Short and Efficient Synthesis of Optically Active N-Tosyl Aziridines from 2-Amino Alcohols
Abstract
:Introduction
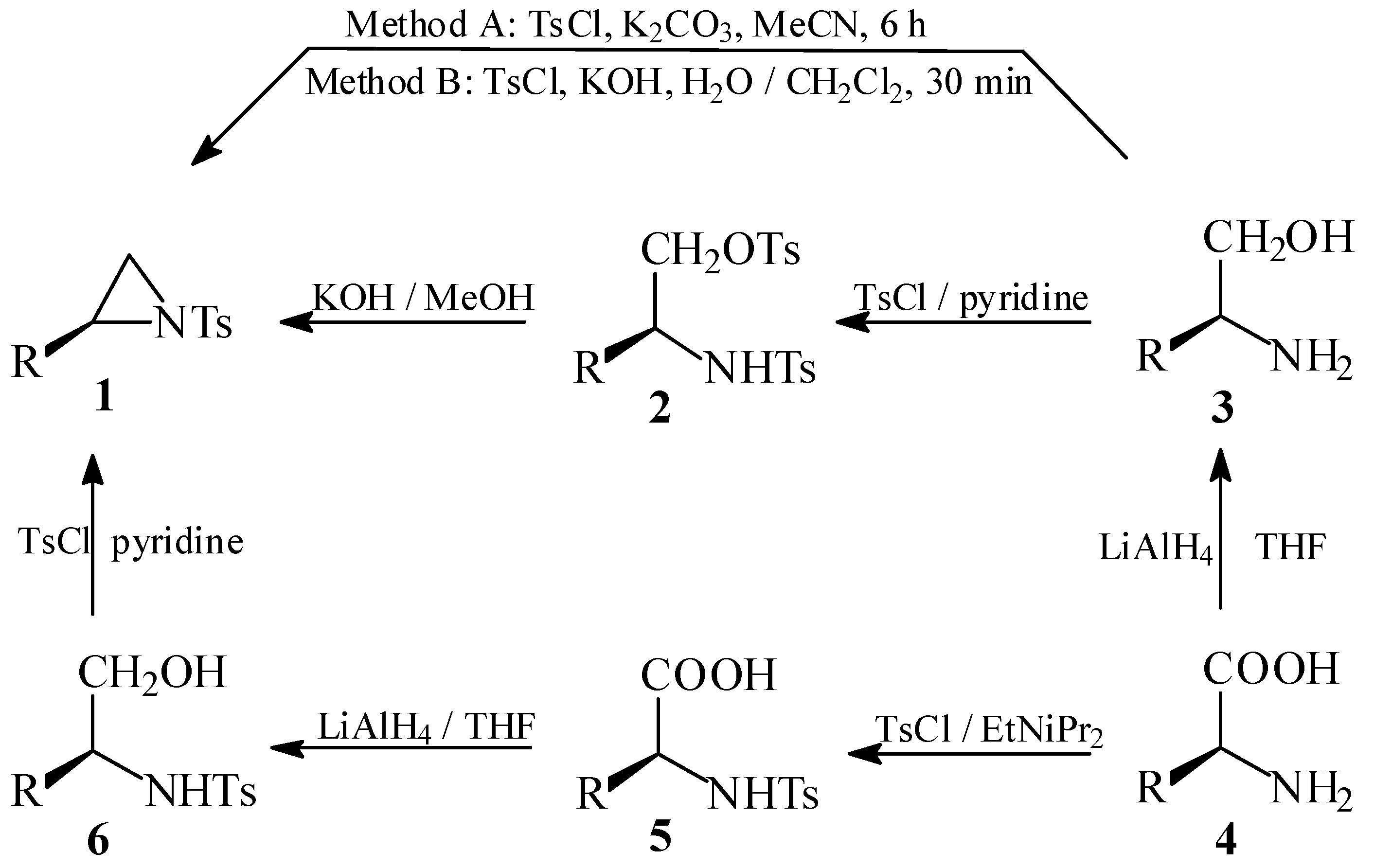
Results and Discussion
| Entry | R = | Method A Yield % | Method B Yield % |
|---|---|---|---|
| 1 | H | 46 | 74 |
| 2 | CH3 | 53 | 78 |
| 3 | C2H5 | 62 | 86 |
| 4 | (CH3)2CH | 75 | 70 |
| 5 | CH3 CH2CH2 | 82 | 73 |
| 6 | (CH3)2CHCH2 | 76 | 52 |
| 7 | C6H5 CH2 | 92 | 64 |
| 8 | CH3SCH2CH2 | 85 | 67 |
| 9 | 4-HO-C6H4CH2a | 87 | 58 |
Conclusions
Experimental
General
Typical Synthetic Procedures
Acknowledgements
References
- Tanner, D. Angew. Chem. Int. Ed. Engl. 1994, 33, 599–619.Osborn, H.M.I.; Sweeney, J. Tetrahedron Asymmetry 1997, 8, 1693–1715.
- Halfen, J.A.; Hallman, J.K.; Schultz, J.A.; Emerson, J.P. Organometallics 1999, 18, 5435–5437.Albone, D.P.; Aujla, P.S.; Taylor, P.C.; Challenger, S.; Derrick, A.M. J. Org. Chem. 1998, 63, 9569–9571.
- Daub, G.W.; Heerding, D.A.; Overman, L.E. Tetrahedron 1988, 44, 3919–3930.
- Berry, M.B.; Craig, D. Synlett 1992, 41–45.
- Craven, A.P.; Dyke, H.J.; Thomas, E.J. Tetrahedron 1989, 45, 2417–2429.Wang, J.Q.; Zhong, M.; Lin, G.Q. Chin. J. Chem. 1998, 16, 65–77.
- Oppolzer, W.; Flaskamp, E.; Bieber, L.W. Helv. Chim. Acta 2001, 84, 141–145.
- Araújo, A.C.V.; Almeida, F.V.; Bieber, L.W. Química Nova 1996, 19, 79–81.
- Martin, A.E.; Ford, T.M.; Bulkowski, J.E. J. Org. Chem. 1982, 47, 412–415. [CrossRef]
- McKennon, M.J.; Meyers, A.I.; Drauz, K.; Schwarm, M. J. Org. Chem. 1993, 58, 3568–3571.Abiko, A.; Masamune, S. Tetrahedron Lett. 1992, 33, 5517.
- Sample Availability: Not available.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Bieber, L.W.; De Araújo, M.C.F. Short and Efficient Synthesis of Optically Active N-Tosyl Aziridines from 2-Amino Alcohols. Molecules 2002, 7, 902-906. https://doi.org/10.3390/71200902
Bieber LW, De Araújo MCF. Short and Efficient Synthesis of Optically Active N-Tosyl Aziridines from 2-Amino Alcohols. Molecules. 2002; 7(12):902-906. https://doi.org/10.3390/71200902
Chicago/Turabian StyleBieber, Lothar W., and Maria C. F. De Araújo. 2002. "Short and Efficient Synthesis of Optically Active N-Tosyl Aziridines from 2-Amino Alcohols" Molecules 7, no. 12: 902-906. https://doi.org/10.3390/71200902
APA StyleBieber, L. W., & De Araújo, M. C. F. (2002). Short and Efficient Synthesis of Optically Active N-Tosyl Aziridines from 2-Amino Alcohols. Molecules, 7(12), 902-906. https://doi.org/10.3390/71200902




