Abstract
Reaction of 1,2-dihydro-2-thioxopteridin-4(3H)-one, 6,7-dimethyl-1,2-dihydro-2-thioxopteridin-4(3H)-one and 6,7-diphenyl-1,2-dihydro-2-thioxopteridin- 4(3H)-one with hydrazonoyl halides affords the title compounds in good yields.
Introduction
Thiourea reacts with hydrazonoyl halides to give thiadiazoles [1,2,3], thiazoles [4,5,6] or triazoles [7] according to the reaction conditions and the type of hydrazonoyl halide used. In a subsequent investigation hydrazonoyl halides were found to react with heterocyclic compounds containing thiourea moieties to give only one of the two isomers possible based on the assumption that ring nitrogen adjacent to carbonyl group is less basic [8,9,10,11]. This communication describes the reaction of hydrazonoyl halides with 1,2-dihydro-2-thioxopteridin-4(3H)-one derivatives yielding triazolopteridin- 5-ones and not the other isomeric triazolopteridin-9-one products.
Results and Discussion
The reaction of 1 with hydrazonoyl halides 2 was carried out in THF under reflux in the presence of triethylamine. After workup of the reaction mixtures only a single product was isolated in each case. The spectral and elemental analysis data of these products are listed in Table I. The pathways leading to the subsequent products 9 or 10 are outlined in Scheme 1 and Scheme 2. In Scheme 1 it is suggested that the studied reactions involve an initial formation of thiohydrazonate esters 4, which undergo a S ° N migration to give the thiohydrazides 6 via the spirocycloadduct 5. Compounds 6 can also be formed via 1,3-dipolar cycloadditions of nitrilimines 3 (generated in situ from the reaction of hydrazonoyl halides 2 with triethylamine) onto the C=S thione group of 1.

Scheme 1.
Scheme 1.
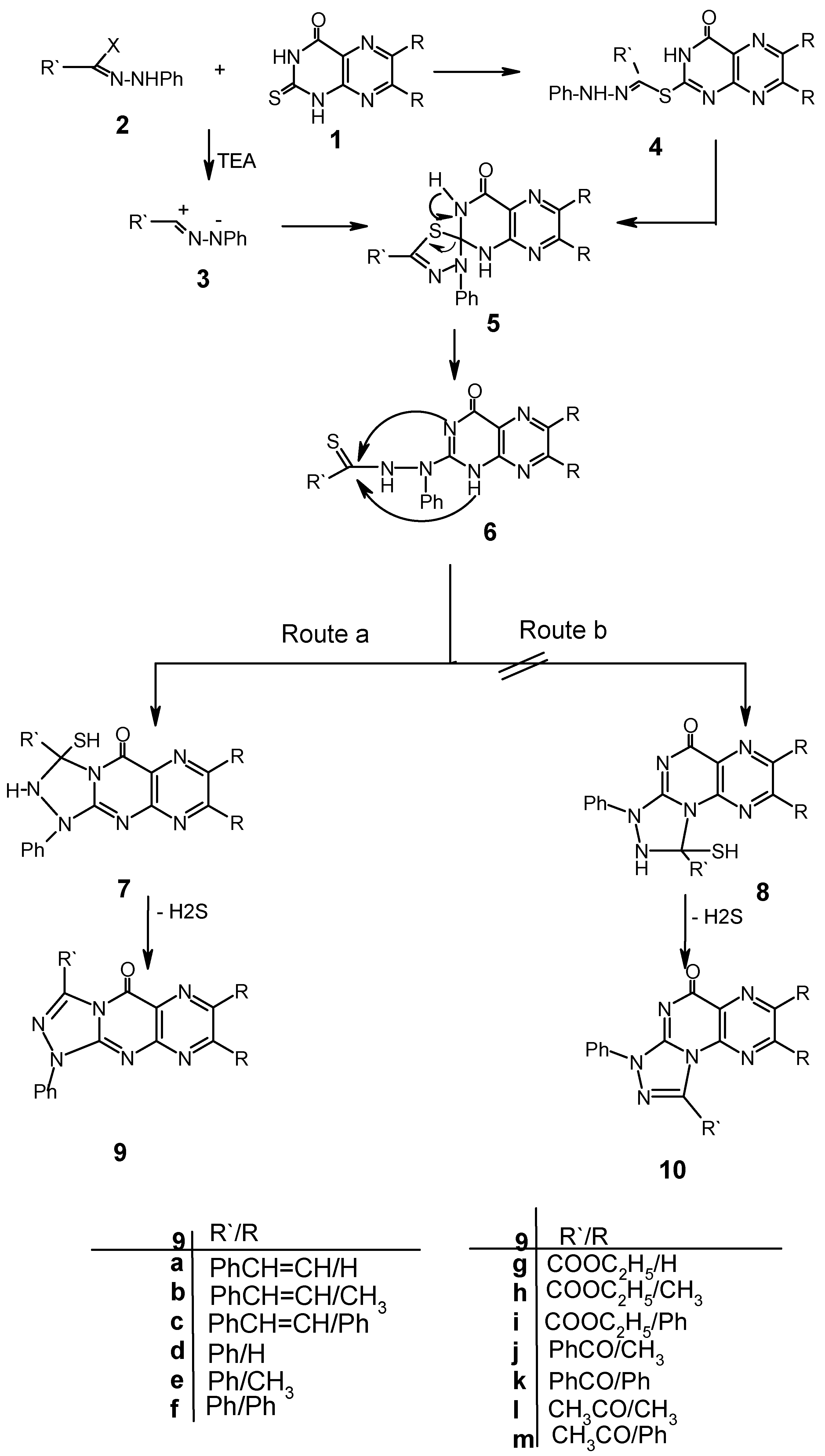
Attempts to isolate the thiohydrazonate ester 4 or the thiohydrazide open chain intermediates 6 did not succeed even under mild conditions as they readily undergo in situ cyclization followed by elimination of hydrogen sulfide to give the final products, that may have either structure 9 or 10 depending on the cyclization step involved (Routes a or b as depicted in Scheme 1).
In Scheme 2 it is suggested that reaction of 1 with 2 starts with nucleophilic attack on N3 or N1 to give the substitution products 11 or 12 respectively. Cyclization of the latter intermediates and elimination of hydrogen sulfide would then give the end products 9 or 10 respectively.
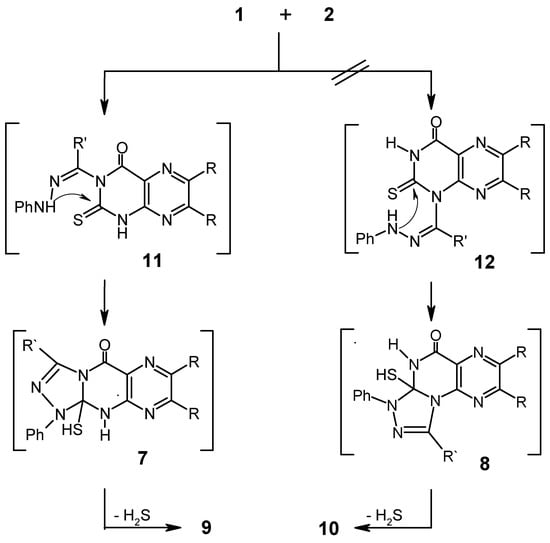
Scheme 2.
Scheme 2.
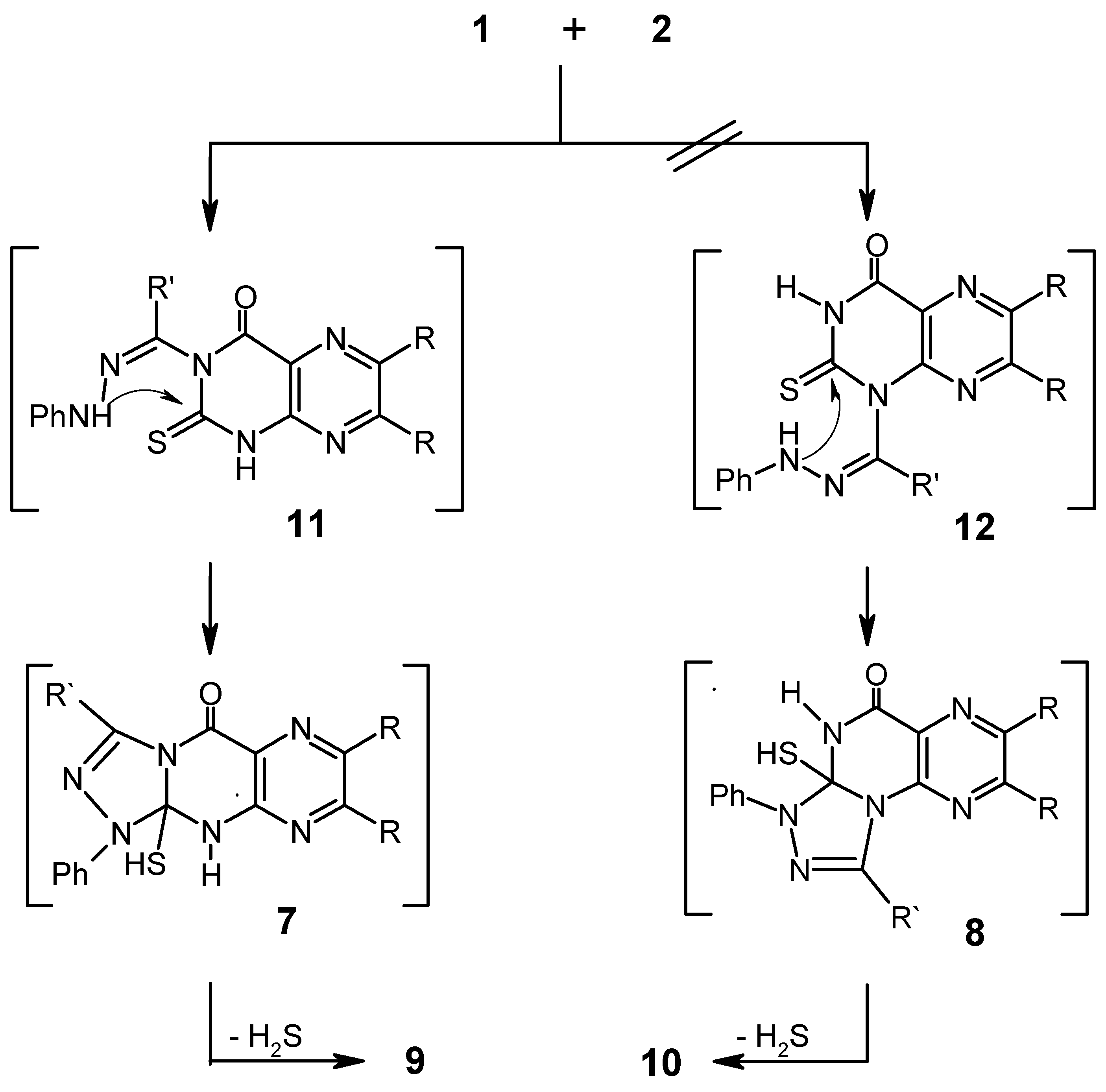
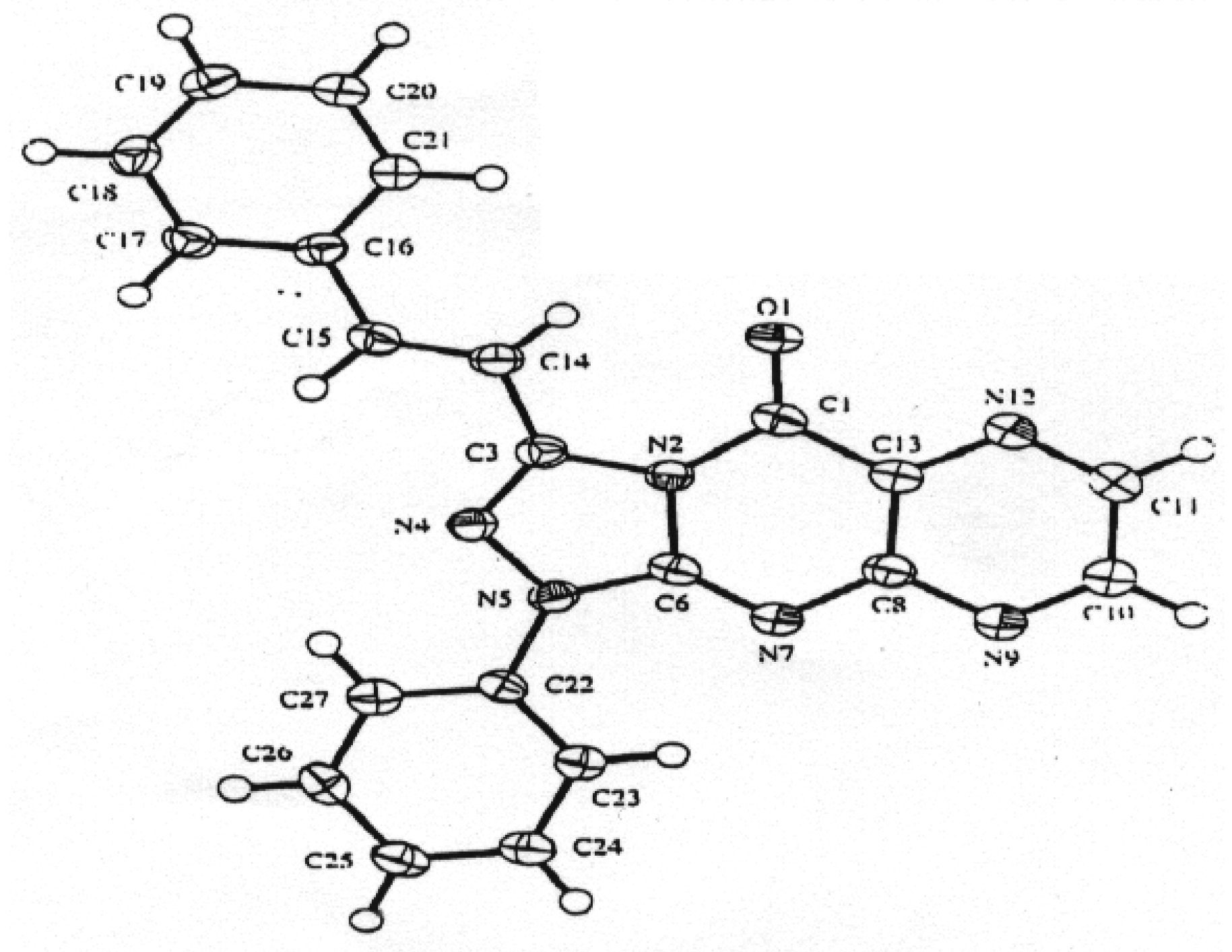
Although it is difficult at present to distinguish between the two mechanisms, the one proposed in Scheme 2 was discarded on the following basis: (1) although amidrazones of type 11 or 12 are known to be stable [12,13,14], all attempts to separate them from the reaction mixture failed; (2) reaction of 2- thiouracil derivatives with various halogen compounds yielded in all reported cases S-substituted products [15,16,17,18]. Thus, the mechanism proposed in Scheme 1 seems to be more acceptable. As the spectroscopic data alone does not allow one to distinguish between possible products 9 or 10, conclusive evidence was obtained by X-ray crystallographic analysis of compound 9a (Figure 1), thus confirming the structure 9 of the products.
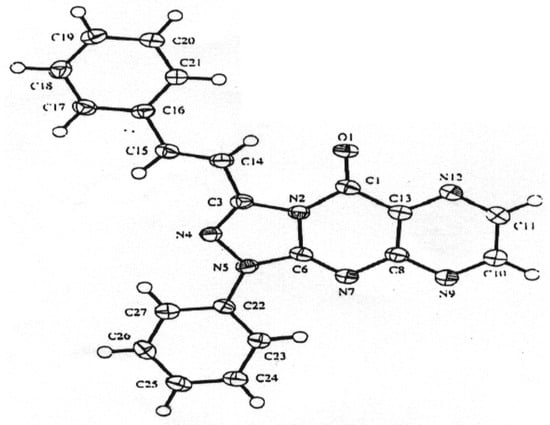
Figure 1.
Diagram of compound 9a with crystallographic numbering system
Figure 1.
Diagram of compound 9a with crystallographic numbering system
Experimental
General
All melting points were determined on an Electrothermal melting point apparatus and are uncorrected. IR spectra were recorded (KBr discs) on a Shimadzu FT-IR 8201 PC spectrophotometer. 1H-NMR spectra were recorded in CDCl3 and (CD3)2SO solutions on a Varian Gemini 200 MHz spectrometer and chemical shifts are expressed in δ units using TMS as internal reference. Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer, operating at 70 eV. Elemental analyses were carried out at Microanalytical Center of the University of Cairo, Giza, Egypt.
General procedure for the synthesis of 2-aryl-3-styryl-1,2,4-triazolo[4,3-a]7,8-pteridin-5-ones (9). A suspension of pteridine 1 [19] (5 mmoles) in tetrahydrofuran (25 mL) was refluxed with hydrazonoyl halides 2 [4,6,20,21,22] (5 mmoles) and triethylamine (0.7 mL, 5 mmoles) for 10 h. The excess solvent was evaporated and the residue was triturated with methanol (10 mL). The solid formed was collected and crystallized from dimethylformamide to give analytically pure product. The physical and spectral data of the compounds 9a-m thus prepared is summarized in Table 1.
X-ray structural analysis of compound 9a. Crystal data C21H14N6O, M=3.664, monoclinic P`1, a=8.874 (3), b=17.978 (4), c=11.229 (3), Å, α=90, β=108.97, (2), γ=90. The alkene substituent has the E conformation. The non-hydrogen atoms were refined anisotropically refinement of the structure was carried out on F using full-matrix least square procedure, which minimized the function Σ w (|Fo| - |Fc|) [23].

Table I.
Physical and spectral data of new Triazolopteridines
| Compound | m.p. (°C) (solvent) | Yield (%) | IR (ν) | MS(70 ev) m/e (M+) | Molecular Formula | Analysis Calcd Found | 1H NMR | ||
|---|---|---|---|---|---|---|---|---|---|
| 9a | 253 (DMF) | 80 | 1709 | 366 | C21H14N6O (366) | C H N | 68.8 3.8 22.9 | 68.8 3.8 22.7 | 7.2-7.6 (m, 8H); 7.9 (d, J=5 Hz, 1H); 8.1 (d, J=5 Hz, 1H); 8.3 (s, 1H); 8.4 (s, 1H); 8.8 (d, J=14 Hz, 1H) and 8.9 (d, J=14 Hz, 1H). |
| 9b | 277 (DMF) | 75 | 1705 | 394 | C23H18N6O (394) | C | 70.0 | 69.8 | 2.7 (s, 3H); 2.8 (s, 3H) and 7.2-8.4 (m, 12H). |
| H | 4.6 | 4.7 | |||||||
| N | 21.3 | 21.4 | |||||||
| 9c | 302 (DMF) | 82 | 1714 | 518 | C33H22N6O (518) | C | 76.4 | 76.4 | 7.3-8.5 (m, Ar-H). |
| H | 4.2 | 4.3 | |||||||
| N | 16.2 | 16.0 | |||||||
| 9d | 314 (DMF) | 78 | 1727 | 340 | C19H12N6O (340) | C | 67.1 | 67.3 | 7.2-7.9 (m, 10H); 8.7 (d, J=14 Hz, 1H) and 8.8 (d, J=14 Hz, 1H). |
| H | 3.5 | 3.3 | |||||||
| N | 24.7 | 24.4 | |||||||
| 9e | 287 (DMF) | 75 | 1708 | 368 | C21H16N6O (368) | C | 68.5 | 68.7 | 2.7 (s, 3H); 2.7 (s, 3H) and 7.3- 8.4 (m, 10H). |
| H | 4.4 | 4.4 | |||||||
| N | 22.8 | 22.6 | |||||||
| 9f | 308 (DMF) | 75 | 1725 | 492 | C31H20N6O (492) | C | 75.6 | 75.6 | 7.3-8.5(m,Ar-H) |
| H | 4.1 | 4.0 | |||||||
| N | 17.1 | 16.9 | |||||||
| 9g | 199 (DMF) | 77 | 17451707 | 336 | C16H12N6O3 (336) | C | 57.1 | 57.1 | 1.5 (t, J=7 Hz, 3H); 4.7 (q, J=7 Hz, 2H); 7.3-7.6 (m, 5H); 8.3 (d, J=14 Hz, 1H) and 8.8 (d, J=14Hz, 1H). |
| H | 3.6 | 3.6 | |||||||
| N | 25.0 | 24.9 | |||||||
| 9h* | 211 (DMF) | 77 | 17141675 | C18H16N6O3 | C | 59.3 | 59.4 | 1.4 (t, J=7 Hz, 3H); 2.7 (s, 3H); 2.8 (s, 3H); 4.6 (q, J=7Hz, 2H) and 7.3-8.3 (m, 5H). | |
| H | 4.4 | 4.2 | |||||||
| N | 23.1 | 22.9 | |||||||
| 9i | 206 (DMF) | 84 | 17301640 | 488 | C28H20N6O3 (488) | C | 68.9 | 68.7 | 1.5 (t, J=7Hz, 3H); 4.6 (q, J=7 Hz, 2H) and 7.2-8.3 (m, 15H). |
| H | 4.1 | 3.9 | |||||||
| N | 17.2 | 16.9 | |||||||
| 9j | 298 (DMF) | 78 | 17231683 | 396 | C22H16N6O2 (396) | C | 66.7 | 66.5 | 2.7 (s, 3H); 2.8 (s, 3H) and 7.3-8.3 (m, 10H). |
| H | 4.0 | 3.9 | |||||||
| N | 21.2 | 21.2 | |||||||
| 9k | 183 (DMF) | 72 | 17731637 | 520 | C32H20N6O2 (520) | C | 73.9 | 73.9 | 7.2-8.3 (m, Ar-H) |
| H | 3.9 | 3.9 | |||||||
| N | 16.2 | 16.1 | |||||||
| 9l | 197 (DMF) | 66 | 17101680 | 334 | C17H14N6O2 (334) | C | 61.1 | 61.1 | 2.6 (s, 3H), 2.7 (s, 6H) and 7.3-8.2 (m, 5H). |
| H | 4.2 | 4.3 | |||||||
| N | 25.1 | 25.0 | |||||||
| 9m | 196 (DMF) | 70 | 17621640 | 458 | C27H18N6O2 (458) | C | 70.7 | 70.8 | 2.6 (s,3H) and 7.2-7.8 (m, 15H). |
| H | 3.9 | 3.6 | |||||||
| N | 18.3 | 18.2 | |||||||
* 13 C-NMR of compound 9h: 15.6, 23.9, 25.2, 66.1, 122.7, 126.2, 129.7, 131.4, 137.9, 138.3, 147.0, 153.5, 156.0, 156.4, 158.4, 163.5).
References
- Fusco, R; Romani, R. Gazz. Chim. Ital. 1946, 76, 419.
- Fusco, R.; Musante, C. Gazz. Chim. Ital. 1938, 68, 147.
- Bacchetti, T. Gazz. Chim. Ital. 1961, 91, 86.
- Eweiss, N.F; Osman, A. J. Heterocycl. Chem. 1980, 17, 1713.
- Eweiss, N.F; Osman, A. Tetrahedron Letts. 1979, 1169.
- Shawali, A.S; Abdelhamid, A.O. Bull. Chem. Soc. Jpn. 1976, 49, 321.
- Gibson, M.S. Can. J. Chem. 1975, 53, 3211.
- Sasaki, T.; Ito, E. J. Heterocycl. Chem. 1981, 18, 1553.
- Dawnis, J.; Lopez, H.; Murry, G. J. Org. Chem. 1977, 42, 1018.
- Abdelhadi, H.A.; Abdallah, T.A.; Hassaneen, H.M. Heterocycles 1995, 41, 1999.
- Mansour, A.K.; Elwan, N.M.; Abdelhadi, H.A.; Abdallah, T.A.; Hassaneen, H.M. Sulfur Letts. 1994, 18, 105.
- Hassaneen, H.M.; Mousa, H.A.H.; Abed, N.M. Heterocycles 1988, 3, 27.
- Shawali, A.S.; Hassaneen, H.M.; Sherif, M.S. J. Heterocycl. Chem. 1980, 17, 1745.
- Hassaneen, H.M.; Abdelhamid, A.O.; Fahmi, A.A.; Shawali, A.S. J. Heterocycl. Chem. 1985, 22, 395.
- Skaric, V.; Skaric, F.; Cizmek, A. J. Chem. Soc. Perkin Tran.1. 1984, 2221.
- Talukdar, P.B.; Sengupta, S.K.; Datta, A.K. Ind. J. Chem. 1986, 25B, 275.
- Mizutani, M.; Sanamitsu, Y.; Tamaru, Y.; Yoshida, Z. J. Org. Chem. 1985, 50, 764.
- Mizutani, M.; Sanamitsu, Y; Tamaru, Y.; Yoshida, Z. J. Org. Chem. 1983, 48, 4585.
- Hübsch, W; Pfleiderer, W. Helv. Chim. Acta 1988, 71, 1379.
- Hassaneen, H.M; Hilal, R.H; Elwan, N.M; Harhash, A; Shawali, A.S. J.Heterocycl. Chem. 1984, 21, 1013.
- Wolkoff, P. Can. J. Chem. 1975, 53, 1333.
- Shawali, A.S; Eweiss, N.F; Hassaneen, H.M; Sami, M. Bull. Chem. Soc. Jpn. 1975, 48, 365.
- North, A.C.T; Philip, D.C; Mathews, F.S. Acta Crystallogr. Sect. A. 1994, 24, 351.
- Sample Availability: Samples of entries 1, 5, 7 and 11 are available from MDPI
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
