Synthesis and Biological Evaluation of Some New Coumarin Derivatives
Abstract
:Introduction
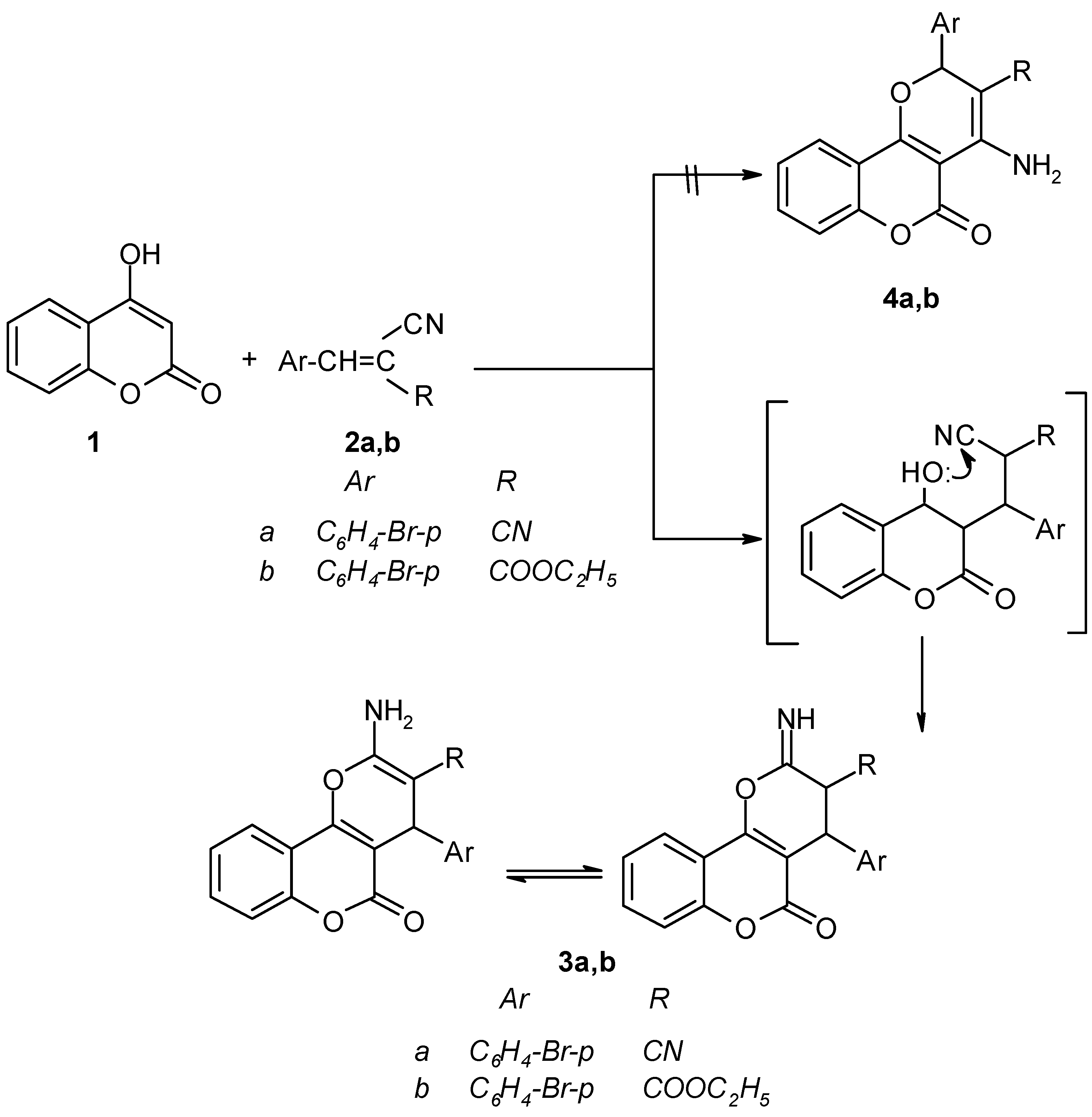
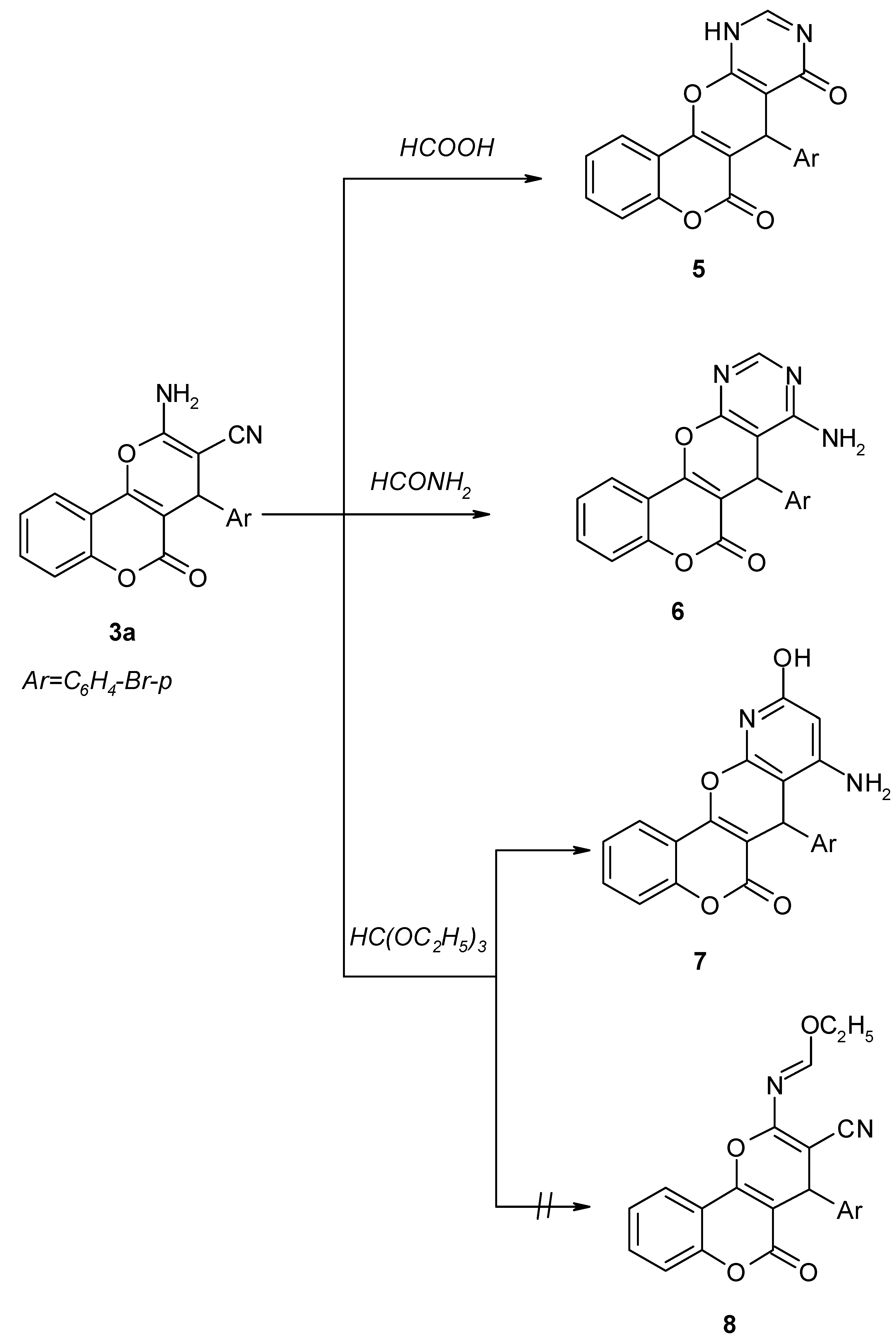
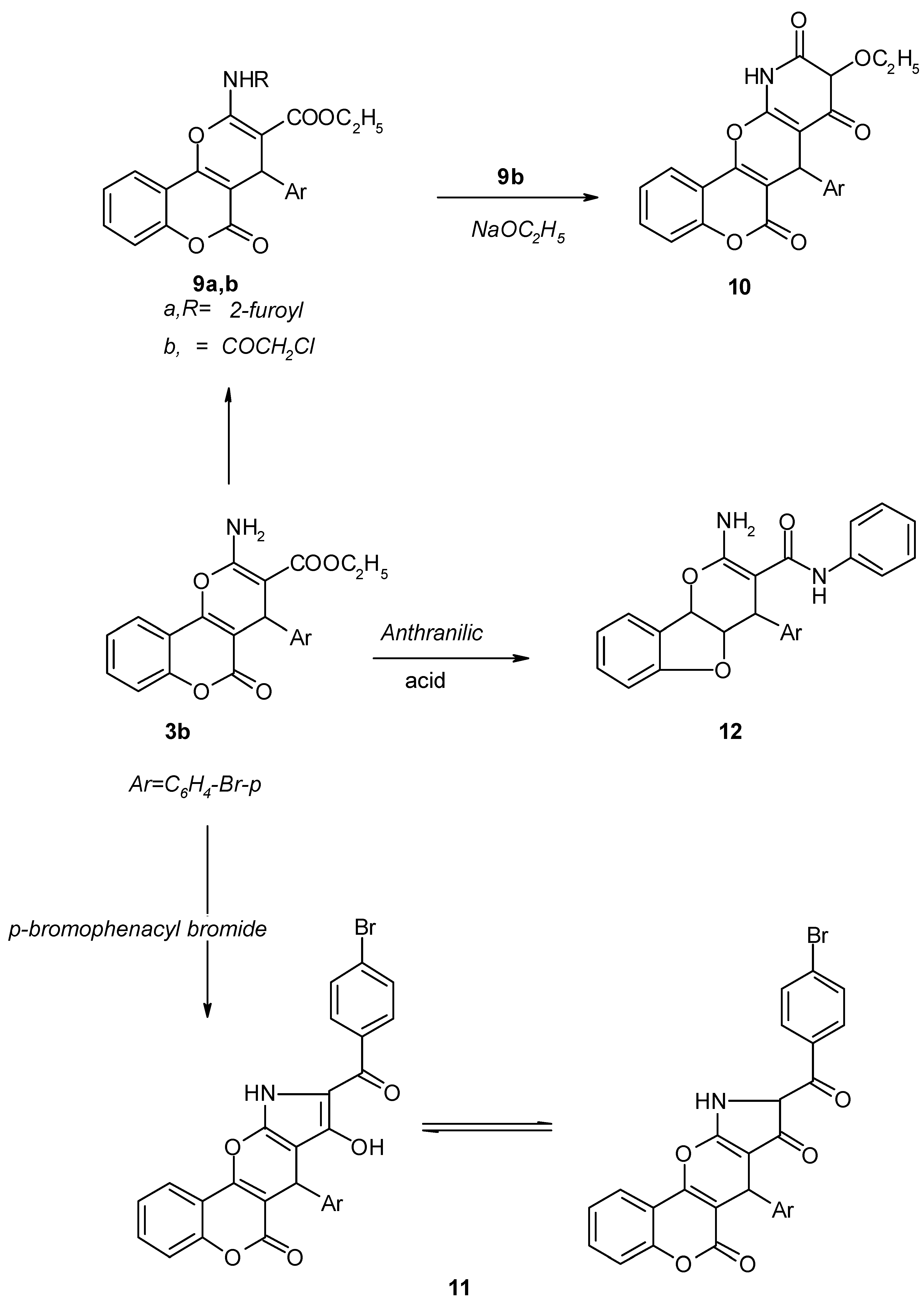
Results and Discussion
Biological Activity
| Compd. | Organisms* | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 3a | 28 | 11 | 19 | 10 | 20 | 10 |
| 3b | 25 | 17 | 15 | 10 | 19 | 11 |
| 5 | 22 | 18 | 16 | 10 | 13 | 12 |
| 6 | 29 | 22 | 18 | 18 | 21 | 20 |
| 7 | 20 | 14 | 16 | 18 | 22 | 11 |
| 9a | 22 | 13 | 10 | 16 | 15 | 15 |
| 9b | 24 | 14 | 10 | 8 | 13 | 13 |
| 10 | 28 | 14 | 19 | 12 | 15 | 15 |
| 11 | 18 | 15 | 14 | 10 | 12 | 11 |
| 12 | 20 | 15 | 13 | 19 | 29 | 16 |
| Amoxicillin | 29 | 12 | 20 | 11 | 36 | 10 |
| Compd. | Organisms* | ||
|---|---|---|---|
| A | B | C | |
| 3a | 12 | 11 | 20 |
| 3b | 15 | 15 | 19 |
| 5 | 21 | 12 | 20 |
| 6 | 14 | 16 | 18 |
| 7 | 16 | 20 | 18 |
| 9a | 12 | 22 | 19 |
| 9b | 18 | 20 | 22 |
| 10 | 10 | 12 | 19 |
| 11 | 10 | 13 | 11 |
| 12 | 12 | 18 | 11 |
| Mycostatin | 12 | 20 | 26 |
Conclusions
Experimental
General
Biological Tests
2-Amino-4- (4`-bromophenyl)-3-cyano-4H,5H-pyrano[3,2-c][1]benzopyran-5-one (3a).
2-Amino-4-(4`-bromophenyl)-3-carboethoxy-4H,5H-pyrano[3,2-c][1]- benzopyran -5-one (3b).
7-(p-Bromophenyl)-11-hydropyrimidino[5`,4`-6,5]4H-pyrano[3,2-c][1]-benzopyran-6,8-dione (5).
8-Amino-7-(p-bromophenyl)pyrimidino[5`,4`-6,5]4H-pyrano[3,2-c][1]-benzopyran-6-one (6).
8-Amino-7-(p-bromophenyl)-10-hydroxypyridino[3`,2`-6,5]4H-pyrano[3,2-c][1]benzopyran-6-one (7).
2-N-furoylamino-3-carboethoxy-4-(p-bromophenyl)-4H,5H-pyrano[3,2-c][1]benzopyran-5-one (9a).
2-N-chloroacetylamino-3-carboethoxy-4-(p-bromophenyl)-4H,5H-pyrano[3,2-c][1]benzopyran-5-one (9b).
7-(p-Bromophenyl)-9-ethoxy-9,11-dihydropyridino[3`,2`-6,5]4H-pyrano[3,2-c][1]benzopyran-6,8,10-trione (10).
7-(p-Bromophenyl)-9-[(p-bromophenyl)carbonyl]-8-hydroxy-pyrrolo[3`,2`-5,6]4H-pyrano[3,2-c][1]-benzopyran-6-one (11).
2-Amino-4-(p-bromophenyl)-3-[N-phenyl carbamido]-dihydrobenzofurano [3,2-b]-4H-pyran (12).
| Compd. | M.P. Co | Yield (%) Colour | Molecular Formula (M. wt.) | Analysis Calcd./Found | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 3a | 254 | 67 White | C19H11BrN2O3 (395.22) | 57.74 57.81 | 2.81 2.70 | 7.09 6.92 |
| 3b | 192 | 40 White | C21H16BrNO5 (442.27) | 57.03 56.99 | 3.65 3.70 | 3.17 3.06 |
| 5 | >300 | 40 White | C20H11BrN2O4 (423.23) | 56.76 56.68 | 2.62 2.46 | 6.62 6.43 |
| 6 | >300 | 50 White | C20H12BrN3O3 (422.24) | 56.89 57.10 | 2.86 2.79 | 9.95 9.90 |
| 7 | >300 | 55 White | C21H13BrN2O4 (437.25) | 57.68 57.93 | 2.99 3.20 | 6.40 6.11 |
| 9a | 140 | 40 White | C26H18BrNO7 (536.33) | 58.22 58.10 | 3.38 3.43 | 2.61 2.36 |
| 9b | 158 | 60 White | C23H17BrClNO6 (518.75) | 53.25 53.56 | 3.30 3.45 | 2.70 2.70 |
| 10 | 196 | 45 Yellow | C23H16BrNO6 (482.28) | 57.27 57.11 | 3.31 3.46 | 2.90 3.00 |
| 11 | 230 | 40 Yellow | C27H15Br2NO5 (593.22) | 54.66 54.87 | 2.54 2.34 | 2.36 2.45 |
| 12 | 282 | 40 Yellow | C24H19BrN2O3 (463.33) | 62.22 62.01 | 4.13 3.98 | 6.05 5.90 |
References
- El-Sayed, A.M.; Abd- Allah, O.A. Synthetic and Biological Studies on Coumarin Hydrazone Derivatives. Phosphorus, Sulfur and Silicon Relat.Elem. 2001, 170, 75–86. [Google Scholar] [CrossRef]
- Kalluraya, B.; Vishwanatha, P.; Isloor, A.M.; Rai, G.; Kotian, M. Synthesis and biological activity of 6-substituted-3-[2-(5-substituted-2-furfurylidenehydrazino)-4-thiazolyl]coumarins. Boll. Chim. Farm 2000, 139, 263–266. [Google Scholar]
- Abd-Allah, O.A. Synthesis and biological studies of some benzopyrano[2,3-c]-pyrazole derivatives. Farmaco 2000, 55, 641–649. [Google Scholar]
- El-Agrody, A.M.; Abd El-Latif, M.S.; El-Hady, N.A.; Fakery, A.H.; Bedair, A.H. Heteroaromatization with 4-Hydroxycoumarin Part II. Molecules 2001, 6, 519–527. [Google Scholar]
- Emmanuel-Giota, A.A.; Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Synthesis and biological evaluation of several 3-(coumarin-4-yl)-tetrahydroisoxazole and 3-(coumarin-4-yl)dihydropyrazole derivatives. J. Heterocyclic Chem. 2001, 38, 717–722. [Google Scholar]
- Manolov, I.; Danchev, N.D. Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarin. Eur. J. Med. Chem. Chim. Ther. 1995, 30, 531–536. [Google Scholar] [CrossRef]
- Nofal, Z.M.; El-Zahar, M.I.; Abd El-Karim, S.S. Novel Coumarin Derivatives with Expected Biological Activity. Molecules 2000, 5, 99–113. [Google Scholar]
- Raev, L.; Voinov, E.; Ivanov, I.; Popov, D. Antitumor activity of some coumarin derivatives. Pharmazie 1990, 45, 696, [Chem. Abstr. 1990, 114, 74711 B]. [Google Scholar]
- Shaker, R.M. Synthesis and reactions of some new 4H-Pyrano[3,2-c] benzopyran-5-one derivatives and their potential biological activities. Pharmazie 1996, 51, 148. [Google Scholar]
- Abd El-Fattah, A.M.; Sherif, S.M.; El-Reedy, A.M. New Synthesis of Imidazo[1,2-a] – and Pyrimido[1,2-a]Pyrimidines. Phosphorus. Sulfur and Silicon 1992, 70, 67–73. [Google Scholar] [CrossRef]
- Nene, Y.L.; Thapliyal, P.N. Fungicides in plant disease control. Oxford & IBH Publ: New Delhi, 1982; p. 192. [Google Scholar]
- Sample Availability: Samples of compounds 3a, 3b, 5, 6, 7, 9a, 9b, 10, 11 and 12 are available from MDPI. For other samples contact the authors.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Al-Haiza, M.A.; Mostafa, M.S.; El-Kady, M.Y. Synthesis and Biological Evaluation of Some New Coumarin Derivatives. Molecules 2003, 8, 275-286. https://doi.org/10.3390/80200275
Al-Haiza MA, Mostafa MS, El-Kady MY. Synthesis and Biological Evaluation of Some New Coumarin Derivatives. Molecules. 2003; 8(2):275-286. https://doi.org/10.3390/80200275
Chicago/Turabian StyleAl-Haiza, M. A., M. S. Mostafa, and M. Y. El-Kady. 2003. "Synthesis and Biological Evaluation of Some New Coumarin Derivatives" Molecules 8, no. 2: 275-286. https://doi.org/10.3390/80200275
APA StyleAl-Haiza, M. A., Mostafa, M. S., & El-Kady, M. Y. (2003). Synthesis and Biological Evaluation of Some New Coumarin Derivatives. Molecules, 8(2), 275-286. https://doi.org/10.3390/80200275




