Crystal Structure of N,N,N’,N’-tetra-[(3,5-dimethyl-1-pyrazolyl) methyl]-para-phenylenediamine
Abstract
:Introduction
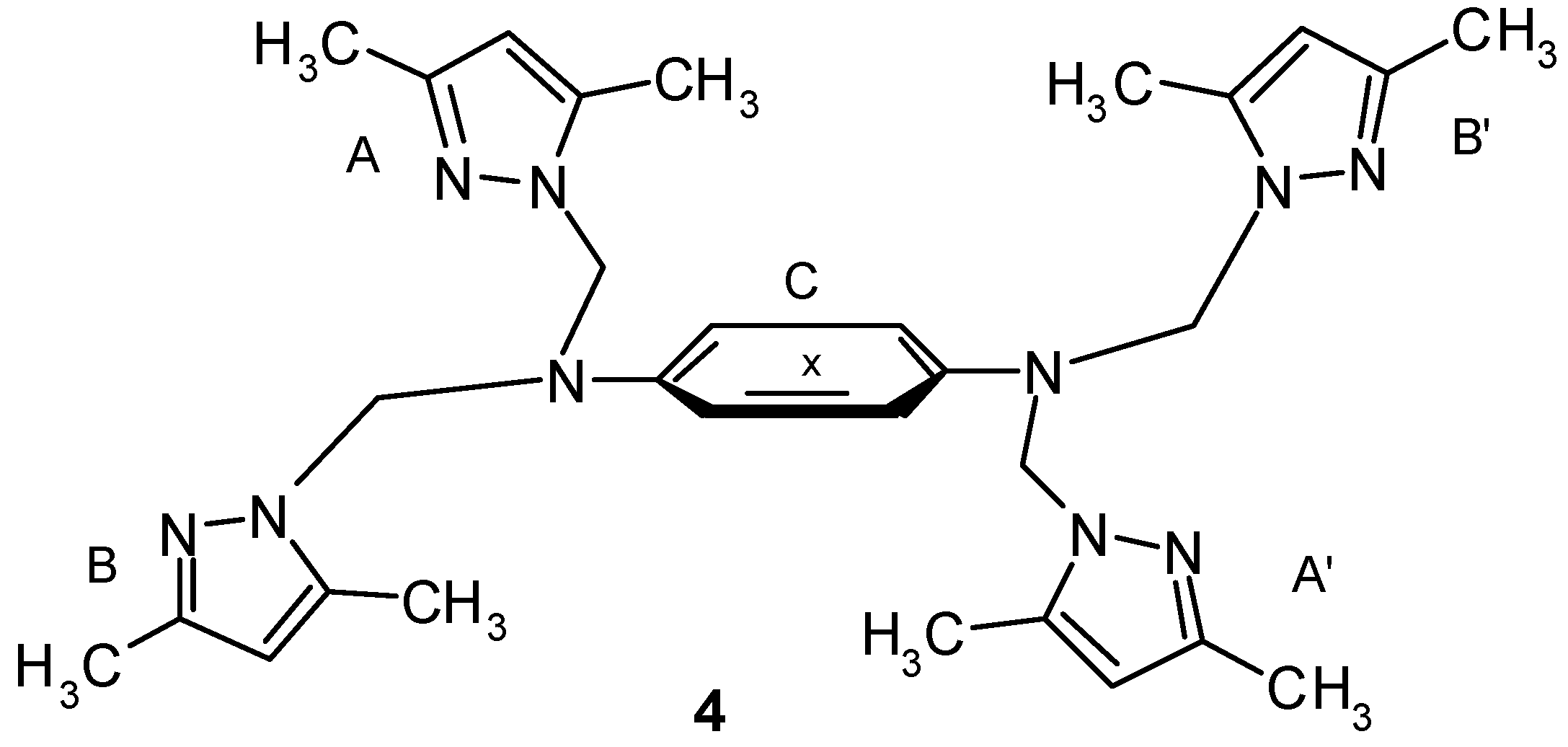
Results and Discussion
Synthesis of bis-tripodal ligand (4).
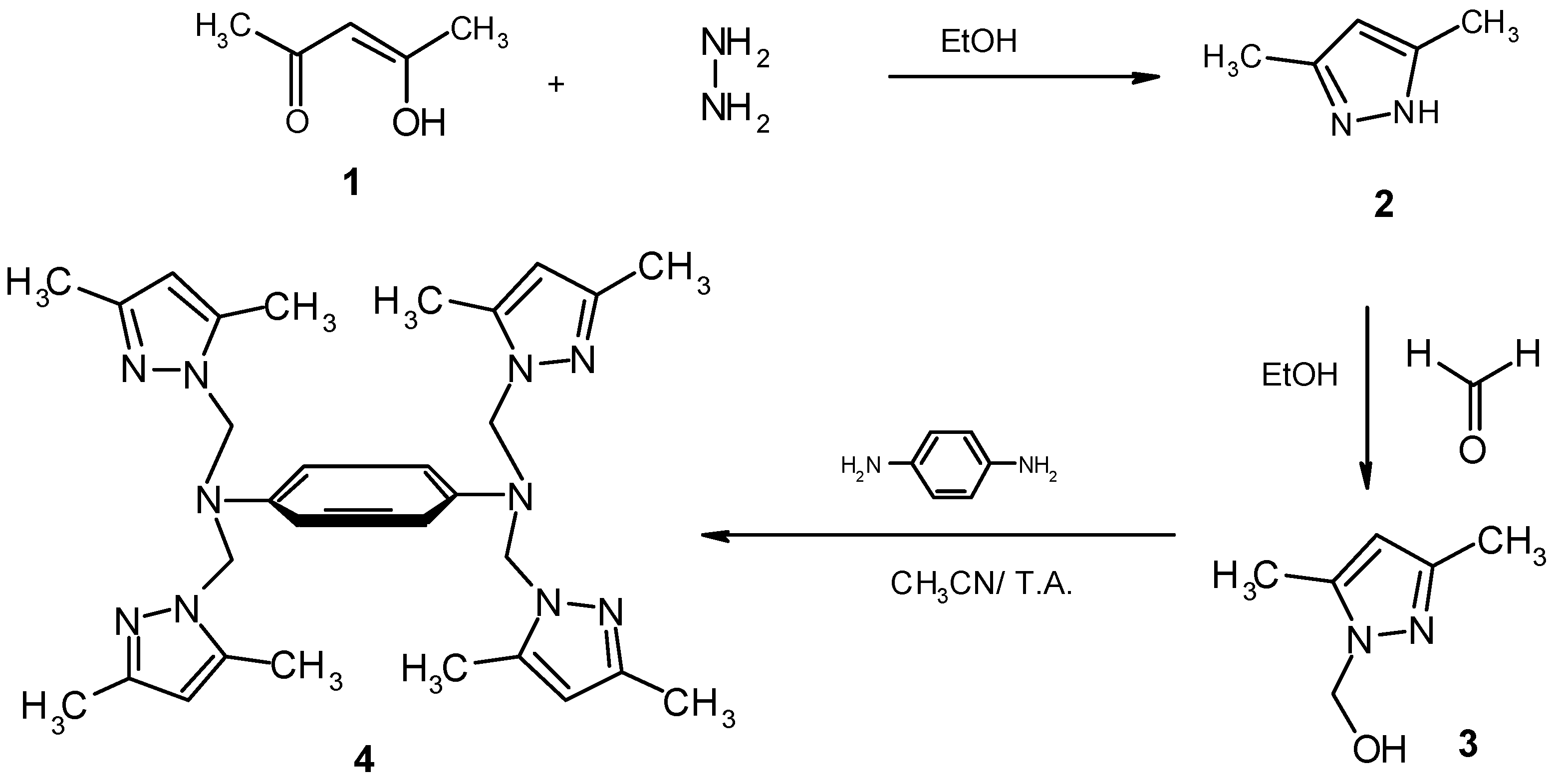
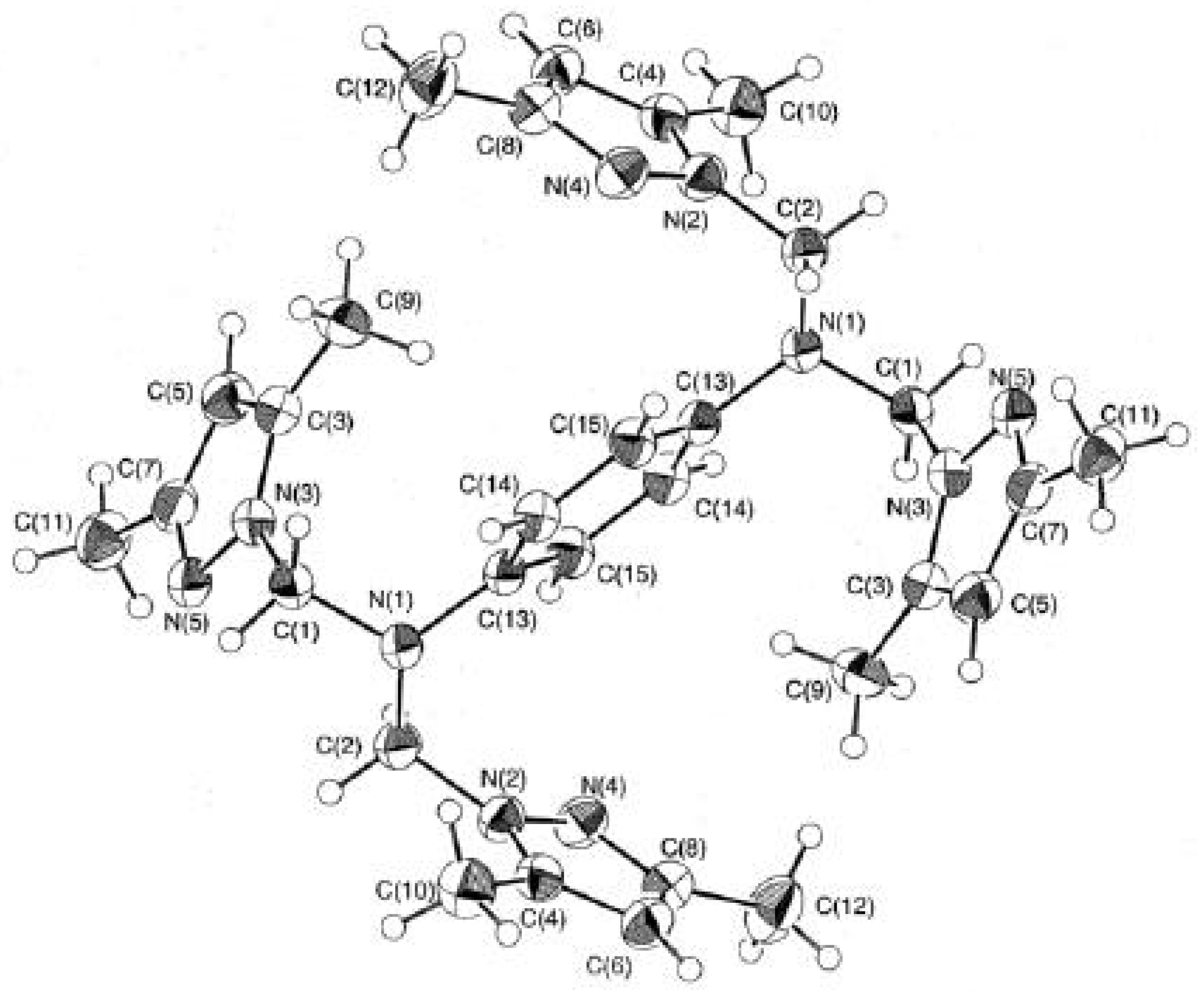
Crystallographic study of bis-tripodal ligand (4).
| Formula weight = 540.43 |
| Crystal system: monoclinic |
| Space group: P21/a Z = 2 |
| a = 11.262(2) Å a = 90.00 (1) ° |
| b = 12.259(3) Å β = 109.8 (8) ° |
| c = 11.876(5) Å γ = 90.00 (1) ° |
| V = 1517.6(5) Å3 |
| Dx = 1.183 g/cm3 |
| μ (Mo Kα) = 0.8 cm-1 |
| Shape = near prismatic form 0.16, 0.17, 0.20 mm |
| Transmission min = 0.691 |
| Transmission max = 1.036 |
| T = 298K |
| F (000) = 580 |
| Radiation: Mo Kα 0.71073 |
| θmax = 29.98 ° |
| R = 0.045 (on F) |
| Rw = 0.093 (on F2) |
| (?P)max = +0.54 |
| (?P)min = -0.47 |
| No. of reflections measured = 4622 |
| No. of reflections used = 1509 |
| No. of parameters = 182 |
| Goodness-of-fit = 0.551 |
| Measurement- Kappa CCD – Nonius Diffractometer |
| Program system: MaXus |
| Absorption correction : Sortav |
Conclusions
Experimental
General
Preparation of N,N,N’,N’-tetra-[(3,5-dimethyl-1-pyrazolyl) methyl]para-phenylenediamine (4).
Acknowledgements
References
- Sorrell, T.N.; Vankai, V.A.; Garrity, M. L. Inorg. Chem. 1991, 30, 207.
- Togni, A.; Venanzi, L. M. Angew. Chem. Int. Ed. Engl. 1994, 33, 497.
- Touzani, R.; Ramdani, A.; Ben-Hadda, T.; El Kadiri, S.; Maury, O.; Le Bozec, H.; Dixneuf, P. H. Synthetic Comm. 2001, 31, 39–45.
- Launay, J.-P. Chem. Soc. Rev. 2001, 30, 386–397. [CrossRef]
- Sondaz, E.; Jaud, J.; Launay, J.-P.; Bonvoisin, J. Eur. J. Inorg. Chem. 2002, in press.
- Bouabdellah, I.; Ramdani, A.; Zidane, I. (2001). Synthése de Nouveaux Ligands Bicentriques à Base de Tripodes et Quinoxalines. Rapport de DESA, Université Med Premier.
- Sheu, S. C.; Tien, M. J.; Cheng, M. C.; Ho, T. I.; Peng, S. M.; Lin, Y. C. J. Chem. Soc. Dalton Trans 1995, 3503–3510.
- Driessen, W. L.; Graaff, R. A. G.; Parlevliet, F. J.; Reedijk, J.; Vos, R. M. Inorg. Chim. Acta 1994, 216, 43–49.
- Dvoretzky, I.; Richter, G.H. J. Org. Chem. 1950, 15, 1285. [CrossRef]
- CCDC 186572 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via the URL http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
- Bol, J. E.; Maase, B.; Gonesh, G.; Driessen, W. L.; Goubitz, K.; Reedijk, J. Heterocycles 1997, 45, 1477–1492.
- Mackay, S.; Gilmore, C. J.; Edwards, C.; Stewart, N.; Shankland, K. maXus Computer Program for the Solution and Refinement of Crystal Structures. Bruker Nonius, The Netherlands, Mac-Science, Japan & The University of Glasgow (1999).
- Johnson, C. K. ORTEP-II. In A Fortran Thermal-Ellipsoid Plot Program; Report ORNL-5138; Oak Ridge National Laboratory: Oak Ridge, Tennessee, USA, (1976).
- Otwinowski, Z.; Minor, W. Methods in Enzymology; Carter, C.W., Jr., Sweet, R.M., Eds.; Academic Press: New York, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M. C.; Polidori, G.; Camalli, M. J. Appl. Cryst. 1994, 27, 435–435.
- Waasmaier, D.; Kirfel, A. Acta Cryst. 1995, A51, 416–431.
- Sample Availability: Samples of compound 4 are available from the authors and MDPI.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Daoudi, M.; Larbi, N.B.; Benjelloun, D.; Kerbal, A.; Launay, J.P.; Bonvoisin, J.; Jaud, J.; Mimouni, M.; Ben-Hadda, T. Crystal Structure of N,N,N’,N’-tetra-[(3,5-dimethyl-1-pyrazolyl) methyl]-para-phenylenediamine. Molecules 2003, 8, 269-274. https://doi.org/10.3390/80200269
Daoudi M, Larbi NB, Benjelloun D, Kerbal A, Launay JP, Bonvoisin J, Jaud J, Mimouni M, Ben-Hadda T. Crystal Structure of N,N,N’,N’-tetra-[(3,5-dimethyl-1-pyrazolyl) methyl]-para-phenylenediamine. Molecules. 2003; 8(2):269-274. https://doi.org/10.3390/80200269
Chicago/Turabian StyleDaoudi, Maria, Najib Ben Larbi, Driss Benjelloun, Abdelali Kerbal, Jean Pierre Launay, Jacques Bonvoisin, Joël Jaud, Mostafa Mimouni, and Taibi Ben-Hadda. 2003. "Crystal Structure of N,N,N’,N’-tetra-[(3,5-dimethyl-1-pyrazolyl) methyl]-para-phenylenediamine" Molecules 8, no. 2: 269-274. https://doi.org/10.3390/80200269
APA StyleDaoudi, M., Larbi, N. B., Benjelloun, D., Kerbal, A., Launay, J. P., Bonvoisin, J., Jaud, J., Mimouni, M., & Ben-Hadda, T. (2003). Crystal Structure of N,N,N’,N’-tetra-[(3,5-dimethyl-1-pyrazolyl) methyl]-para-phenylenediamine. Molecules, 8(2), 269-274. https://doi.org/10.3390/80200269




