ** | 0.360 ± 0.040 | 0.080 ± 0.010 *** |
| Sum of PPs a | 247 ± 10 | 1774 ± 69 *** | 411 ± 35 | 984 ± 71 *** | 26.4 ± 2.3 | 850 ± 44 *** | 79 ± 21 | 493 ± 54 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, J.V.; Esteban-Muñoz, A.; Fernández-Espinar, M.T. Changes in the Polyphenolic Profile and Antioxidant Activity of Wheat Bread after Incorporating Quinoa Flour. Antioxidants 2022, 11, 33. https://doi.org/10.3390/antiox11010033
Gil JV, Esteban-Muñoz A, Fernández-Espinar MT. Changes in the Polyphenolic Profile and Antioxidant Activity of Wheat Bread after Incorporating Quinoa Flour. Antioxidants. 2022; 11(1):33. https://doi.org/10.3390/antiox11010033
Chicago/Turabian StyleGil, José Vicente, Adelaida Esteban-Muñoz, and María Teresa Fernández-Espinar. 2022. "Changes in the Polyphenolic Profile and Antioxidant Activity of Wheat Bread after Incorporating Quinoa Flour" Antioxidants 11, no. 1: 33. https://doi.org/10.3390/antiox11010033






