Preparation of Substituted Methyl o-Nitrophenyl Sulfides
Abstract
:Introduction
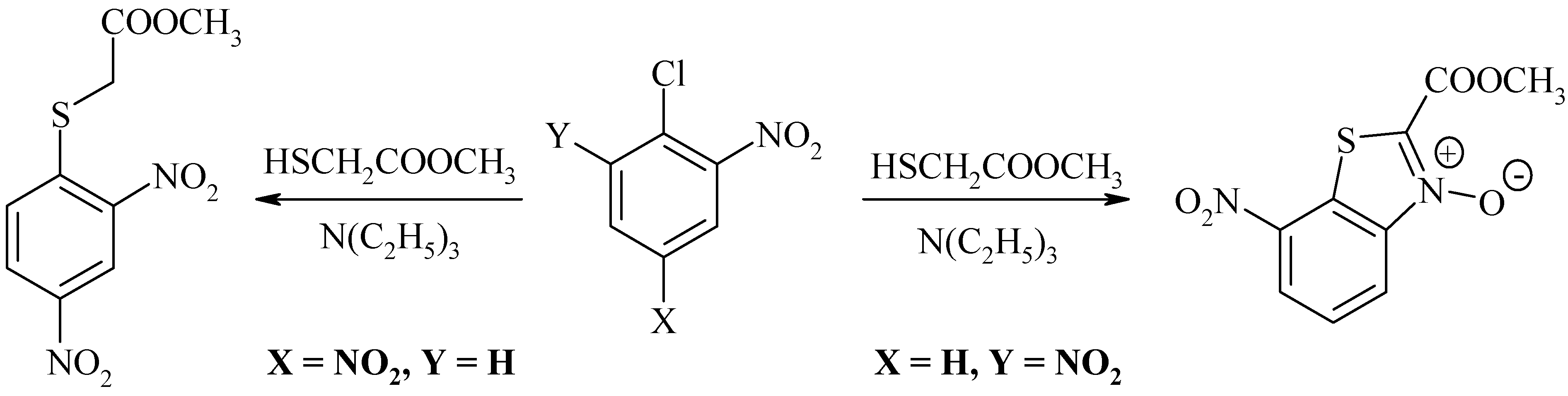
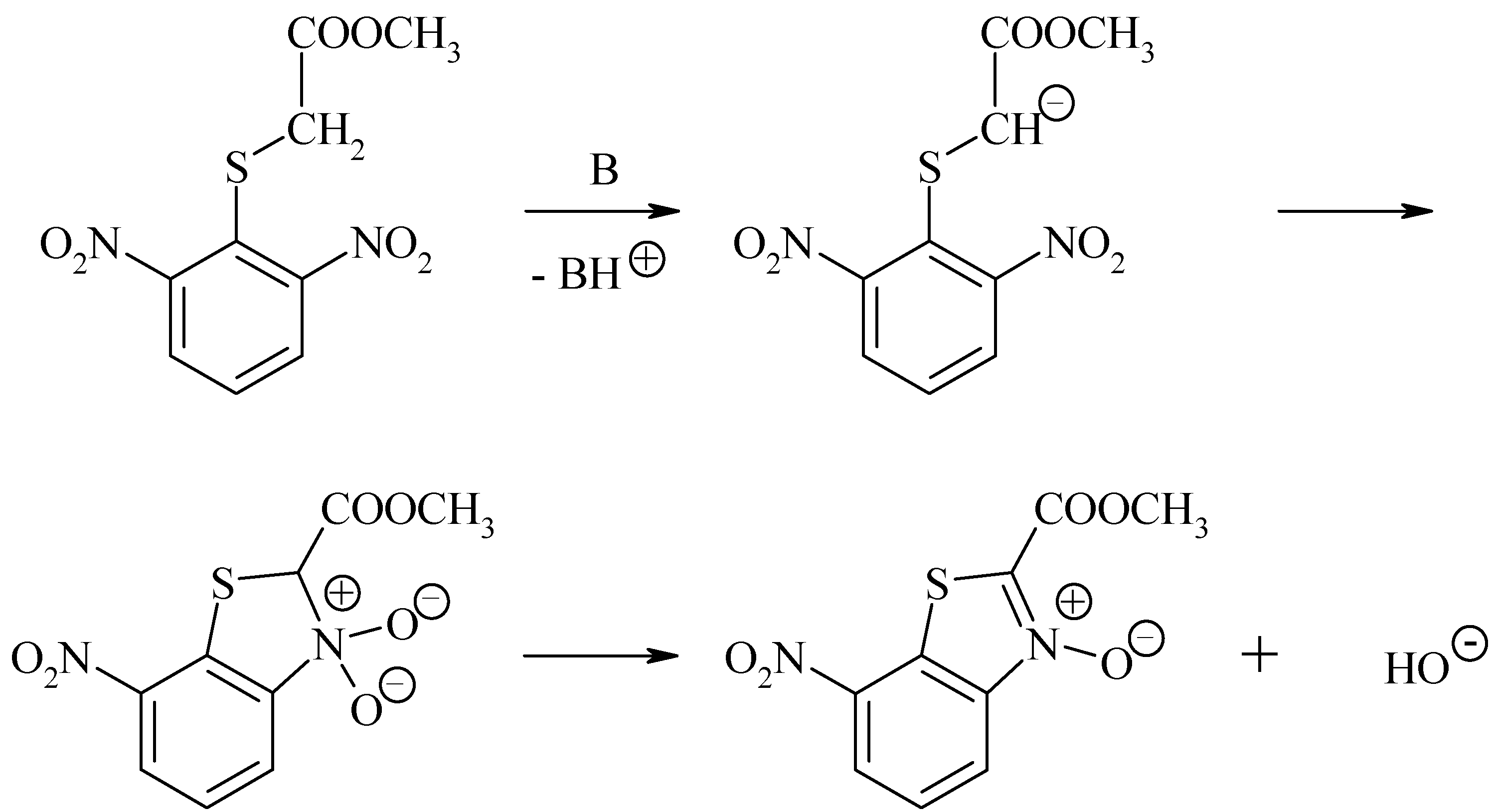
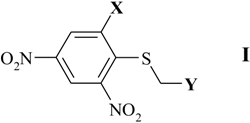
| Comp. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| X | CH3 | CH(CH3)2 | Br | NO2 | COOH | COOH | COOH | COOCH3 | COOCH3 | COOCH3 |
| Y | COOCH3 | COOCH3 | COOCH3 | p-NO2-Ph | COOCH3 | C6H5 | p-NO2-Ph | p-NO2-Ph | COOCH3 | C6H5 |
Results and discussion

Experimental
General methods
Methyl (2-methyl-4,6-dinitrophenylsulfanyl)ethanoate (1).
(4-Nitrophenylmethyl) (2,4,6-trinitrophenyl) sulfide (4).
2-(Methoxycarbonylmethylsulfanyl)-3,5-dinitrobenzenecarboxylic acid (5).
2-(4-Nitrophenylmethylsulfanyl)-3,5-dinitrobenzencarboxylic acid (7).
Methyl 2-(4-nitrophenylmethylsulfanyl)-3,5-dinitrobenzenecarboxylate (8).
Methyl 2-(methoxycarbonylmethylsulfanyl)-3,5-dinitrobenzenecarboxylate (9).
Reaction of methyl 2-chloro-3,5-dinitrobenzenecarboxylate with methyl sulfanylethanoate
Methyl 2-(phenylmethylsulfanyl)-3,5-dinitrobenzenecarboxylate (10).
| Comp. | Solvent for crystallisation | M.p. | Yield | Formula / | Elemental analysis Calculated / Found (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| (°C) | (%) | M.w. | |||||||
| C | H | N | S | Br | |||||
| 1 | Methanol | 48 - 50 | 88 | C10H10N2O6S | 41.96 | 3.52 | 9.79 | 11.20 | |
| 286.3 | 41.94 | 3.57 | 9.78 | 11.39 | |||||
| 2 | Chloroform– Cyclohexane | 93.5 -95 | 81 | C12H14N2O6S | 45.86 | 4.49 | 8.91 | 10.20 | |
| 314.3 | 45.66 | 4.72 | 8.68 | 10.26 | |||||
| 3 | Methanol | 103 - 104 | 78 | C9H7N2O6SBr | 30.79 | 2.01 | 7.98 | 9.13 | 22.76 |
| 351.1 | 30.60 | 2.09 | 7.92 | 9.35 | 22.79 | ||||
| 4 | Chloroform | 161.5 -162.5 | 52 | C13H8N4O8S | 41.06 | 2.12 | 14.73 | 8.43 | |
| 380.3 | 41.27 | 2.03 | 14.69 | 8.43 | |||||
| 5 | Chloroform | 109 - 111 | 62 | C10H8N2O8S | 37.98 | 2.55 | 8.86 | 10.14 | |
| 316.2 | 37.99 | 2.56 | 9.13 | 10.16 | |||||
| 6 | Chloroform | 144 - 146.5 | 60 | C14H10N2O6S | 50.30 | 3.01 | 8.38 | 9.59 | |
| 334.3 | 50.00 | 3.23 | 8.20 | 9.59 | |||||
| 7 | Chloroform | 179.5 - 180 | 60 | C14H9N3O8S | 44.33 | 2.39 | 11.08 | 8.45 | |
| 379.3 | 44.48 | 2.36 | 10.87 | 8.51 | |||||
| 8 | Methanol– Chloroform | 128-129 | 24 | C11H10N2O8S | 45.81 | 2.82 | 10.68 | 8.15 | |
| 393.3 | 45.90 | 2.78 | 10.52 | 8.22 | |||||
| 9 | Toluene | 76 - 77 | 81 | C15H12N2O6S | 40.00 | 3.05 | 8.48 | 9.71 | |
| 330.215 | 40.27 | 3.02 | 8.37 | 9.49 | |||||
| 10 | Methanol | 114.5 - 115 | 86 | C15H11N3O8S | 51.72 | 3.47 | 8.04 | 9.20 | |
| 348.3 | 51.64 | 3.5 | 7.89 | 9.05 | |||||
| Comp. | Solvent | H3 | H5 | CH2 | OCH3 | Ph | Other | |
|---|---|---|---|---|---|---|---|---|
| 1 | CDCl3 | 8.30 | 8.31 | 3.59 | 3.66 | – | δ(ArCH3) 2.76 s | |
| AB, J = 2.4 Hz | s | s | ||||||
| 2 | CDCl3 | 8.27 | 8.34 | 3.58 | 3.68 | – | δ(ArCH) 3.91 sp | |
| d, J = 2.3 Hz | s | s | δ((CH3)2) 1.33 s | |||||
| 3 | CDCl3 | 8.45 | 8.67 | 3.73 | 3.66 | – | − | |
| d, J = 2.4 Hz | s | s | ||||||
| 4 | CDCl3 | 8.69 | 4.26 | – | 8.16 | 7.41 | – | |
| s | s | AA'XX' | ||||||
| 5 | DMSO-d6 | 8.69 | 8.94 | 3.92 | 3.60 | – | – | |
| d, J = 2.5 Hz | s | s | ||||||
| 6 | DMSO-d6 | 8.66 | 8.86 | 4.25 | – | 7.40-7.15 | – | |
| d, J = 2.5 Hz | m | |||||||
| 7 | DMSO-d6 | 8.68 | 8.87 | 4.37 | – | 8.16 | 7.41 | – |
| d, J = 2.5 Hz | s | AA'XX' | ||||||
| 8 | CDCl3 | 8.72 | 8.93 | 4,37 | – | 8.15 | 7.41 | δ(OCH3) 4.01 s |
| d, J = 2.5 Hz | s | AA'XX' | ||||||
| 9 | CDCl3 | 8.62 | 8.70 | 3.74 | 3.66 | – | δ(OCH3) 4.03 s | |
| d, J = 2.5 Hz | s | s | ||||||
| 10 | DMSO-d6 | 8.72 | 8.93 | 4.20 | – | 7.13-7.73 | δ(OCH3) 4.01 s | |
| d, J = 2.5 Hz | s | m | ||||||
| Comp. | Solvent | Ar-S- | X | CH2 | Y = | Y = C6H5, |
|---|---|---|---|---|---|---|
| COOCH3 | C6H4NO2 | |||||
| 1 | CDCl3 | 155.19 (C6); 147.63 (C4); 146.93 (C2); 132.99 (C1) 126.74 (C3); 116.14 (C5) | 21.52 (CH3) | 36.62 | 168.31 (CO) 52.52 (CH3) | – |
| 2 | CDCl3 | 158.42 (C2); 155.75 (C6) | 31.65 (CH) 23.63 (CH3)2 | 38.26 | 168.35 (CO) 52.64 (CH3) | – |
| 148.15 (C4); 131.37 (C1) | ||||||
| 123.13 (C3); 115.98 (C5) | ||||||
| 3 | CDCl3 | 158.42 (C2); 155.75 (C6) | – | 36.30 | 168.35 (CO) 52.64 (CH3) | – |
| 148.15 (C4);131.37 (C1) | ||||||
| 123.13 (C3); 115.98 (C5) | ||||||
| 4 | CDCl3 | 154.92 (C2);147.82 (C4) 141.61 (C1); 124.51 (C3) | – | 40.92 | – | 148.18 (C10) 147.33 (C7) |
| 130.41 (C8) | ||||||
| 121.65 (C9) | ||||||
| 5 | DMSO-d6 | 155.42 (C6); 147.91 (C4) | 169.27 (CO) | 38.60 | 166.61 (CO) 53.44 (CH3) | – |
| 141.48 (C1); 134.26 (C3) | ||||||
| 127.35 (C2); 121.61 (C5) | ||||||
| 6 | DMSO-d6 | 154.04 (C6); 146.51 (C4) | 166.04 (CO) | 40.84 | – | 135.89 (C7) |
| 140.58 (C1); 134.58 (C3) | 128.78 (C8) | |||||
| 127.95 (C9) | ||||||
| 129.08 (C2); 120.52 (C5) | 126.19 (C10) | |||||
| 7 | DMSO-d6 | 155.43 (C6); 147.80 (C4) | 166.73 (CO) | 40.84 | – | 147.95 (C10) |
| 142.04 (C1); 133.52 (C3) | ||||||
| 131.12 (C2); 124.73 (C5) | 145.16 (C7) | |||||
| 127.02 (C8) | ||||||
| 121.26 (C9) | ||||||
| 8 | CDCl3 | 155.23 (C6); 147.11 (C4) | 164.62 (CO) 53.97 (CH3) | 41.39 | – | 147.85 (C10) |
| 140.61 (C1); 135.20 (C3) | ||||||
| 142.83 (C7) | ||||||
| 126.64 (C8) | ||||||
| 130.29 (C2); 120.80 (C5) | ||||||
| 124.24 (C9) | ||||||
| 9 | CDCl3 | 155.13 (C6); 146.96 (C4) | 164.42 (CO) 53.85 (CH3) | 38.37 | 168.34 (CO) 52.94 (CH3) | – |
| 139.94 (C1); 135.66 (C3) | ||||||
| 126.95 (C2); 120.99 (C5) | ||||||
| 10 | CDCl3 | 154.56 (C6); 146.29 (C4) | 164.81 (CO) 53.80 (CH3) | 42.10 | – | 139.91 (C7) |
| 137.58 (C1); 135.21 (C3) | 128.99 (C8) | |||||
| 129.32 (C2); 120.84 (C5) | 128.33 (C9) | |||||
| 126.60 (C10) |
Acknowledgements
References
- Wagner, K.; Heitzer, H.; Oehlmann, L. Chem. Ber. 1973, 106, 640.
- Janík, M.; Macháček, V.; Pytela, O. Collect. Czech. Chem. Commun. 1997, 62, 1429. [CrossRef]
- Janík, M.; Macháček, V. Chem. Listy 1997, 91, 672.
- Kunz, W.; Shurter, R.; Maetzke, T. Pestic. Sci. 1997, 50, 275.
- Rossi, R.H.; Vargas, E. B. J. Org. Chem. 1979, 44, 4100.
- Borsche, W.; Fiedler, A. Chem. Ber. 1912, 45, 271.
- Sarnin, G.P.; Buzykin, B.I.; Nurgatin, V.V.; Mojsak, I.E. Zh. Org. Khim. 1967, 3, 82.
- Gibson, G.P. J. Chem. Soc. 1925, 127, 45. [CrossRef]
- Heller, G. J. Prakt. Chem. 1931, 129, 211.
- Gasparič, J. Collect. Czech. Chem. Commun. 1964, 29, 1374.
- Sane, S.M.; Joshi, S.S. J. Chem. Soc. 1924, 125, 2482.
- Weissberger, A.; Bach, H.; Strasser, E. J. Chem. Soc. 1935, 68, 70.
- Boothoyd, B.; Clark, E.R. J. Chem. Soc. 1953, 1499.
- McCubbin, J.A.; Moir, R.Y.; Neville, G.A. Can. J. Chem. 1970, 48, 934.
- Liu, X.; Reese, C.B. J. Chem. Soc., Perkin Trans. I 1995, 1685.
- Sample Availability: Samples are available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Dudova, K.; Castek, F.; Machacek, V.; Simunek, P. Preparation of Substituted Methyl o-Nitrophenyl Sulfides. Molecules 2002, 7, 7-17. https://doi.org/10.3390/70100007
Dudova K, Castek F, Machacek V, Simunek P. Preparation of Substituted Methyl o-Nitrophenyl Sulfides. Molecules. 2002; 7(1):7-17. https://doi.org/10.3390/70100007
Chicago/Turabian StyleDudova, Katerina, Frantisek Castek, Vladimir Machacek, and Petr Simunek. 2002. "Preparation of Substituted Methyl o-Nitrophenyl Sulfides" Molecules 7, no. 1: 7-17. https://doi.org/10.3390/70100007
APA StyleDudova, K., Castek, F., Machacek, V., & Simunek, P. (2002). Preparation of Substituted Methyl o-Nitrophenyl Sulfides. Molecules, 7(1), 7-17. https://doi.org/10.3390/70100007






