Nitrogen Heterocycles as Building Blocks for New Metallo-supramolecular Architectures
Abstract
:Introduction
N,N'-Chelating Bis-heterocycles
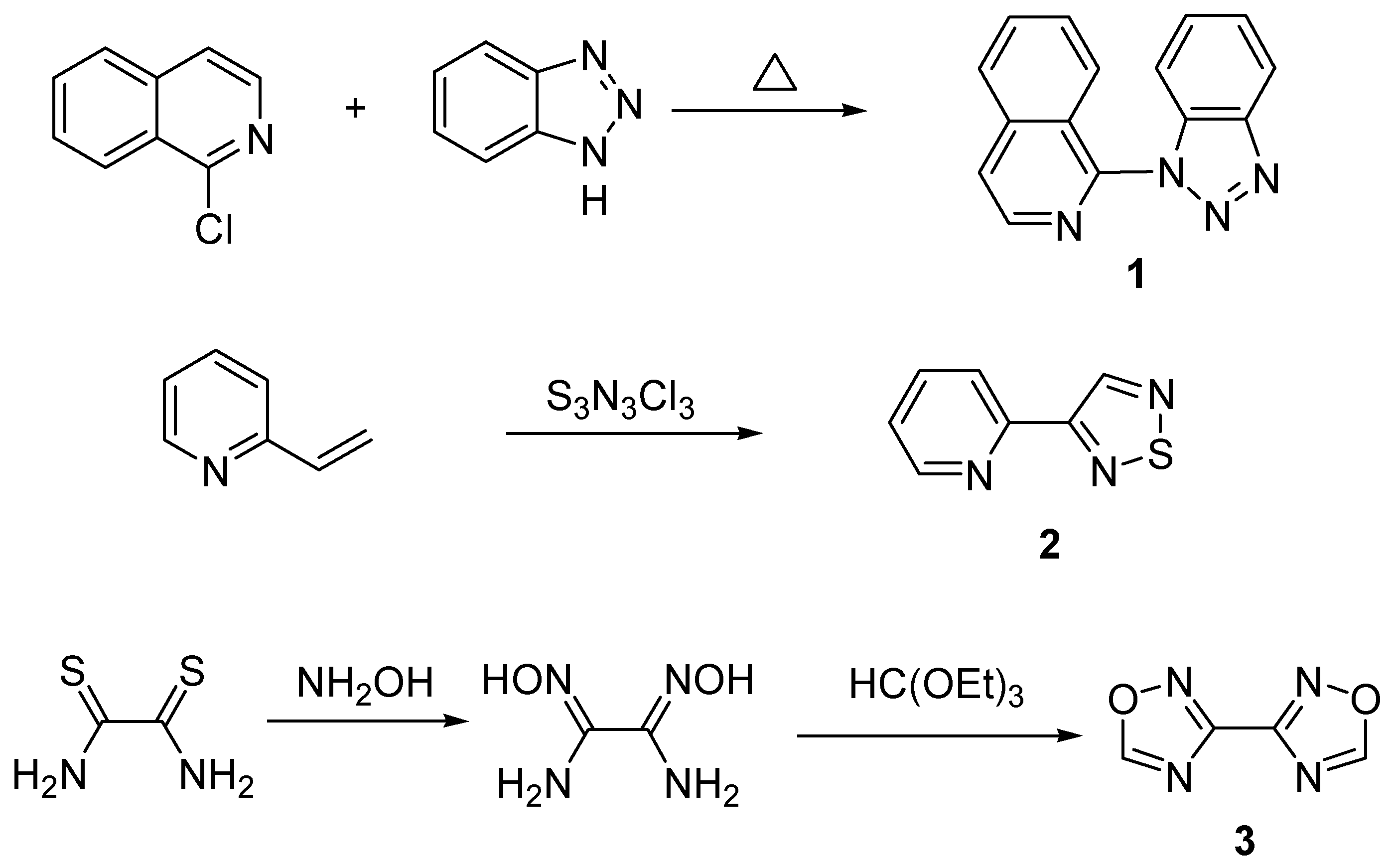
Binucleating Ligands
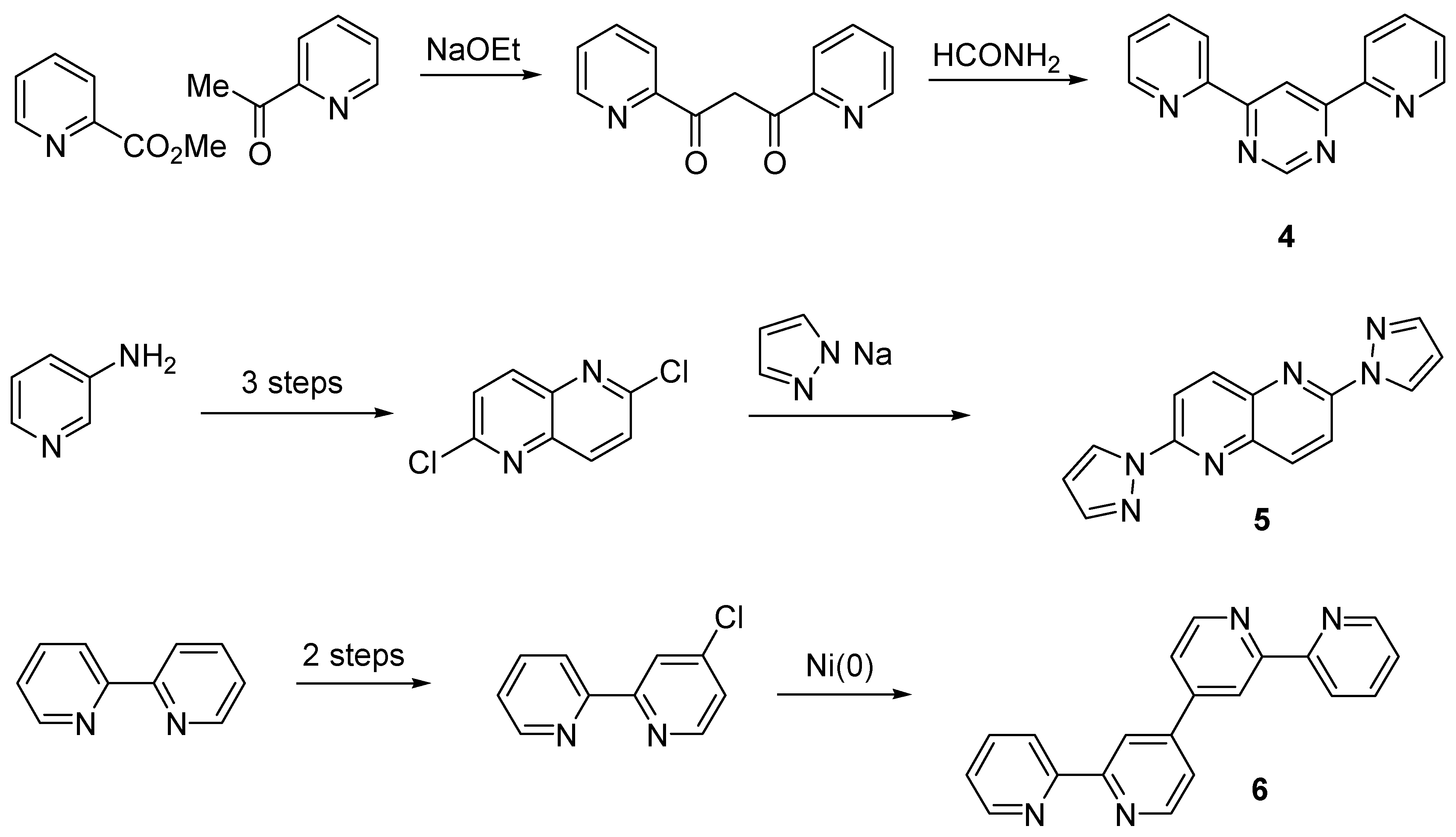
Cyclometallated Analogues

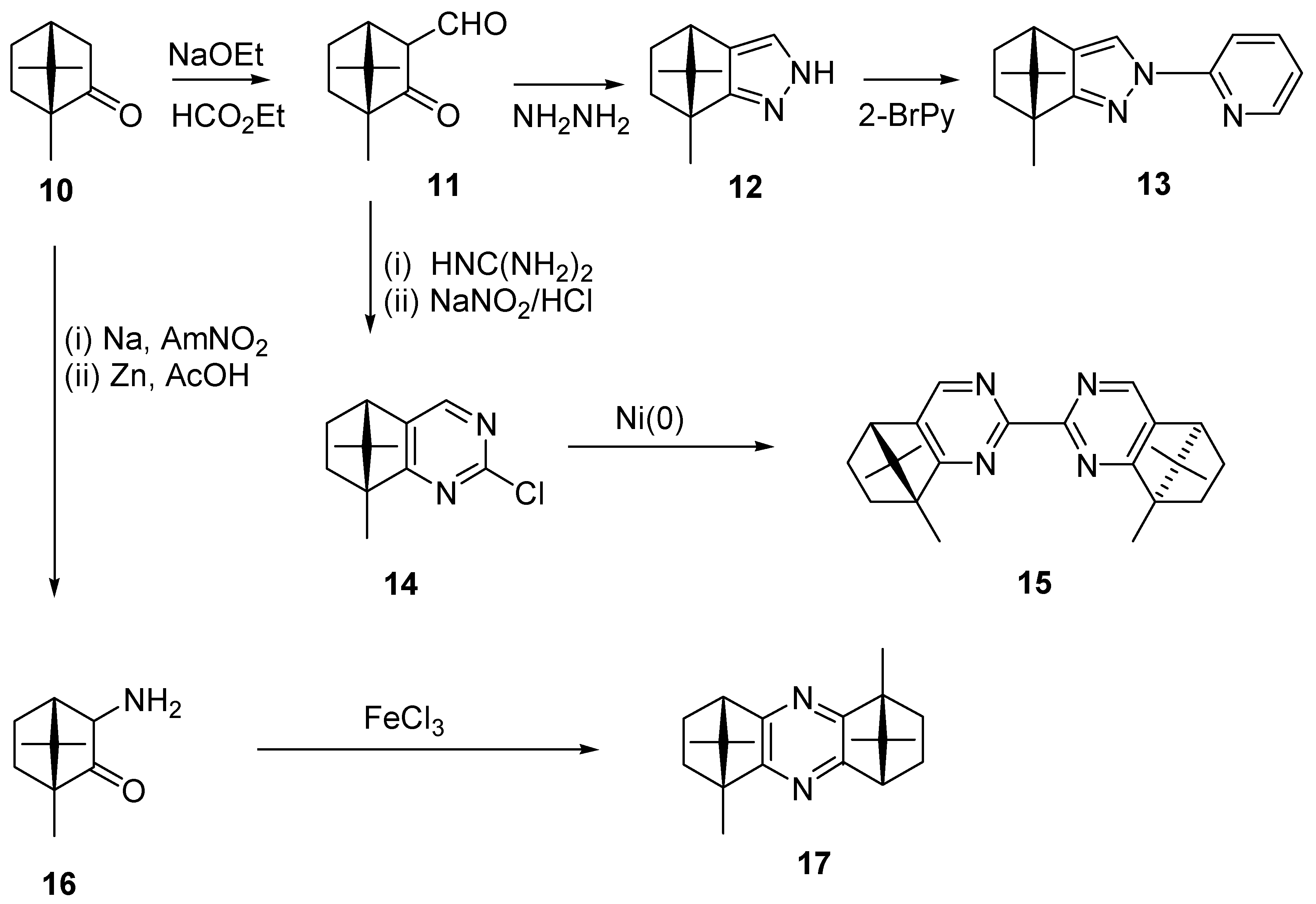
Chiral Ligands
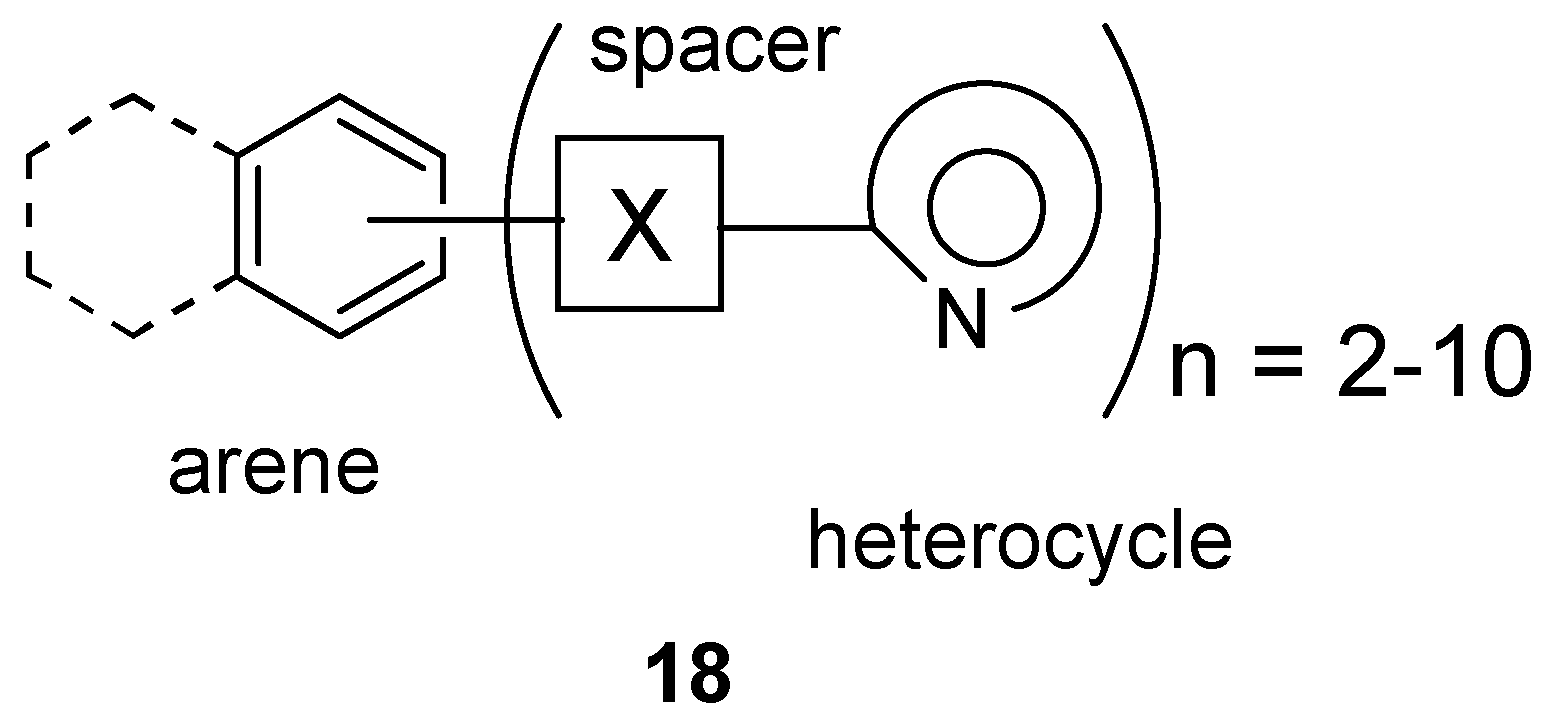
Polyheteroaryl-linked Arenes

Conclusions
Acknowledgements
References
- Blau, F. Die destillation pyridinmonocarbonsaurer salze. Chem. Ber. 1888, 21, 1077–1078. [Google Scholar]
- Constable, E. C. Homoleptic complexes of 2,2'-bipyridine. Adv. Inorg. Chem. 1989, 34, 1–63. [Google Scholar]
- Constable, E. C.; Steel, P. J. N,N'-Chelating biheteroaromatic ligands; a survey. Coord. Chem. Rev. 1989, 93, 205–223. [Google Scholar] [CrossRef]
- Richardson, C.; Steel, P. J. Benzotriazole as a structural component in chelating and bridging heterocyclic ligands; ruthenium, palladium, copper and silver complexes. J. Chem. Soc., Dalton Trans. 2003, 992, 992–1000. [Google Scholar] [CrossRef]
- Richardson, C.; Steel, P. J. Unpublished results. 2000.
- Steel, P. J. Aromatic nitrogen heterocycles as bridging ligands; a survey. Coord. Chem. Rev. 1990, 106, 227–265. [Google Scholar] [CrossRef]
- Kaes, C.; Katz, A.; Hosseini, M. W. Bipyridine: the most widely used ligand. A review of molecules comprising at least two 2,2'-bipyridine units. Chem. Rev. 2000, 100, 3553–3590. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, A. L.; Bosnich, B. Principles of mononucleating and binucleating ligand design. Chem. Rev. 2004, 104, 349–383. [Google Scholar] [CrossRef] [PubMed]
- Balzani, V.; Juris, A.; Venturi, M.; Campagna, S.; Serroni, S. Luminescent and redox-active polynuclear transition metal complexes. Chem. Rev. 1996, 96, 759–833. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I. G.; Steel, P. J. Mono- and bi-nuclear complexes of the doubly bidentate, bridging ligand 4,6-di(2-pyridyl)pyrimidine. Aust. J. Chem. 1998, 51, 371–382. [Google Scholar] [CrossRef]
- Phillips, I. G. Syntheses and complexes of heterocyclic ligands. Ph.D. Thesis, University of Canterbury, 1995. [Google Scholar]
- Downard, A. J.; Honey, G. E.; Phillips, L. F.; Steel, P. J. Synthesis and properties of a tris(2,2'- bipyridine)ruthenium(II) dimer directly coupled at the C4 carbon. Inorg. Chem. 1991, 30, 2259–2260. [Google Scholar] [CrossRef]
- Constable, E. C. Cyclometallated complexes incorporating a heterocyclic donor atom; the interface of coordination chemistry and organometallic chemistry. Polyhedron 1984, 3, 1037–1057. [Google Scholar] [CrossRef]
- Steel, P. J.; Caygill, G. B. Cyclometallated compounds. II. Proton and carbon-13 nuclear magnetic resonance spectral assignments of cyclopalladated compounds. J. Organomet. Chem. 1987, 327, 101–114. [Google Scholar] [CrossRef]
- de Geest, D. J.; O'Keefe, B. J.; Steel, P. J. Cyclometallated compounds. XIII. Cyclopalladation of 2-phenoxypyridine and structurally-related compounds. J. Organomet. Chem. 1999, 579, 97–105. [Google Scholar] [CrossRef]
- Caygill, G. B.; Hartshorn, R. M.; Steel, P. J. Cyclometallated compounds. IV. Cyclopalladation of phenyl pyrimidines and X-ray structure of a doubly cyclopalladated derivative of 4,6- diphenylpyrimidine. J. Organomet. Chem. 1990, 382, 455–469. [Google Scholar] [CrossRef]
- de Geest, D. J.; Steel, P. J. Cyclometallated compounds. XII. Syntheses of two new doublycyclometallated compounds. Inorg. Chem. Commun. 1998, 1, 358–360. [Google Scholar] [CrossRef]
- O'Keefe, B. J.; Steel, P. J. Cyclometallated compounds. XV. A tetranuclear, acetate-bridged, cyclopalladated molecular box. Acta Crystallogr. 2000, C56, 1440–1441. [Google Scholar]
- O'Keefe, B. J.; Steel, P. J. Cyclometallated compounds. XVI. Double cyclopalladations of bis(2- pyridyloxy)naphthalenes. Kinetic versus thermodynamic control over regioselectivity. Organometallics 2003, 22, 1281–1292. [Google Scholar] [CrossRef]
- Slater, J. W.; Lydon, D. P.; Rourke, J. P. Doubly cyclopalladated pyridazines: chiral liquid crystals. J. Organomet. Chem. 2002, 645, 246–255. [Google Scholar] [CrossRef]
- Sumby, C. J.; Steel, P. J. Cyclometallated compounds. XVII. The first three-fold cyclopalladation of a single benzene ring. Organometallics 2003, 22, 2358–2360. [Google Scholar] [CrossRef]
- Ghosh, A. K.; Mathivanan, P.; Cappiello, J. C2-symmetric chiral bis(oxazoline)-metal complexes in catalytic asymmetric synthesis. Tetrahedron: Asymmetry 1998, 9, 1–45. [Google Scholar] [CrossRef]
- Mamula, O; von Zelewsky, A. Supramolecular coordination compounds with chiral pyridine and polypyridine ligands derived from terpenes. Coord. Chem. Rev. 2003, 242, 87–95. [Google Scholar]
- Steel, P. J. Dichloro[(4S,7R)-7,8,8-trimethyl-2-(2-pyridyl)-4,5,6,7-tetrahydro-4,7-methano-2H-indazole]palladium(II), C16H19C12N3Pd. Acta Crystallogr. 1983, C39, 1623–1625. [Google Scholar] [CrossRef]
- House, D. A.; Steel, P. J.; Watson, A. A. Chiral heterocyclic ligands. IV. Synthesis and metal complexes of 2,6-bis(pyrazol-1-ylmethyl)-pyridine and chiral derivatives. Inorg. Chim. Acta 1987, 130, 167–176. [Google Scholar]
- Watson, A. A.; House, D. A.; Steel, P. J. Chiral heterocyclic ligands. VII. Syntheses of some chiral 2,6-bis(N-pyrazolyl)pyridines. J. Org. Chem. 1991, 56, 4072–4074. [Google Scholar] [CrossRef]
- Watson, A. A.; House, D. A.; Steel, P. J. Chiral heterocyclic ligands. VIII. Syntheses and complexes of new chelating ligands derived from camphor. Aust. J. Chem. 1995, 48, 1549–1572. [Google Scholar] [CrossRef]
- Downard, A. J.; Phillips, I. G.; Steel, P. J. Chiral heterocyclic ligands. X. Synthesis and metal complexes of hindered and chiral 2,2'-bipyrimidines. Aust. J. Chem. submitted for publication. [CrossRef]
- Fitchett, C. M.; Steel, P. J. Chiral heterocyclic ligands. Part 9. Homoconfigurational coordination polymers based on a C2-symmetric, linear-bridging ligand. New J. Chem. 2000, 24, 945–947. [Google Scholar] [CrossRef]
- Hennrich, G.; Anslyn, E. V. 1,3,5-2,4,6-Functionalized, facially segregated benzenes - exploitation of sterically predisposed systems in supramolecular chemistry. Chem. Eur. J. 2002, 8, 2218–2224. [Google Scholar]
- Hartshorn, C. M.; Steel, P. J. Self-assembly and X-ray structure of a ten-component, three-dimensional, metallosupramolecular cage. Chem. Commun. 1997, 541–542. [Google Scholar] [CrossRef]
- Hartshorn, C. M.; Steel, P. J. Cœlenterands: a new class of metal-encapsulating ligands. Angew. Chem., Int. Ed. Engl. 1996, 35, 2655–2657. [Google Scholar] [CrossRef]
- Hartshorn, C. M.; Steel, P. J. Bis(3-(2-pyridyl)pyrazol-1-ylmethyl)benzenes, doubly-chelating binucleating ligands and encapsulation of a linear trisilver(I) moiety. Inorg. Chem. Commun. 2000, 3, 476–481. [Google Scholar] [CrossRef]
- O'Keefe, B. J. Synthesis and complexes of heterocyclic ligands. Ph.D. Thesis, University of Canterbury, 1999. [Google Scholar]
- Piguet, C.; Bernardinelli, G.; Hopfgartner, G. Helicates as versatile supramolecular complexes. Chem. Rev. 1997, 97, 2005–2062. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. "Let's twist again"-double-stranded, triple-stranded, and circular helicates. Chem. Rev. 2001, 101, 3457–3497. [Google Scholar] [CrossRef] [PubMed]
- O'Keefe, B. J.; Steel, P. J. Self-assembly and X-ray structure of a triple helicate with two trigonal silver(I) termini. Inorg Chem. Commun. 2000, 3, 473–475. [Google Scholar] [CrossRef]
- McMorran, D. A.; Steel, P. J. The first coordinatively-saturated quadruply-stranded helicate and its encapsulation of a hexafluorophosphate anion. Angew. Chem., Int. Ed. 1998, 37, 3295–3297. [Google Scholar] [CrossRef]
© 2004 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Steel, P.J. Nitrogen Heterocycles as Building Blocks for New Metallo-supramolecular Architectures. Molecules 2004, 9, 440-448. https://doi.org/10.3390/90600440
Steel PJ. Nitrogen Heterocycles as Building Blocks for New Metallo-supramolecular Architectures. Molecules. 2004; 9(6):440-448. https://doi.org/10.3390/90600440
Chicago/Turabian StyleSteel, Peter J. 2004. "Nitrogen Heterocycles as Building Blocks for New Metallo-supramolecular Architectures" Molecules 9, no. 6: 440-448. https://doi.org/10.3390/90600440
APA StyleSteel, P. J. (2004). Nitrogen Heterocycles as Building Blocks for New Metallo-supramolecular Architectures. Molecules, 9(6), 440-448. https://doi.org/10.3390/90600440



