Sesquiterpenes from Cymbopogon proximus
Abstract
:Introduction
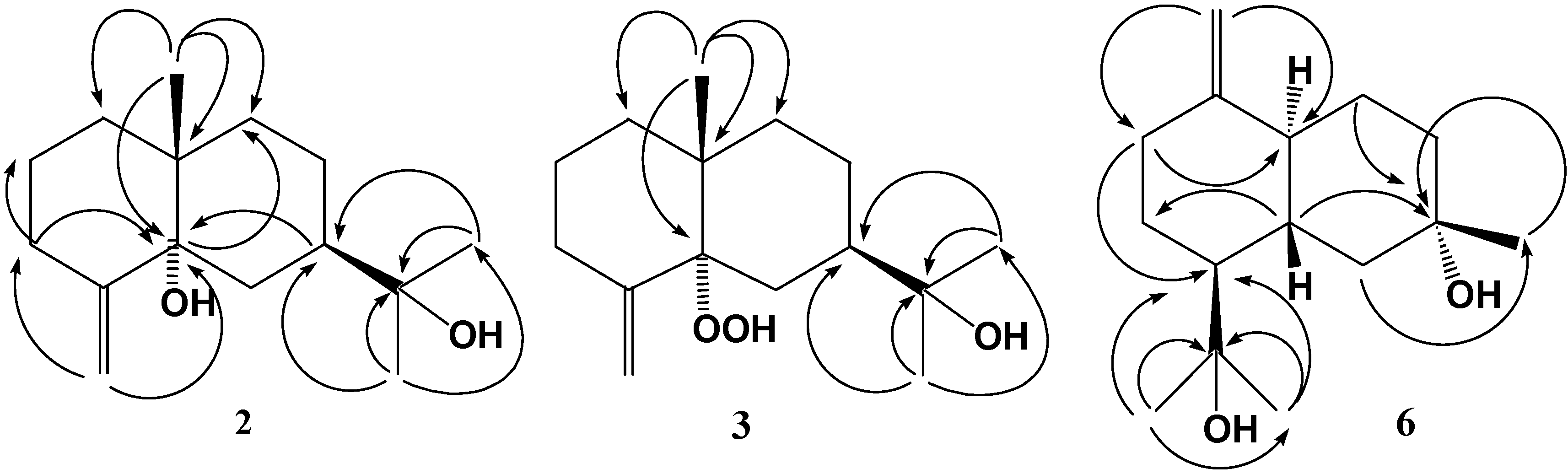
Results and Discussion
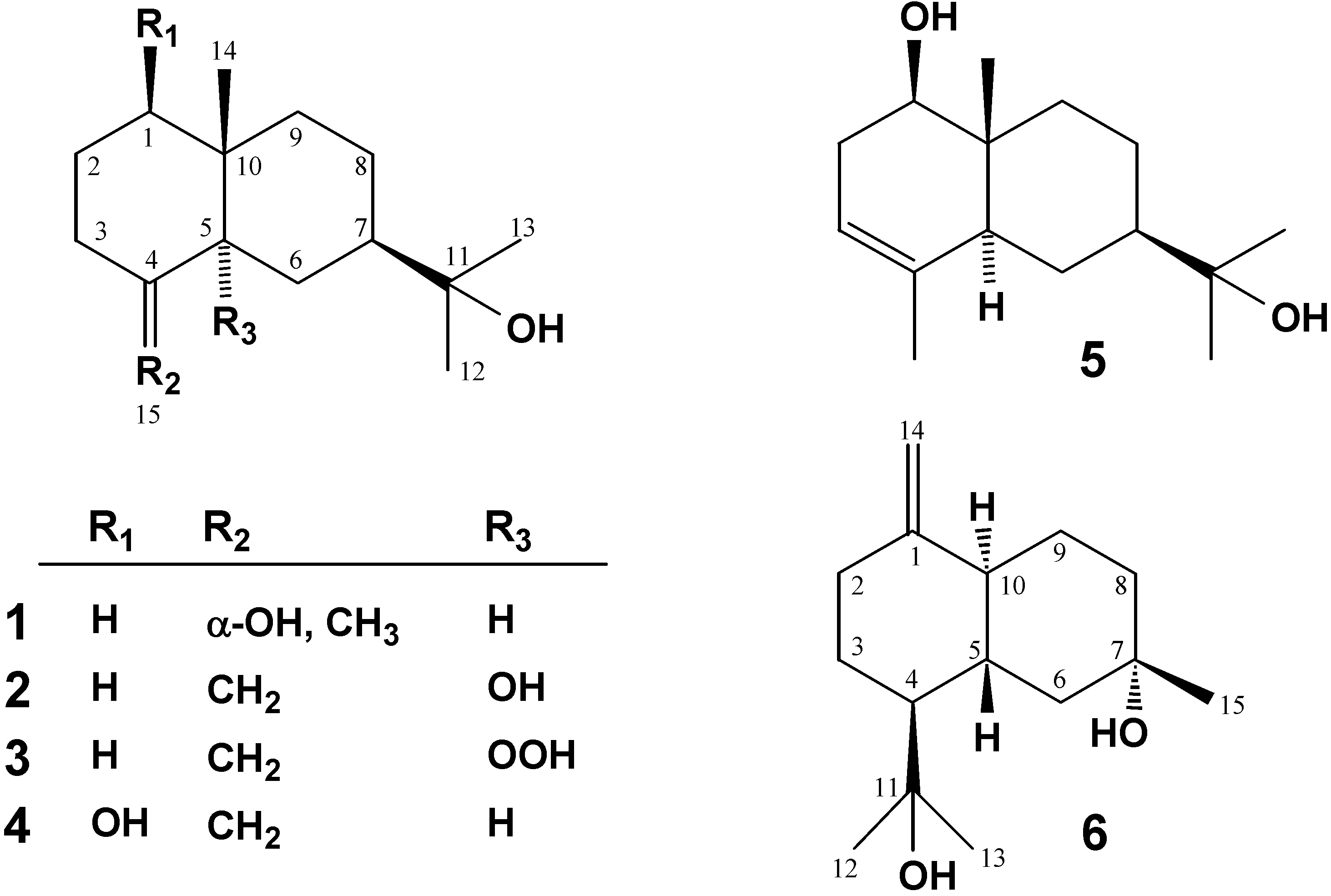
| Position | 2 | 3 | 10-epi-5β-hydro- peroxy-β-eudesmol [14] | 10-epi-5α-hydro- peroxy-β-eudesmol [14] | 4 | 6 | |
| 1 | 34.9 t | 34.4 t | 33.5 t | 35.8 t | 79.3 d | 153.8 s | |
| 2 | 21.9 t | 21.5 t | 20.1 t | 23.5 t | 31.4 t | 37.8 t | |
| 3 | 31.7 t | 32.0 t | 32.4 t | 34.2 t | 34.2 t | 25.6 t | |
| 4 | 152.1 s | 148.8 s | 148.7 s | 148.8 s | 148.9 s | 47.4 d | |
| 5 | 75.5 s | 87.2 s | 87.0 s | 89.5 s | 47.5 d | 52.5 d | |
| 6 | 31.0 t | 24.7 t | 23.4 t | 28.0 t | 24.4 t | 40.6 t | |
| 7 | 43.5 d | 43.2 d | 40.7 d | 43.7 d | 48.9 d | 81.1 s | |
| 8 | 22.3 t | 22.5 t | 22.7 t | 22.4 t | 22.1 t | 27.2 t | |
| 9 | 34.2 t | 34.3 t | 35.2 t | 35.4 t | 36.9 t | 27.5 t | |
| 10 | 37.8 s | 38.7 s | 38.1 s | 38.9 s | 40.1 s | 48.3 d | |
| 11 | 72.8 s | 72.9 s | 74.1 s | 72.9 s | 72.8 s | 74.2 s | |
| 12 | 26.8 q | 26.3 q | 28.7 q | 26.5 q | 27.0 q | 26.9 q | |
| 13 | 27.8 q | 28.0 q | 29.9 q | 27.6 q | 27.2 q | 27.1 q | |
| 14 | 19.9 q | 21.2 q | 22.8 q | 21.3 q | 10.2 q | 106.5 t | |
| 15 | 107.6 t | 111.6 t | 111.1 t | 108.0 t | 106.8 t | 23.8 q | |
Experimental
General
Plant material
Extraction and Isolation
Acknowledgments
References
- Taeckholm, V. "Students Flora of Egypt", 2nd Ed. ed; Cairo University Press: Cairo, 1974; p. 759. [Google Scholar]
- Boulos, L. "Medicinal Plants of North Africa"; Reference Publication Inc.: Michigan, 1983; p. 92. [Google Scholar]
- Abdel-Moneim, F.M. “Chemical Investigation of Certain Species of Egyptian Desert Plants”. Ph.D. Thesis, Faculty of Science, Cairo University, Egypt, 1966. [Google Scholar]
- Abdel-Moneim, F.M.; Ahmed, Z.F.; Fayez, M.B.E.; Ghaleb, H. Constituents of local plants XIV. The antispasmodic principle in Cymbopogon proximus. Planta Med. 1969, 3, 209. [Google Scholar] [Green Version]
- El-Deeb, K.S. A “Pharmacognostical study of certain Cymbopogon species, growing in Egypt”. Master Thesis, Faculty of Pharmacy, Cairo University, Egypt, 1981. [Google Scholar]
- Radwan, A.S. An analytical method for proximadiol, the active principle of Cymbopogon proximus. Planta Med. 1975, 27, 93. [Google Scholar] [Green Version]
- Evens, F.E.; Miller, D.W.; Cairns, T.; Baddeley, G.V.; Wenkert, E. Structure analysis of proximadiol (Cryptomeridiol) by 13C-NMR spectroscopy. Phytochemistry 1982, 21, 937. [Google Scholar] [Green Version]
- Locksley, H.D.; Fayez, M.B.E.; Radwan, A.S.; Chari, V.M.; Cordell, G.A.; Wagner, H. Constituents of Local Plants XXV, Constitution of the antispasmodic principle of Cymbopogon proximus. Planta Med. 1982, 45, 20. [Google Scholar] [Green Version]
- Su, W-C.; Fang, J.-M.; Cheng, Y.-S. Sesquiterpenes from leaves of Cryptomeria japonica. Phytochemistry 1995, 39, 603. [Google Scholar] [Green Version]
- Elgamal, M.H.; Wolff, P. A Further Contribution to the Sesquiterpenoid constituents of Cymbopogon proximus. Planta Med. 1987, 53, 293. [Google Scholar] [Green Version]
- Suzuki, M.; Segawa, M.; Kikuchi, H.; Suzuki, T.; Kurosawa, E. (5S, 7R, 10R)-Selin-4(14)-en-5α-ol, A sesquiterpene alcohol from the red algae Laurencia nipponica. Phytochemistry 1985, 24, 2011. [Google Scholar] [Green Version]
- Nyasse, B.; Ghogomu Tih, R.; Sondengam, B.L.; Martin, M.T.; Bodo, B. Isolation of α—corymbolol, an eudesmane sesquiterpene diol from Cyperus articulatus. Phytochemistry 1988, 27, 179. [Google Scholar] [Green Version]
- Ahmed, A.A.; Jakupovic, J. Sesqui- and monoterpenes from Jasonia Montana. Phytochemistry 1990, 29, 3658. [Google Scholar] [Green Version]
- Itokawa, H.; Morita, H.; Watanabe, K. Novel eudesmane-type sesquiterpenes from Alpinia japonica (THUNB.)MIQ. Chem. Pharm. Bull. 1987, 35, 1460. [Google Scholar] [Green Version]
- Adinarayana, D.; Syamasundar, K.V. A new sesquiterpene alcohol from Pterocarpus marsupium. Phytochemistry 1982, 21, 1083. [Google Scholar] [Green Version]
- Van Beek, T.A.; Kleis, R.; Posthumus, M.A.; Van Veldhuizen, A. Essential oil of Amyris balsamifera. Phytochemistry 1989, 28, 1909. [Google Scholar] [Green Version]
- Thappa, R.K.; Dhar, K.L.; Atal, C.K. sointermedeol, A new sesquiterpene alcohol from Cymbopogon flexuosus. Phytochemistry 1979, 18, 671. [Google Scholar] [Green Version]
- Itokawa, H.; Nakanishi, H.; Mihashi, S. Acid-catalyzed rearrangement of hinesol and related compounds to eudesmane derivatives. Chem. Pharm. Bull. 1983, 31, 1991. [Google Scholar] [Green Version]
- Raharivelomanana, P.; Bianchini, J.P.; Ramanoelina, A.R.P.; Rasoarahona, J.R.E.; Faure, R.; Cambon, A. Eudesmane sesquiterpenes from Laggera alata. Phytochemistry 1998, 47, 1085. [Google Scholar] [Green Version]
- Schmeda-Hirschmann, G.; Papastergiou, F. Sesquiterpenes from Fabiana imbricata. Phytochemistry 1994, 36, 1439. [Google Scholar] [Green Version]
- Queiroga, C.L.; Ferracini, V.L.; Marsaioli, A.J. Three new oxygenated cadinanes from Baccharis species. Phytochemistry 1996, 42, 1097. [Google Scholar] [Green Version]
- Nagashima, F.; Suda, K.; Askawa, Y. Cadinane-Type sesquiterpenoids from the liverwort Scapania undulata. Phytochemistry 1994, 37, 1323. [Google Scholar] [Green Version]
- De Rosa, S.; De Giulio, A.; Iodice, C.; Zavodink, N. Sesquiterpenes from the brown alga Taonia atomaria. Phytochemistry 1994, 37, 1327. [Google Scholar] [Green Version]
- Wagner, H.; Bladt, S.; Zgainski, E. M. “Plant Drug Analysis”; Springer Verlag: Berlin, 1984; p. 304. [Google Scholar]
- Sample Availability: Available from the authors
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
El-Askary, H.I.; Meselhy, M.R.; Galal, A.M. Sesquiterpenes from Cymbopogon proximus. Molecules 2003, 8, 670-677. https://doi.org/10.3390/80900670
El-Askary HI, Meselhy MR, Galal AM. Sesquiterpenes from Cymbopogon proximus. Molecules. 2003; 8(9):670-677. https://doi.org/10.3390/80900670
Chicago/Turabian StyleEl-Askary, Hesham I., Meselhy R. Meselhy, and Ahmed M. Galal. 2003. "Sesquiterpenes from Cymbopogon proximus" Molecules 8, no. 9: 670-677. https://doi.org/10.3390/80900670
APA StyleEl-Askary, H. I., Meselhy, M. R., & Galal, A. M. (2003). Sesquiterpenes from Cymbopogon proximus. Molecules, 8(9), 670-677. https://doi.org/10.3390/80900670




