Abstract
3-Aryl-2-chloropropylisothiocyanates (1) are formed by interaction of arene-diazonium chlorides with allyl isothiocyanate. Adducts 1 react with monoacylhydrazines to form 1-acyl-4-(3-aryl-2-chloropropyl)thiosemicarbazides (2a–d). Thiosemicarbazides 2a–d in the presence of bases selectively transform into 2-(2-R1-hydrazino)-5-(R2-benzyl)-2-thiazolines (3a–d).
Introduction
There are different approaches to the synthesis of 2-thiazoline derivatives [1,2]. One of the most convenient methods is cyclization of compounds containing the CH(X)CH2NHC(S) fragment that take place when they are heated or when they are subjected to base-catalysis. Another well-known method involves an addition of an electrophilic reagent to the double bond of the allylic fragment of the thiourea or a related compound.
To obtain five- or six-membered heterocycles, allyl isothiocyanate or its dibromo-derivative [3] were used as starting materials. We now offer a new approach to the synthesis of such compounds by the use of chloro- or bromoarylation products of allyl isothiocyanate in reaction with arenediazonium halides. Taking into account that Ar and Hal add to the double bond as the result of catalytic dediazoniation of arenediazonium halides in the presence of unsaturated compounds [4], we used this approach with allyl isothiocyanate to obtain suitable reagents for the synthesis of 2-thiazolines [5,6].
Results and Discussion
It had been shown before that allyl isothiocyanate takes part in a catalytic reaction with arenediazonium chlorides [4,5]. The result is formation of the addition products of an aryl group and a chlorine atom to the C=C double bond (Meerwein reaction).
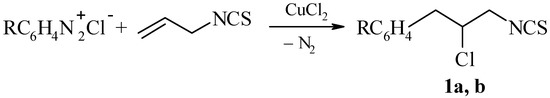
Scheme 1.
Scheme 1.
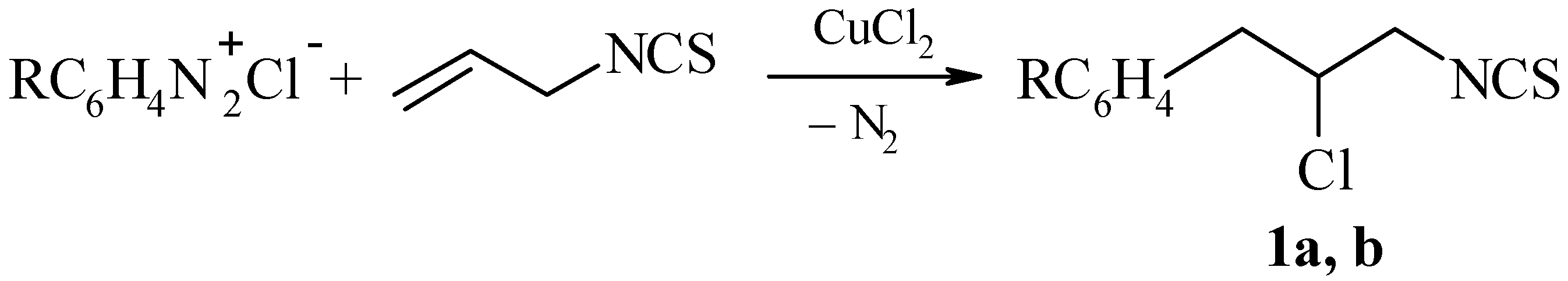
R = H (1a) and 3-Cl (1b).
Compounds 1a,b are suitable reagents for obtaining five-membered heterocycles. Specifically, these compounds react with primary and secondary amines to form 1,3-disubstituted thioureas, which were converted to 2-thiazoline-derivatives in the presence of bases [5,6]. In this work the interaction of chloroarylation products of allyl isothiocyanate 1a,b with some monoacylhydrazines was investigated. It was shown that the result of this reaction was formation of 1,4-disubstituted thiosemicarbazides 2a–d.
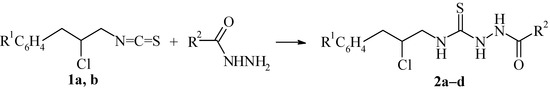
Scheme 2.
Scheme 2.
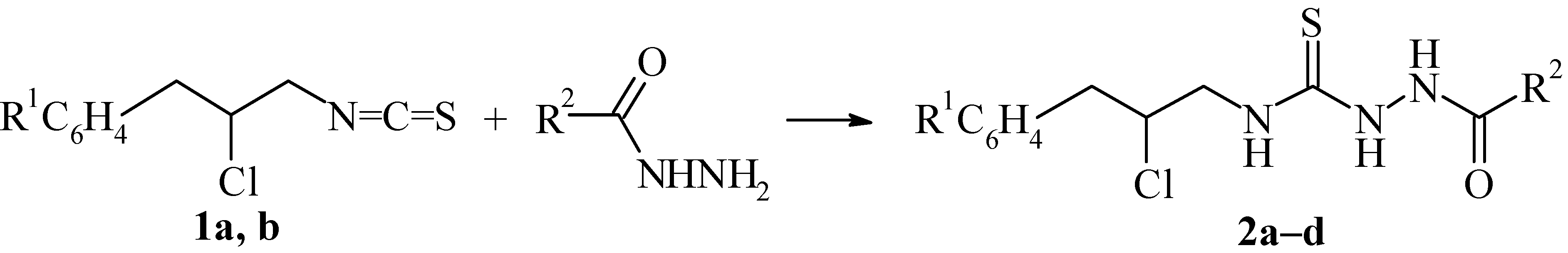

| 2a | 2b | 2c | 2d | |
|---|---|---|---|---|
| R1 | H | H | 3-Cl | 3-Cl |
| R2 | C5H11 | Ph | C5H11 | Ph |
Thiosemicarbazides 2a–d contain in the side chain the chlorine atom and several other nucleophilic centers. For this reason the can be used for intramolecular cyclisations with subsequent formation of heterocycles. Actually, compounds 2a–d cyclize in the presence of bases to form 2-thiazoline derivatives 3a–d, which contain hydrazino groups in the 2 position of the ring (pathway a). It has been noted that other variants of this cyclization – formation of imidazolidine-2-thione derivatives (pathway b), or hexahydro-1,2,4-thiazine-3-thione derivatives (pathway c) do not take place. This may be caused by the high nucleophility of the sulfur atom relative to the nitrogen atoms:
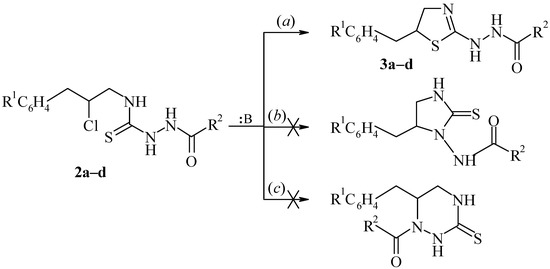
Scheme 3.
Scheme 3.
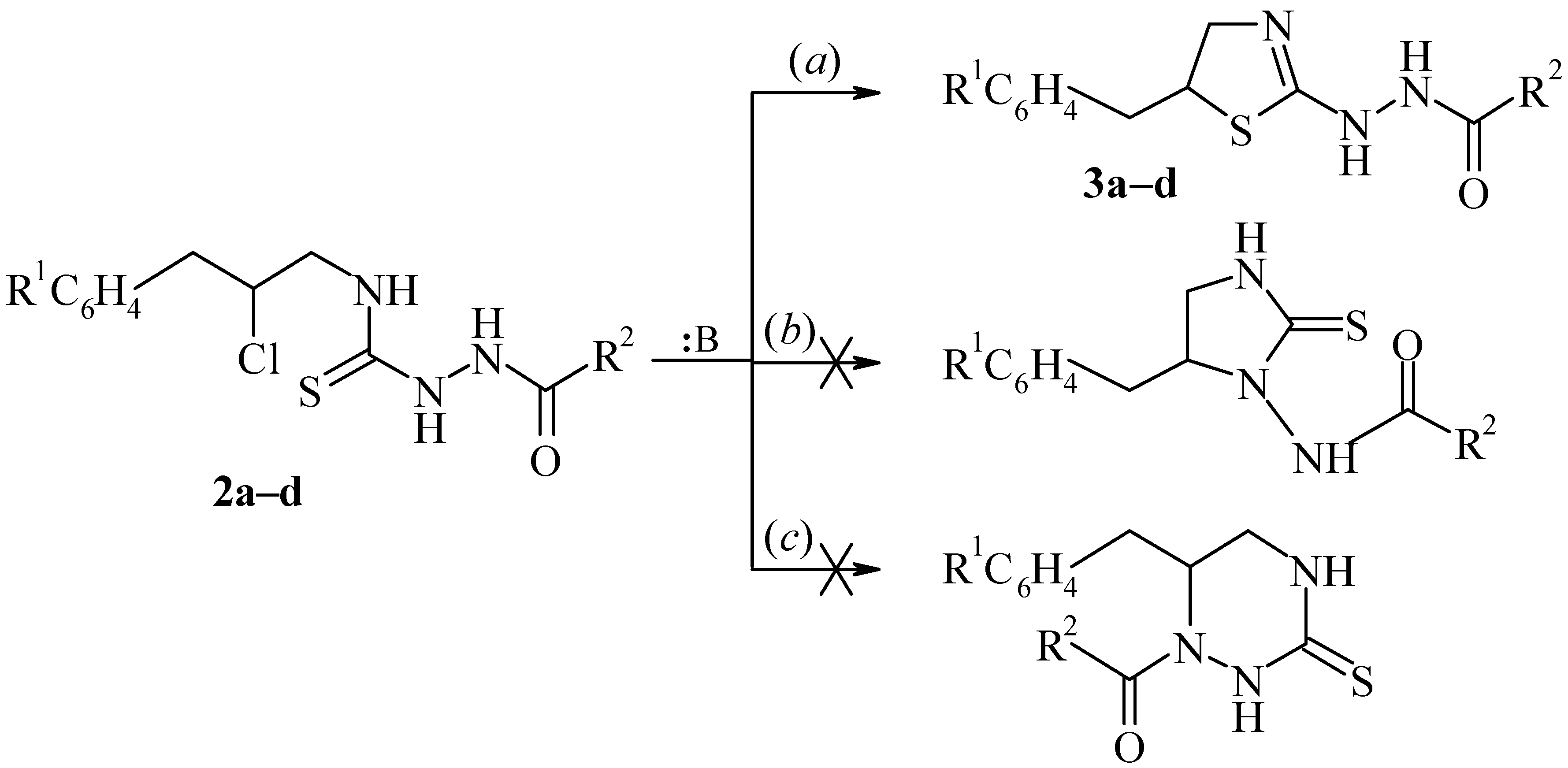
The heterocyclization reactions of thiosemicarbazides 2a–d were carried out in acetone, in the presence of organic bases (triethylamine or N-methylmorpholine). It is important to note that 2-thiazoline derivatives 3a–d, which contain the hydrazino group in the 2 position of the ring, are difficult to obtain by other methods. The structure of the synthesized compounds has been confirmed by 1H-NMR-spectroscopy. The spectra of the compounds 2a–d and 3a–d (see Experimental) contain two ABX spin systems (CH2CHXCH2). It is significant that the chemical shifts of the proton in the CHCl group (thiosemicarbazides 2a–d) and those of the protons in the CHS group (thiazolines 3a–d) differ by ~ 0.5 ppm. The protons of the NHC(S)NH fragments in compounds 2a–d and the NHNH in thiazolines 3a–d do not give sharp signals in the 1H-NMR spectra.
Conclusions
We have presented a facile route for the formation of 1-acyl-4-(3-aryl-2-chloropropyl)-thiosemicarbazides and 2-(2-R1-hydrazino)-5-(R2-benzyl)-2-thiazolines.
Experimental
General
All melting points are uncorrected. 1H-NMR spectra were obtained using a Bruker AC-200 spectrometer and were recorded at 200 MHz in DMSO-d6. Chemical shifts are reported in ppm relative to the residual signal of the solvent. When the signals of the CH2N or CH2Ar groups in compounds 2a–d and 3a–d were not sharp, coupling constants were not determined. For narrow multiplets the centres of the signals is indicated. The chloroarylation of allyl isothiocyanate was carried by the method described in [4]. The data of 3-phenyl-2-chloropropylisothiocyanate (1a) are presented there.
3-(3-Chlorophenyl)-2-chloropropylisothiocyanate (1b); yield 27%, b.p. 108–110°C (0.5 mm), n20D 1.6066; Found: C, 48.58; H, 3.77; S, 13.25. C10H9Cl2NS requires C, 48.79; H, 3.69; S, 13.03%; 1H-NMR: δ 2.98 (dd, 1H, CH2Ar, J13 8.0 Hz, J12 14.2 Hz), 3.19 (dd, 1H, CH2Ar, J13 5.0 Hz), 3.89 (dd, 1H, CH2N, J13 5.8 Hz, J12 15.2 Hz), 4.04 (dd, 2H, CH2N, J13 4.0 Hz), 4.53 (m, 1H, CH), 7.20–7.60 (m, 4H, C6H4).
1-Acyl-4-(3-aryl-2-chloropropyl)thiosemicarbazides (2a-d). A mixture of 3-aryl-2-chloropropyl-isothiocyanate (1, 5 mmole) and the corresponding monoacylhydrazine (5 mmole) was boiled in benzene (5 mL) for 1 hour. The precipitates obtained were filtered and recrystallized from the solvent indicated.
1-Caproyl-4-(3-phenyl-2-chloropropyl)thiosemicarbazide (2a); yield 61%, m.p. 170–171°C (from dioxane); Found: C, 56.14; H, 7.22; S, 9.21; C16H24ClN3OS requires C, 56.21; H, 7.08; S, 9.38%; 1H-NMR: δ 0.90 (t, 3H, CH3), 1.24–1.36 (m, 4H, CH2CH2CH3), 1.50–1.64 (m, 2H, CH2CH2C=O), 2.22 (t, 2H, CH2C=O), 3.01(dd, 1H, CH2Ar), 3.17 (dd, 1H, CH2Ar), 3.78 (dd, 1H, CH2N), 4.03 (dd, 1H, CH2N), 4.49 (m, 1H, CH), 7.26 (m, 5H, C6H5), 10.98 (s, 1H, NHC=O).
1-Benzoyl-4-(3-phenyl-2-chloropropyl)thiosemicarbazide (2b); yield 72%, m.p. 172–173°C (from toluene); Found: C, 58.81; H, 5.32; S, 9.43; C17H18ClN3OS requires C, 58.69; H, 5.21; S, 9.22%; 1H-NMR: δ 3.05 (dd, 1H, CH2Ar), 3.19 (dd, 1H, CH2Ar), 3.77 (m, 1H, CH2N), 4.02 (m, 1H, CH2N), 4.50 (m, 1H, CH), 7.04–7.36 (m, 5H, C6H5), 7.44–7.63 (m, 3H, C6H5), 7.98–8.08 (m, 2H, C6H5 – o-H), 11.69 (s, 1H, NHC=O).
1-Caproyl-4-[3-(3-chlorophenyl)-2-chloropropyl]thiosemicarbazide (2c); yield 53%, m.p. 161–162°C (from toluene); Found: C, 50.89; H, 6.04; S, 8.64; C16H23Cl2N3OS requires C, 51.06; H, 6.16; S, 8.52%); 1H-NMR: δ 0.88 (t, 3H, CH3), 1.20–1.40 (m, 4H, CH2CH2CH3), 1.48–1.67 (m, 2H, CH2CH2C=O), 2.23 (t, 2H, CH2C=O), 3.01 (dd, 1H, CH2Ar, J13 8.6 Hz, J12 13.6 Hz), 3.19 (dd, 2H, CH2Ar, J13 7.0 Hz), 3.80 (dd, 1H, CH2N), 4.06 (dd, 1H, CH2N), 4.50 (m, 1H, CH), 7.20–7.45 (m, 4H, C6H4), 11.00 (s, 1H, NHC=O).
1-Benzoyl-4-[3-(3-chlorophenyl)-2-chloropropyl]thiosemicarbazide (2d); yield 46%, m.p. 188–189°C (from benzene); Found: C, 53.57; H, 4.31; S, 8.23; C17H17Cl2N3OS requires C, 53.41; H, 4.48; S, 8.39%; 1H-NMR: δ 3.09 (dd, 1H, CH2Ar, J13 8.0 Hz, J12 14.4 Hz), 3.21 (dd, 1H, CH2Ar, J13 6.4 Hz), 3.80 (dd, 1H, CH2N), 4.04 (dd, 1H, CH2N), 4.55 (m, 1H, CH), 7.20–7.40 (m, 4H, Ar), 7.56 (m, 3H, Ar), 8.04 (m, 2H, C6H5 – o-H), 11.64 (s, 1H, NHC=O).
2-(2-Acylhydrazino)-5-benzyl-2-thiazolines (3a–d). Triethylamine (or N-methylmorpholine) (5 mmoles) was added to a solution of the corresponding thiosemicarbazide 2a–d (2 mmoles) in acetone (5 mL) and the mixture was boiled for 30 min. After cooling water (15 mL) was added to the mixture. The precipitate was filtered, air dried and recrystallized from the appropriate solvent.
2-(2-Caproylhydrazino)-5-benzyl-2-thiazoline (3a); yield 86%, m.p. 156–157°C (from benzene – cyclohexane, 3:1); Found: C, 62.80; H, 7.70; S, 10.61; C16H23N3OS requires C, 62.92; H, 7.59; S, 10.50%); 1H-NMR: δ 0.90 (t, 3H, CH3), 1.18–1.38 (m, 4H, CH2CH2CH3), 1.42–1.60 (m, 2H, CH2CH2C=O), 2.02 (t, 2H, CH2C=O), 2.92 (dd, 1H, CH2Ar, J13 9.2 Hz, J12 14.4 Hz), 3.04 (dd, 1H, CH2Ar, J13 6.8 Hz), 3.37 (dd, 1H, CH2N, J13 6.6 Hz, J12 8.0 Hz), 3.62 (dd, 1H, CH2N, J13 6.4 Hz), 3.94 (m, 1H, CH), 7.19–7.35 (m, 5H, C6H5).
2-(2-Benzoylhydrazino)-5-benzyl-2-thiazoline (3b); yield 56%, m.p. 143–144°C (from toluene); Found: C, 65.41; H, 5.35; S 10.11; C17H17N3OS requires C, 65.57; H, 5.50; S, 10.30%); 1H-NMR: δ 2.93 (dd, 1H, CH2Ar, J13 8.6 Hz, J12 14.0 Hz), 3.06 (dd, 1H, CH2Ar, J13 6.0 Hz), 3.40 (dd, 1H, CH2N, J13 6.4 Hz, J12 11.2 Hz), 3.66 (dd, 1H, CH2N, J13 7.0 Hz), 3.98 (m, 1H, CH), 7.10–7.33 (m, 5H, C6H5), 7.38–7.48 (m, 3H, C6H5), 7.77–7.87 (m, 2H, C6H5 – o-H).
2-(2-Caproylhydrazino)-5-(3-chlorobenzyl)-2-thiazoline (3c); yield 69%, m.p. 149–150°C (from petroleum ether – benzene, 1:5); Found: C, 56.74; H, 6.68; S, 9.33; C16H22ClN3OS requires C, 56.54; H, 6.52; S, 9.43%); 1H-NMR: δ 0.89 (t, 3H, CH3), 1.21–1.37 (m, 4H, CH2CH2CH3), 1.45–1.60 (m, 2H, CH2CH2C=O), 2.03 (t, 2H, CH2C=O), 2.88 (dd, 1H, CH2Ar, J13 8.2 Hz, J12 13.6 Hz), 3.05 (dd, 1H, CH2Ar, J13 6.4 Hz), 3.38 (dd, 1H, CH2N, J13 5.4 Hz, J12 10.4 Hz), 3.63 (dd, 2H, CH2N, J13 6.6 Hz), 3.97 (m, 1H, CH), 7.16–7.36 (m, 4H, C6H4).
2-(2-Benzoylhydrazino)-5-(3-chlorobenzyl)-2-thiazoline (3d); yield 56%, m.p. 176–177°C (from toluene); Found : C, 59.21; H, 4.44; S, 9.37; C17H16ClN3OS requires C, 59.04; H, 4.66; S, 9.27%); 1H-NMR: δ 2.92 (dd, 1H, CH2Ar, J13 8.0 Hz, J12 14.4 Hz), 3.08 (dd, 2H, CH2Ar, J13 6.2 Hz), 3.41 (dd, 1H, CH2N, J13 5.6 Hz, J12 10.2 Hz), 3.66 (dd, 1H, CH2N, J13 5.8 Hz), 4.01 (m, 1H, CH), 7.15–7.50 (m, 7H, Ar), 7.78–7.88 (m, 2H, C6H5 – o-H).
References and Notes
- Heterocyclic Compounds; Elderfield, R. C. (Ed.) Moscow, 1961; Vol. 5, pp. 531–544.
- Staninets, V. I.; Shilov, Ye. A. Addition Reactions with intramolecular ring closure. Uspekhi Khimii (Chem. Rev. USSR) 1971, 40, 491–512. [Google Scholar]
- Tkachenko, S. E.; Pushin, A. N.; Fedoseev, V. M. Course of the addition of amines to 2,3-dibromopropyl isothiocyanate. J. Gen. Chem. USSR 1987, 57, 2146–2147. [Google Scholar]
- Obushak, N. D.; Karpyak, V. V.; Ganushchak, N. I.; Koval’chuk, E. P.; Tikhonov, V. P. Chloroarylation of allyl compounds. Russ. J. Org. Chem. 1993, 29, 1149–1156. [Google Scholar]
- Obushak, M. D.; Karpyak, V. V.; Ganushchak, M. I. Synthesis of Heterocycles on the Basis of Anionoarylation Products of Unsaturated Compounds. 6. Halogenoarylation of Allyl Isothiocyanate: Synthesis of 2,5-Disubstituted 2-Thiazolines. Heteroatom Chem. 1999, 10, 517–525. [Google Scholar] [CrossRef]
- Karpyak, V. V.; Obushak, N. D.; Ganushchak, N. I. Preparation of 2,5-disubstituted 2-thiazolines. Chem. Heterocycl. Compds. 1997, 1278–1279. [Google Scholar]
- Sample Availability: Compounds 1-3 are available from the authors.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
