Influence of the Mixture of Carrageenan Oligosaccharides and Egg White Protein on the Gelation Properties of Culter alburnus Myofibrillar Protein under Repeated Freezing–Thawing Cycles
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Methods
2.2.1. Preparation for the Mixture of Carrageenan Oligosaccharide and Egg White Protein (CGO/EWP)
2.2.2. Extraction of Myofibrillar Protein and Preparation of Samples
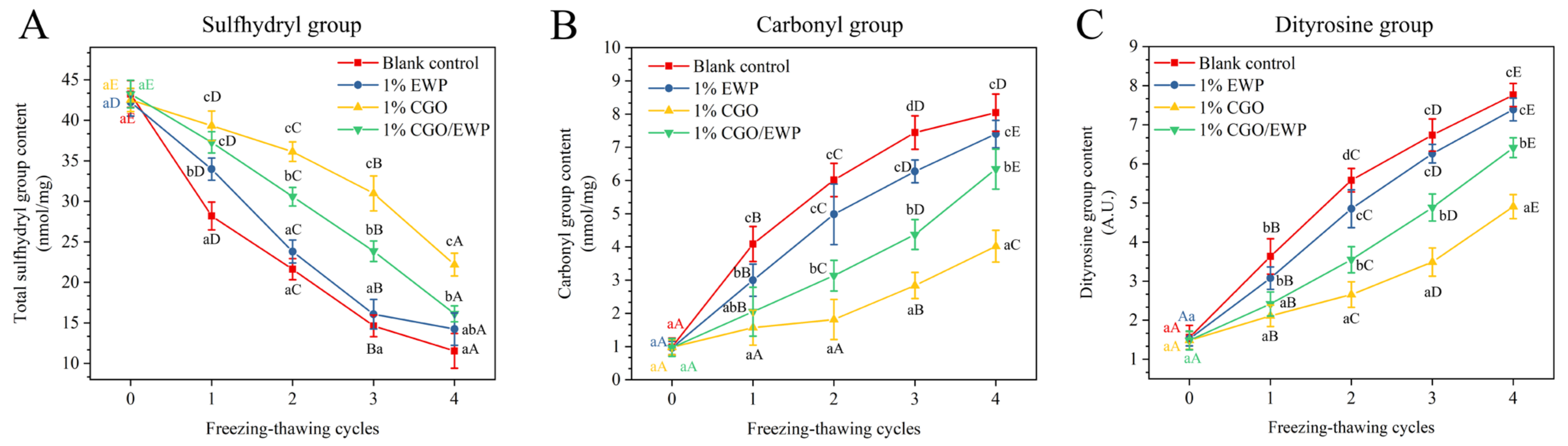
2.2.3. Sulfhydryl Group Content
2.2.4. Carbonyl Group Content
2.2.5. Dityrosine Content
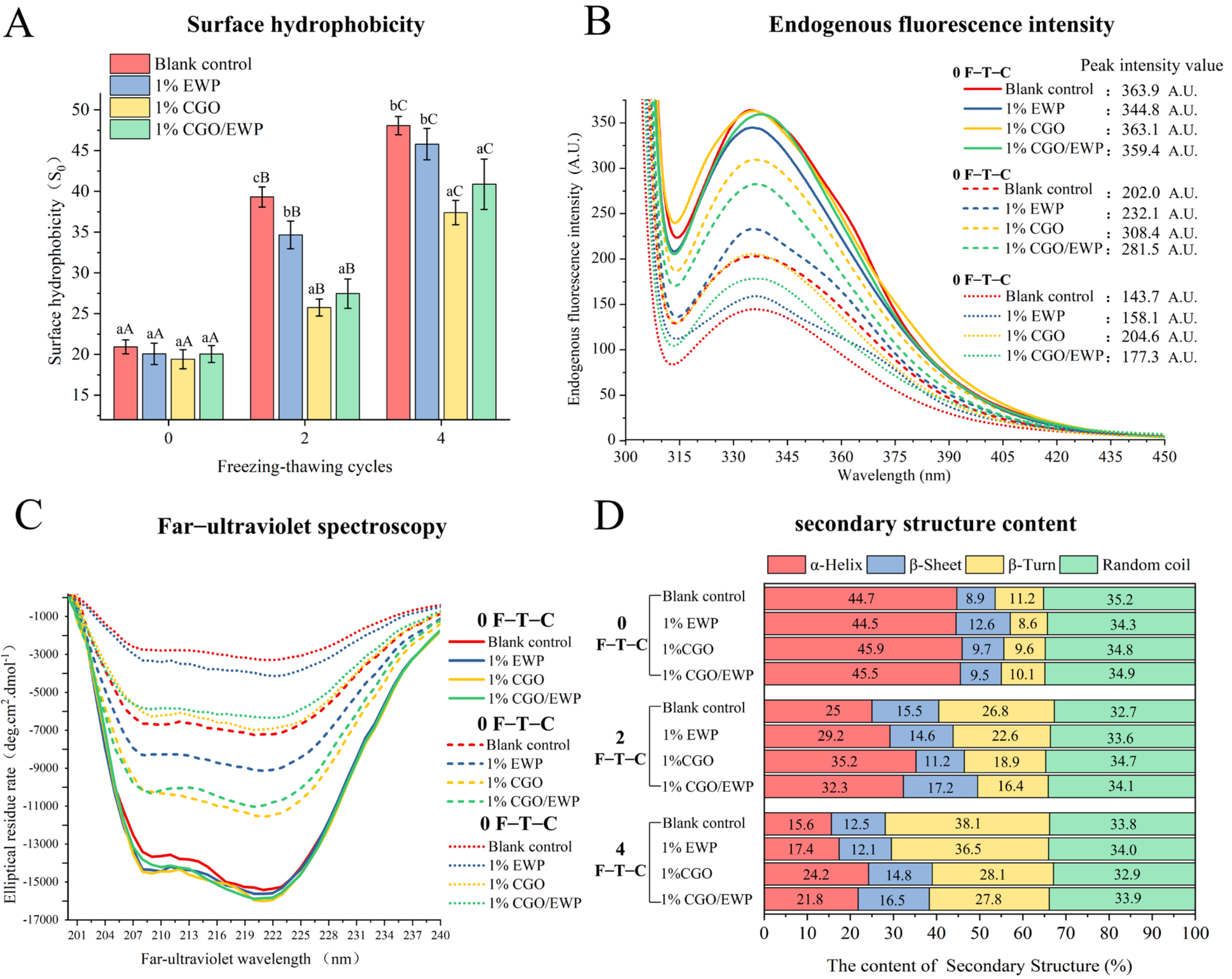
2.2.6. Surface Hydrophobicity (S0)
2.2.7. Endogenous Fluorescence Intensity
2.2.8. Circular Dichroism
2.2.9. Preparation of Heat-Induced MP Gel
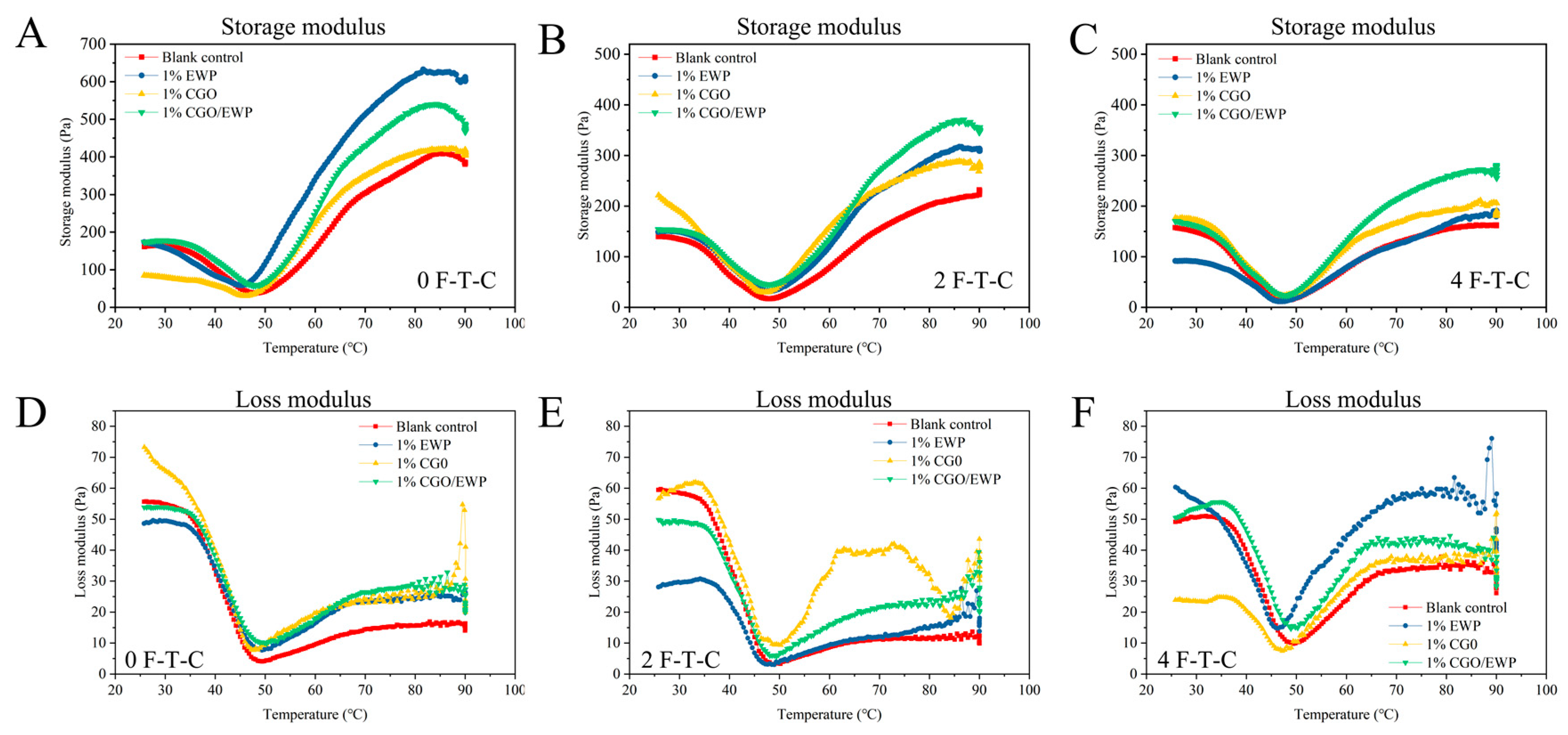
2.2.10. Dynamic Rheological Analysis
2.2.11. Gel Strength
2.2.12. Water-Holding Capacity
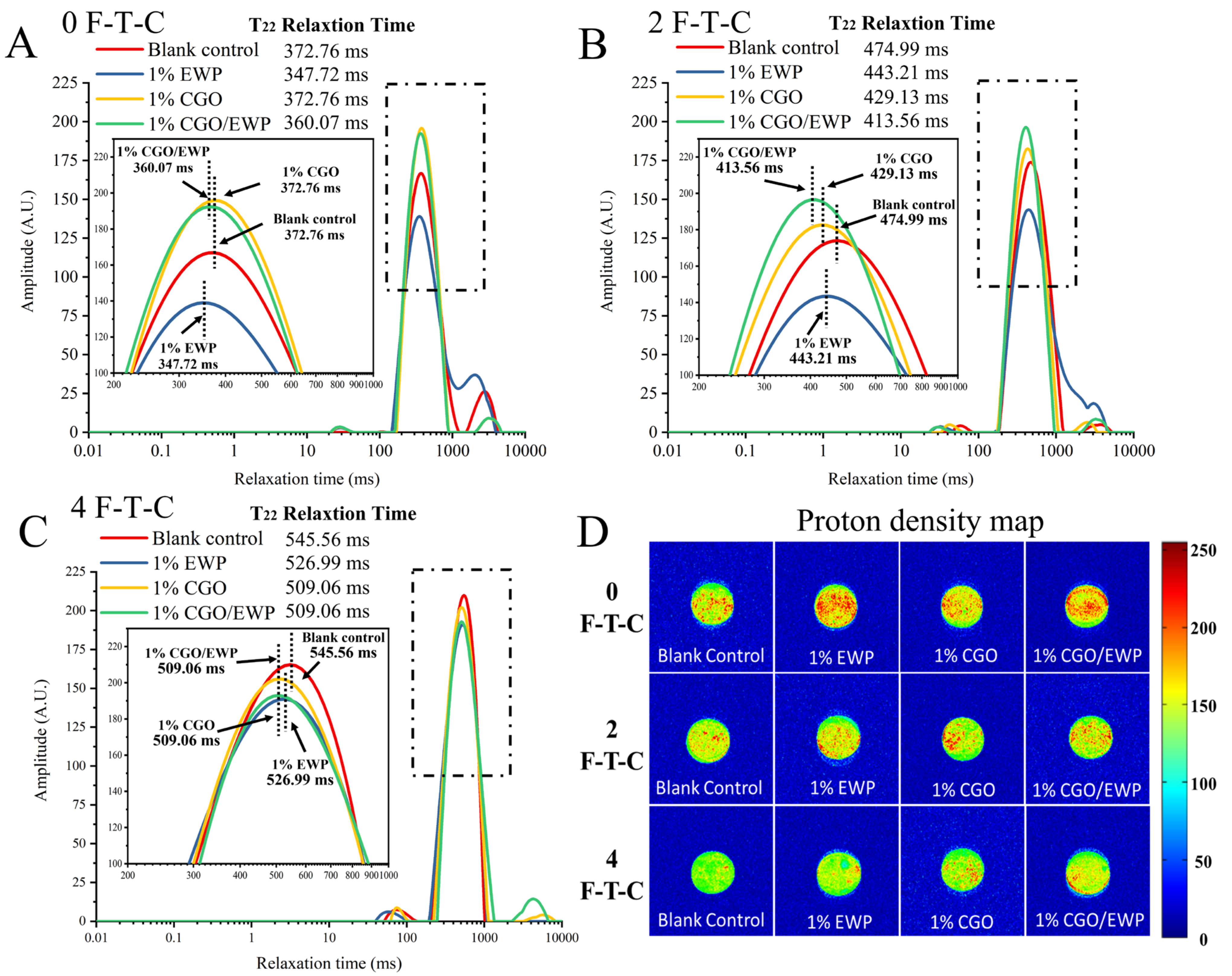
2.2.13. T2 Relaxation Time
2.2.14. Proton Density Image
2.2.15. MP Gel Selective Solubility
2.2.16. Statistical Analysis
3. Results and Discussion
3.1. Functional Group
3.2. Surface Hydrophobicity (S0)
3.3. Endogenous Fluorescence Intensity
3.4. Circular Dichroism
3.5. Rheological Properties
3.6. Gel Strength and Water Holding Capacity in MP Gel
3.7. T2 Relaxation Time and Proton Density Map in MP Gel
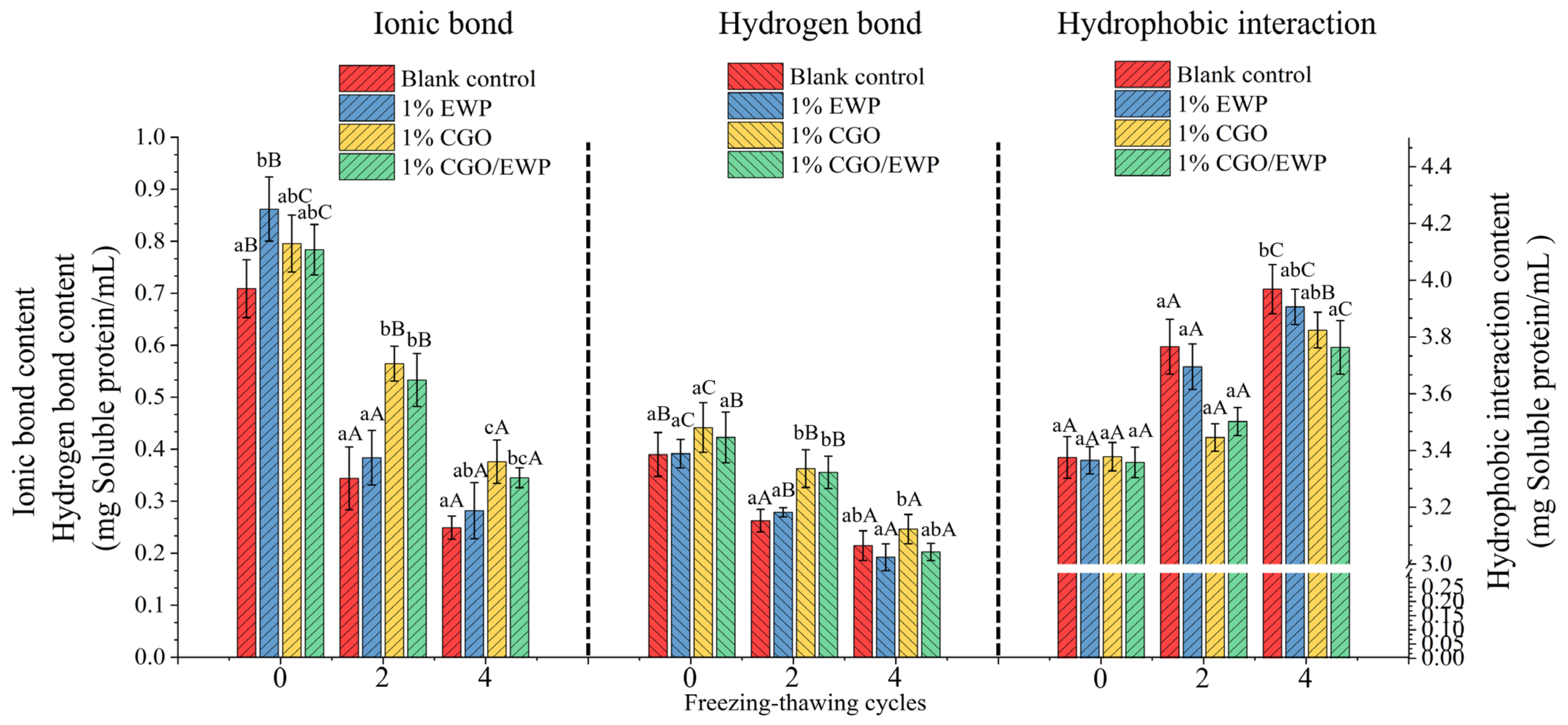
3.8. Selective Solubility in MP Gel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, X.; Li, H.; Nuerjiang, M.; Rui, L.; Kong, B.; Xia, X.; Shao, M. Influence of repeated freeze-thaw treatments on the functional and structural properties of myofibrillar protein from mirror carp (Cyprinus carpio L). Food Biophys. 2021, 16, 492–501. [Google Scholar] [CrossRef]
- Weiqing, L.; Yanan, Z.; Xiaoyu, H.; Xi, Z.; Jing, X. Effects of carrageenan oligosaccharide on lipid, protein oxidative changes, and moisture migration of Litopenaeus vannamei during freeze-thaw cycles. J. Food Process. Preserv. 2020, 44, e14675. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, L.; Walayat, N.; Xiong, H. Effects of oxidative modification on the functional, conformational and gelling properties of myofibrillar proteins from Culter alburnus. Int. J. Biol. Macromol. 2020, 162, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Walayat, N.; Wang, X.; Liu, J.; Nawaz, A.; Zhang, Z.; Khalifa, I.; Rincon Cervera, M.A.; Pateiro, M.; Lorenzo, J.M.; Nikoo, M.; et al. Kappa-carrageenan as an effective cryoprotectant on water mobility and functional properties of grass carp myofibrillar protein gel during frozen storage. Lwt Food Sci. Technol. 2022, 154, 112675. [Google Scholar] [CrossRef]
- Walayat, N.; Wang, X.; Nawaz, A.; Zhang, Z.; Abdullah, A.; Khalifa, I.; Saleem, M.H.; Mushtaq, B.S.; Pateiro, M.; Lorenzo, J.M.; et al. ovalbumin and kappa-carrageenan mixture suppresses the oxidative and structural changes in the myofibrillar proteins of Grass Carp (Ctenopharyngodon idella) during frozen storage. Antioxidants 2021, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, L.; Chen, C.; Xiong, G.; Hu, Y.; Qiao, Y.; Wu, W.; Li, X.; Wang, J.; Liao, L.; et al. Antioxidant capacity of fermented soybeans and their protective effect on protein oxidation in largemouth bass (Micropterus salmoides) during repeated freezing-thawing (FT) treatments. Lwt Food Sci. Technol. 2018, 91, 213–221. [Google Scholar] [CrossRef]
- Tian, J.; Walayat, N.; Ding, Y.; Liu, J. The role of trifunctional cryoprotectants in the frozen storage of aquatic foods: Recent developments and future recommendations. Compr. Rev. Food Sci. Food Saf. 2021. [CrossRef]
- Walayat, N.; Xiong, H.; Xiong, Z.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Randhawa, M.A. Role of cryoprotectants in surimi and factors affecting surimi gel properties: A review. Food Rev. Int. 2020, 1–20. [Google Scholar] [CrossRef]
- Hunt, A.; Getty, K.J.K.; Park, J.W. Roles of Starch in Surimi Seafood: A Review. Food Rev. Int. 2009, 25, 299–312. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, T.; Lin, H.; Chen, H.; Liu, J.; Lyu, F.; Ding, Y. Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocoll. 2019, 90, 82–89. [Google Scholar] [CrossRef]
- Zhouyi, X.; Maojie, Z.; Meihu, M. Emulsifying properties of ovalbumin: Improvement and mechanism by phosphorylation in the presence of sodium tripolyphosphate. Food Hydrocoll. 2016, 60, 29–37. [Google Scholar] [CrossRef]
- Montero, P.; Gomen-Guillen, M.C. Frozen storage of minced prawn flesh: Effect of sorbitol, egg white and starch as protective ingredients. Eur. Food Res. Technol. 1999, 208, 349–354. [Google Scholar] [CrossRef][Green Version]
- Colmenero, F.J.; Barreto, G.; Fernandez, P.; Carballo, J. Frozen storage of bologna sausages as a function of fat content and of levels of added starch and egg white. Meat Sci. 1996, 42, 325–332. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 234. [Google Scholar] [CrossRef]
- Bin, Z.; Chuan-Dong, F.; Gui-Juan, H.; Yang-Yang, Z. Effect of kappa-carrageenan oligosaccharides on myofibrillar protein oxidation in peeled shrimp (Litopenaeus vannamei) during long-term frozen storage. Food Chem. 2018, 245, 254–261. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Rahmanifarah, K. Hydrolysates from marine sources as cryoprotective substances in seafoods and seafood products. Trends Food Sci. Technol. 2016, 57, 40–51. [Google Scholar] [CrossRef]
- Salgo, M.G.; Bermudez, E.; Squadrito, G.L.; Pryor, W.A. DNA damage and oxidation of thiols peroxynitrite causes in rat thymocytes. Arch. Biochem. Biophys. 1995, 322, 500–505. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Lin, W.; Min, Z.; Zhongxiang, F.; Bhesh, B. Gelation properties of myofibrillar protein under malondialdehyde-induced oxidative stress. J. Sci. Food Agric. 2017, 97, 50–57. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Niaz, N.; Ahmad, M.N.; Hassan, A.; Nawaz, A.; Ahmad, I.; Wang, P.-K. Cryoprotective effect of egg white proteins and xylooligosaccharides mixture on oxidative and structural changes in myofibrillar proteins of Culter alburnus during frozen storage. Int. J. Biol. Macromol. 2020, 158, 865–874. [Google Scholar] [CrossRef]
- Liu, J.; Fang, C.; Luo, Y.; Ding, Y.; Liu, S. Effects of konjac oligo-glucomannan on the physicochemical properties of frozen surimi from red gurnard (Aspitrigla cuculus). Food Hydrocoll. 2019, 89, 668–673. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Li, Q.; Nawaz, A.; Zhang, Z.; Wang, P.; Niaz, N. The effectiveness of egg white protein and beta-cyclodextrin during frozen storage: Functional, rheological and structural changes in the myofibrillar proteins of Culter alburnus. Food Hydrocoll. 2020, 105, 105842. [Google Scholar] [CrossRef]
- Perez-Mateos, M.; Lourenco, H.; Montero, P.; Borderias, A.J. Rheological and biochemical characteristics of high-pressure- and heat-induced gels from blue whiting (Micromesistius poutassou) muscle proteins. J. Agric. Food Chem. 1997, 45, 44–49. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of protein—Modification of lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.-l.; Shen, C.-l.; Deng, S.-G. Understanding the influence of carrageenan oligosaccharides and xylooligosaccharides on ice-crystal growth in peeled shrimp (Litopenaeus vannamei) during frozen storage. Food Funct. 2018, 9, 4394–4403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yan, H.B.; Su, L.J.; Chen, X.N. Kappa-carrageenan oligosaccharides retard the progression of protein and lipid oxidation in mackerel (Scomber japonicus) fillets during frozen storage. RSC Adv. 2020, 10, 20827–20836. [Google Scholar] [CrossRef]
- Fangfei, L.; Bo, W.; Baohua, K.; Shuo, S.; Xiufang, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Feng, H.; Jin, H.; Gao, Y.; Yan, S.; Zhang, Y.; Zhao, Q.; Xu, J. Effects of freeze-thaw cycles on the structure and emulsifying properties of peanut protein isolates. Food Chem. 2020, 330, 127215. [Google Scholar] [CrossRef]
- Yu-Bi, W.; Kuo-Wei, L. Influences of xylooligosaccharides on the quality of Chinese-style meatball (kung-wan). Meat Sci. 2011, 88, 575–579. [Google Scholar]
- Yin, T.; He, Y.; Liu, L.; Shi, L.; Xiong, S.; You, J.; Hua, Y.; Huang, Q. Structural and biochemical properties of silver carp surimi as affected by comminution method. Food Chem. 2019, 287, 85–92. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Z.; Wang, J.; Zheng, B. Gelation properties and thermal gelling mechanism of golden threadfin bream myosin containing CaCl2 induced by high pressure processing. Food Hydrocoll. 2019, 95, 43–52. [Google Scholar] [CrossRef]
- Hunt, A.; Park, J.W.; Handa, A. Effect of Various types of egg white on characteristics and gelation of fish myofibrillar proteins. J. Food Sci. 2009, 74, C683–C692. [Google Scholar] [CrossRef]
- Xiong, Z.; Ma, M.; Jin, G.; Xu, Q. Effects of site-specific phosphorylation on the mechanical properties of ovalbumin-based hydrogels. Int. J. Biol. Macromol. 2017, 102, 1286–1296. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, L.; Jiang, X.; Chen, Y.; Zhou, G. The effects of three polysaccharides on the gelation properties of myofibrillar protein: Phase behaviour and moisture stability. Meat Sci. 2020, 170, 108228. [Google Scholar] [CrossRef]
- Fangfei, L.; Xin, D.; Bo, W.; Nan, P.; Xiufang, X.; Yihong, B. Inhibiting effect of ice structuring protein on the decreased gelling properties of protein from quick-frozen pork patty subjected to frozen storage. Food Chem. 2021, 353, 129104. [Google Scholar] [CrossRef]
- Huang, J.; Bakry, A.M.; Zeng, S.; Xiong, S.; Yin, T.; You, J.; Fan, M.; Huang, Q. Effect of phosphates on gelling characteristics and water mobility of myofibrillar protein from grass carp (Ctenopharyngodon idellus). Food Chem. 2019, 272, 84–92. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y. Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp (Hypophthalmichthys molitrix) fillets during setting and heating: Effects of different washing solutions. Food Hydrocoll. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Eissa, A.S.; Khan, S.A. Modulation of hydrophobic interactions in denatured whey proteins by transglutaminase enzyme. Food Hydrocoll. 2006, 20, 543–547. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xiong, Z.; Walayat, N.; Lorenzo, J.M.; Liu, J.; Nawaz, A.; Xiong, H. Influence of the Mixture of Carrageenan Oligosaccharides and Egg White Protein on the Gelation Properties of Culter alburnus Myofibrillar Protein under Repeated Freezing–Thawing Cycles. Antioxidants 2022, 11, 32. https://doi.org/10.3390/antiox11010032
Zhang Z, Xiong Z, Walayat N, Lorenzo JM, Liu J, Nawaz A, Xiong H. Influence of the Mixture of Carrageenan Oligosaccharides and Egg White Protein on the Gelation Properties of Culter alburnus Myofibrillar Protein under Repeated Freezing–Thawing Cycles. Antioxidants. 2022; 11(1):32. https://doi.org/10.3390/antiox11010032
Chicago/Turabian StyleZhang, Zhongli, Zhouyi Xiong, Noman Walayat, Jose M. Lorenzo, Jianhua Liu, Asad Nawaz, and Hanguo Xiong. 2022. "Influence of the Mixture of Carrageenan Oligosaccharides and Egg White Protein on the Gelation Properties of Culter alburnus Myofibrillar Protein under Repeated Freezing–Thawing Cycles" Antioxidants 11, no. 1: 32. https://doi.org/10.3390/antiox11010032
APA StyleZhang, Z., Xiong, Z., Walayat, N., Lorenzo, J. M., Liu, J., Nawaz, A., & Xiong, H. (2022). Influence of the Mixture of Carrageenan Oligosaccharides and Egg White Protein on the Gelation Properties of Culter alburnus Myofibrillar Protein under Repeated Freezing–Thawing Cycles. Antioxidants, 11(1), 32. https://doi.org/10.3390/antiox11010032










