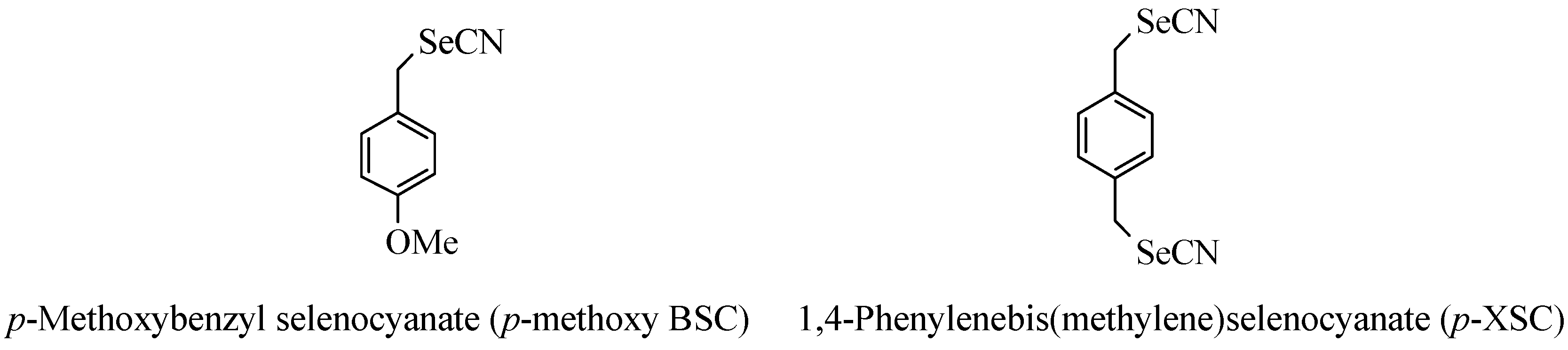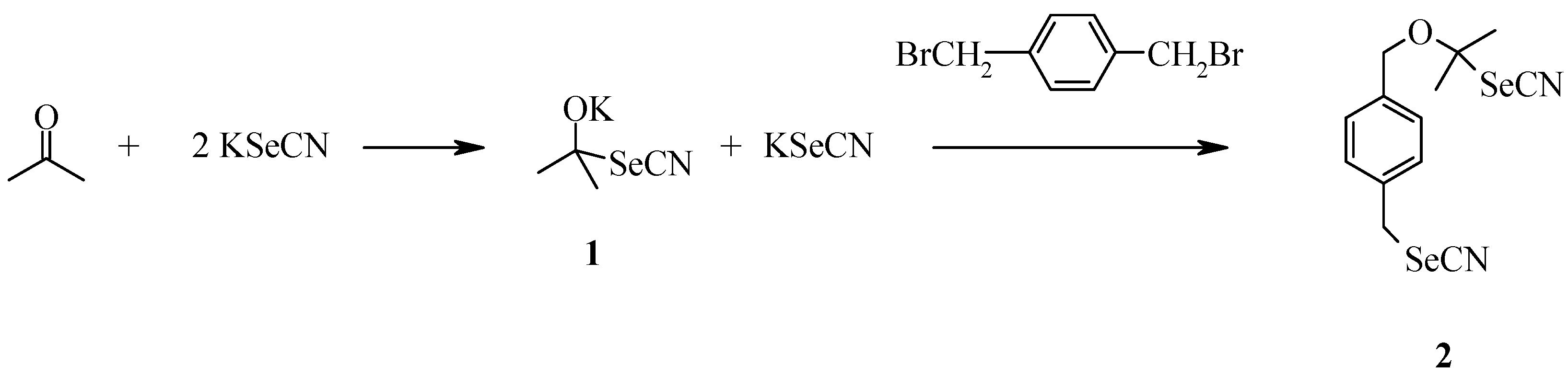Introduction
Organoselenium compounds have shown promising results in a variety of chemopreventative studies by Reddy
et al. focusing on mammary and colon tumors in rats [
1,
2,
3], in which tumor formation was induced with 7,12-dimethylbenz[a]anthracene (mammary tumors) or azoxymethane (colon tumors). Inhibition of tumor formation by dietary organoselenium supplementation was compared to controls and animals fed inorganic selenium supplements. Compounds studied were benzyl selenocyanate (BSC);
o-,
m-,
p-methoxybenzyl selenocyanate (methoxy-BSC),
o-,
m-,
p-nitrobenzyl selenocyanate (nitro-BSC); and
o-,
m-,
p-phenylenebis(methylene)selenocyanate (XSC). Among these the
p‑methoxy-BSC and
p-XSC (
Figure 1) seemed the most effective at preventing azoxymethane induced colonic aberrant crypt foci and
p-XSC best suppressed induced mammary tumors.
Some of the potential mechanisms for chemoprevention due to selenium’s effects on the activity of cellular proteins have recently been reviewed by Ganther [
4]. Possibilities include the effect selenium compounds have on increasing apoptosis, supplementing the activity of selenoproteins such as glutathione peroxidase or thioredoxin reductase, and inhibition of enzymes including protein kinase C and thymidine kinase [
5].
Our laboratory is pursuing the investigation of the effects and mechanism of thymidine kinase inhibition by organoselenium compounds. To start this project, we wanted to synthesize a variety of derivatives of BSC and XSC. Since more of the BSC derivatives could potentially be made from commercially available starting materials, it was decided to begin with these compounds. This would allow us to study the effect of ring substituents on the compounds’ activity and gain an understanding of which types of substituents enhanced activity and/or lowered toxicity.
Results and Discussion
Our first efforts to synthesize organoselenium compounds actually began with an attempt to make
p‑XSC according to the published procedure [
6]. In our hands, this method failed to give the desired product in sufficient yield and it was accompanied by an impurity which could only be separated from the desired
p‑XSC using chromatography. Since this was something we wanted to avoid due to increased waste disposal costs, we looked at other methods in the literature. The method of Tamura
et al. [
7] also required a chromatographic purification, and therefore was not attempted.
Upon examination of the major impurity by
1H-NMR it appeared that it was the result of the KSeCN also attacking the solvent, acetone, thus creating a second nucleophile,
1. This species then reacted with one of the benzylic halide groups to form a yellow solid,
2 (
Scheme 1). This compound could not be separated from the desired product by repeated recrystallizations. We hypothesized that the reactivity of acetone and the long reaction time were the sources of this problem.
To circumvent the problems encountered with the use of acetone, it was necessary to find another solvent that would be less reactive, but still favorable for substitution reactions. Our choice was acetonitrile. The starting materials appeared much more soluble in this medium. In addition, KCl or KBr, which are also produced in this reaction, are insoluble in acetonitrile. This allowed the reaction to be followed visually. The lower reactivity of acetonitrile towards nucleophiles prevented its reaction with KSeCN. The reactions were carried out at room temperature and the time for the completion of the reactions was always under an hour. A single recrystallization afforded analytically pure material, thus eliminating the need for chromatography. The results of our syntheses of benzyl selenocyanates using this procedure are summarized in
Table 1. The starting materials were all conveniently purchased benzylic bromides or chlorides.
Table 1.
The Conversion of Benzylic Halides to Selenocyanates
![Molecules 09 00622 i001]()
Table 1.
The Conversion of Benzylic Halides to Selenocyanates ![Molecules 09 00622 i001]()
| Starting Material | % yield* | m.p. (oC) | 1H-NMR (60 MHz, CD3CN) |
|---|
| Benzyl bromide | 54 | 71-72** | δ 4.28 (s, 2H), 7.40 (s, 5H) |
| 4-Fluorobenzyl bromide | 50 | 64-65 | δ 4.30 (s, 2H), 7.32 (m, 4H) |
| 4-Methylbenzyl bromide | 54 | 51-52 | δ 2.37 (s, 3H), 4.25 (s, 2H), 7.23 (s, 4H) |
| 4-Methoxybenzyl chloride | 70 | 56-57** | δ 3.75 (s, 3H), 4.28 (s, 2H), 7.20 (dd, J=13 Hz, J=9 Hz, 4 H) |
| 4-tert-Butylbenzyl bromide | 52 | 90-91 | δ 1.27 (s, 9H), 4.25 (s, 2H), 7.37 (s, 4H) |
We also found that benzyl selenocyanate can be prepared from benzyl tosylate using the same reaction conditions. However, the yield is lower and is accompanied by formation of red, colloidal selenium. The same problem was noted when commercially available 4-methoxybenzyl chloride was used. These problems can be greatly reduced by distilling these compounds prior to their use in the reaction.
Conclusions
The method presented in this paper offers a facile route to the synthesis of arylselenocyanates. The use of acetone or other solvents that can be attacked by cyanides, sulfides, cyanates, or thiocyanates would not be recommended. The yields are acceptable and could be improved if the use of chromatography is acceptable.
Experimental
General
The benzylic halides were purchased from Aldrich Chemical Company and used without further purification, except for 4-methoxybenzyl chloride, which should be distilled prior to use. The acetonitrile was dried using CaSO4 prior to use. All reactions were carried out in a nitrogen atmosphere. Melting points are uncorrected. All thin layer chromatography was carried out using silica gel on polyester with a fluorescent indicator and dichloromethane as an eluent. Proton NMR were obtained using acetonitrile-d3 (CD3CN) as solvent. All yields reported are after crystallization. Elemental analyses were performed by Quantitative Technologies, Inc. or Galbraith Laboratories, Inc.
General synthetic procedure
A solution of KSeCN (2.2 mmol) in acetonitrile (5 mL) was added to a stirred solution of the benzylic halide (2.0 mmol) in acetonitrile (15 mL). The flask containing the KSeCN was rinsed with additional solvent (1-2 mL) after the end of the addition and this was also added to the reaction. The formation of a fine white precipitate (KBr or KCl) signaled the progress of the reaction. Reactions were deemed complete when no additional precipitate formed (30 – 60 min.). This was verified by thin layer chromatography. The reaction was poured into distilled water (200 mL) and stirred for about 30 minutes. After this time, the mixture was cooled in ice and vacuum filtered. The solid precipitate was washed generously with water to remove any excess salts. The crude solid was then purified using a mixed crystallization from benzene/toluene (1:1) to dissolve the solid and heptane to induce the cloud point. This generally required about 1-4 mL of the benzene/toluene mixture and 20-40 mL of the heptane. The mixture was then sealed and cooled at –20oC for 1-2 hours to induce crystallization. In the case of the compounds that melted below 60oC, a cloud point was not always obtained. In these cases, the reaction was placed in the freezer when the amount of heptane added was 10 to 20 times the amount of the benzene/toluene mixture used. Scratching after the cooling period readily produced crystallization if solid was not already apparent. No attempt was made to obtain a second crop of crystals from either filtrate.
Elemental Analyses
4-Fluorobenzeneselenocyanate: Calcd. for C8H6FNSe; C, 44.88; H, 2.82; F, 8.87; N, 6.54; Se, 36.88. Found: C, 44.81; H, 2.79; F, 8.79; N, 6.46; Se, 36.63.
4-Methylbenzeneselenocyanate: Calcd. for C9H9NSe; C, 51.44; H, 4.32; N, 6.67; Se, 37.58. Found: C, 51.45; H, 4.27; N, 6.73; Se, 37.29.
4-(1,1-Dimethylethyl)benzeneselenocyanate: Calcd. for C12H15NSe; C, 57.14; H, 5.99; N, 5.55; Se, 31.60. Found: C, 57.24; H, 6.15; N, 5.60; Se, 31.64.